Cardiovascular diseases (CVD) continue to be the main cause of death in our country. Adequate control of lipid metabolism disorders is a key challenge in cardiovascular prevention that is far from being achieved in real clinical practice. There is a great heterogeneity in the reports of lipid metabolism from Spanish clinical laboratories, which may contribute to its poor control. For this reason, a working group of the main scientific societies involved in the care of patients at vascular risk, has prepared this document with a consensus proposal on the determination of the basic lipid profile in cardiovascular prevention, recommendations for its realization and unification of criteria to incorporate the lipid control goals appropriate to the vascular risk of the patients in the laboratory reports.
Las enfermedades cardiovasculares (ECV) siguen siendo la principal causa de muerte en nuestro país. El control adecuado de las alteraciones del metabolismo lipídico es un reto clave en prevención cardiovascular que está lejos de alcanzarse en la práctica clínica real. Existe una gran heterogeneidad en los informes del metabolismo lipídico de los laboratorios clínicos espanoles, lo que puede contribuir al mal control del mismo. Por ello, un grupo de trabajo de las principales sociedades científicas implicadas en la atención de los pacientes de riesgo vascular hemos elaborado este documento con una propuesta básica de consenso sobre la determinación del perfil lipídico básico en prevención cardiovascular, recomendaciones para su realización y unificación de criterios para incorporar los objetivos de control lipídico adecuados al riesgo vascular de los pacientes en los informes de laboratorio.
Cardiovascular diseases (CVDs), including coronary heart disease and cerebrovascular disease are the leading cause of mortality and disability in the world.1 In Spain, CVDs continue to be the leading cause of death, followed by tumors and COVID-19 even during the height of the pandemic.2 As the underlying pathological process in most CVDs, arteriosclerosis is a gradual process that occurs over decades. The main associated risk factors are widely known. Among them, dislipidaemia is a well-known risk factor whose control has proven to reduce Cardiovascular morbimortality.3,4 While there is a large therapeutic armamentarium for dyslipidaemia the level of control over lipid abnormalities is clearly suboptimal, particularly in patients with a (very) high cardiovascular risk, where reducing absolute risk is crucial.5–8
An update of ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice was recently published.9 These guidelines are supported by the major Spanish scientific societies involved in cardiovascular disease, including the CEIPV (Comité Español Interdisciplinario de Prevención Vascular).10–13
Therapeutic targets have been established and widely accepted for lipid-lowering therapies. However, the reference values provided on laboratory biochemistry reports continue to be based on the distribution of values in the general population; unfortunately, the "desirable" values according to the level of cardiovascular risk of the patient all-too-often not provided. In spife of the SEA (Spanish Society of Arteriosclerosis) and 2018 SEC (Spanish Society of Cardiology) recommendations,14,15 lipid values largely exceeding "desirable" values in terms of cardiovascular prevention16 are often reported as "normal", whereas "desirable" values are reported as "abnormally low". This information may be misleading and result in therapeutic abstention in patients with "normal" values, and dose reduction in patients with "abnormally low" values. This is the reason by which a task force of experts from the main scientific societies involved in the prevention and treatment of CVDs For this reason, a working group of the main scientific societies involved in the care of patients at vascular risk have prepared this document with a basic consensus proposal on the determination of the basic lipid profile in cardiovascular prevention, recommendations for its implementation and unification of criteria to incorporate the objectives of lipid control appropriate to the vascular risk of the patients in the laboratory reports.17,18
Pre-analytical considerationsHow, when, and in which cases should lipid profile testing be ordered?Lipid profile is necessary to assess the risk of developing a cardiovascular disease in apparently-healthy subjects and in high-risk clinical conditions, including candidates for cardiovascular surgery patients s. Lipid profile is also required to assess the therapeutic effectiveness of and adherence to lipid-lowering treatments. It is essential in the prevention of CVDs, especially in subjects with a high cardiovascular risk or having relatives with a high cardiovascular risk. Likewise, it is part of the global assessment of other disorders causing secondary dyslipidaemias. Our Task Force deems those recent recommendations from the Spanish Society of Cardiology,9 recently translated,10 and supported by the Spanish Committee of Vascular Prevention,13 provide appropriate reference values (Table 1).
| A. |
| Patients not receiving with lipid-lowering drugs |
|
| Monitoring of therapeutic efficacy and adherence to lipid lowering treatment |
|
| B. |
|
Suspect for familial hypercholesterolemia in patients who developed arteriosclerotic cardiovascular disease before 55 years (men) or 60 years of age (women); subjects with a relative who had early CVD; subjects with relatives with tendon xamtomata; patients with very elevated LDL chol. (adults, >5 mmol/L [190 mg/dL]; children >4 mmol/L [150 mg/dL]) and first-degree relatives with familial hypercholesterolemia.
In children, perform testing from 5 years of age or younger, upon suspicion for homozygous familial hypercholesterolemia (HFHo).
CVD, cardiovascular disease; CVR, cardiovascular risk; LDL-c, cholesterol associated to low density lipoproteins; Apo B, apolipoprotein B.
Laboratory parameters may be influenced by multiple factors. Thus, samples should be collected when the patient is in a "stable metabolic status".19
Recommendation 1: Testing lipid profile is not recommended in a context of non-cardiovascular acute inflammatory process. Lipid profile should be determined during the first 24 h after an acute arteriosclerotic ischemic event.
Lifestyle and pathophysiological status of the patient- a)
The patient should maintain regular habits in the two previous weeks prior to the blood test.
- b)
The patient should not do strenuous exercise before a blood test.
- c)
The patient should remain in sitting position 15 min before the blood test.
- d)
Phlebotomy should be standarised: Venous blood should be drawn with the patient in sitting position (levels of TC and LDL cholesterol may be lower in supine position).
- e)
Exclude secondary dyslipidaemias and dyslipidaemias related to drug therapy. Annex 1.20,21
- f)
In case of an acute inflammatory process, phlebotomy should be performed at least 2–4 weeks after the process, since the process may cause a decrease in total cholesterol, LDL cholesterol, and HDL cholesterol, and an increase in triglycerides.22–25
- g)
In case of acute coronary syndrome (or other acute ischemic atherosclerotic event), lipid profile should be obtained within the first 24 h.26–28 If it is performed > 24 h after the acute process, levels of total cholesterol and LDL cholesterol may be reduced with respect to the values normally found in the patient, a phenomenon that should be taken into account in clinical decision-making. In patients that have never undergone a lipid profile test, Lp (a) testing is recommended. Although Lp (a) values may increase in acute processes, variation is modest,29,30 which enables the detection of patients with significantly elevated Lp (a) in early stages.
- •
Most lipid parameters offer little variation regardless of the patient being in fasting conditions or not.31
- •
The main clinical guidelines do not require fasting lipid profile, at least for an initial cardiovascular risk assessment or for diagnosis of isolated hypercholesterolemia, i.e. familial hypercholesterolemia or elevated Lp(a) in the absende of increased triglycerides. Non-fasting lipid values may better predict the risk for ASCVD, since they provide a more accurate insight into the postprandial status of the patient and the influence of residual risk.32
- •
Triglyceride concentration is the only parameter that changes substantially after food intake.32 Given that the Friedewald’s formula is significantly inaccurate in patients with Tg >150 mg/dL, it is recommended that LDL cholesterol is calculated using Martin/Hopkins formula33 (Annex. Supplementary Material Table S3) or that Non-HDL cholesterol is calculated instead in these patients.
- •
Fasting is recommended if Tg ≥4.5 mmol/L (≥398 mg/dL) prior to the initiation of a drug therapy that can cause severe hypertriglyceridaemia (i.e. isotretinoin) and in genetically predisposed subjects with a history of hypertriglyceridaemic pancreatitis. Fasting is also advised when the laboratory request includes additional laboratory tests that require that samples are collected in fasting conditions, or when morning samples are required (i.e. fasting glucose or parameters affected by circadian rhythm).
- •
Fasting and non-fasting lipid profile should be considered as complementary rather than as mutually exclusive.
- •
Cholesterol and triglycerides are generally tested using enzymatic methods, which determinations having a variability <10% (Annex. Supplementary Material Table S2).18 However, within-subject variability and variability resulting from sample collection conditions (≈20% for triglycerides and ≈10% for HDL cholesterol and LDL cholesterol), make it necessary that lipid profile testing is repeated in primary prevention patients without a clear indication for immediate initiation of a lipid-lowering therapy.18
Recommendation 2: Fasting is not required for lipid profile evaluation as an initial cardiovascular risk assessment. If levels of triglycerides exceed Tg ≥ 4.5 mmol/L (≥398 mg/dL), a second determination is recommended in fasting conditions for confirmation of results.
Analytical considerationsShould the analytical method be reported?Lipid profile determination should always be performed using the same methods, and a change in the testing method should always be reported. It is necessary that physicians are aware of the laboratory method used in lipid profile testing, since interferences or misinterpretations may occur.
Recommendation 3: Reporting the laboratory technique or a change of units is essential for a correct interpretation of laboratory results.
Methods for the determination of LDL cholesterolThe method of reference for testing LDL cholesterol involves separating lipoproteins by density-gradient ultracentrifugation, a time-consuming method that is only available in specialized laboratories. For this reason, LDL cholesterol is often estimated by measuring total cholesterol and triglycerides (using enzymatic methods), and by direct HDL cholesterol determination. Friedewald's is the most frequently used formula.34
Friedewald’s formula for the estimation of LDL cholesterol (in mg/dL)
Friedewald’s formula assumes the absence of chylomicrons and a specific of cholesterol/Tg ratio in VLDL (1/5 in mg/dL; 1/2.2 in mmol/L). In VLDL, the Tg/cholesterol ratio progressively increases as hypertriglyceridaemia exacerbates; therefore, in patients with hypertriglyceridaemia, the formula overestimates VLDL cholesterol and thus underestimates LDL cholesterol. The formula has an acceptable accuracy when Tg concentration is <200 mg/dL and it should not be used if Tg > 400 mg/dL (Recommendation 4).
The Martin-Hopkins formula replaces number 5 in the Friedewald’s formula (c-VLDL = Tg/5) with divisors that vary according to the levels of Tg and non-HDL cholesterol of the patient (Annex. Supplementary Material Table).33 The Martin–Hopkins formula is more accurate than Friedewald's when Tg > 150 mg/dL, LDL cholesterol <100 mg/dL, and especially when <70 mg/dL.
The Sampson formula is more complex and provides similar results to those of Martin-Hopkins for patients with Tg < 400 mg/dL. As a result, the former is less frequently used. In patients with Tg > 400 mg/dL, the use of formulas for the estimation of LDL cholesterol is not recommended, due to their poor reliability.
Ultracentrifugation, the classical reference method for LDL cholesterol determination, is labour intensive one and is only used in very specialized laboratories. There is a direct, accurate, and widely available measurement method. The use of this marker is recommended if Tg > 400 mg/dL or LDL < 70 mg/dL, when LDL cholesterol determination methods are more inaccurate.33
When a direct method cannot be used to determine LDL cholesterol, the use of non-HDL cholesterol as a marker of "atherogenic" cholesterol is recommended.35 Determination of apolipoprotein B can also be used (Apo B). Non-HDL cholesterol does not require Tg determination nor is it influenced by fasting. Moreover, it strongly correlates with levels of Apo B.
Recommendation 4: The Friedewald formula is accurate in most patients with LDL cholesterol >100 mg/dL and Tg < 150 mg/dL. The modified Martin–Hopkins formula is superior for the estimation of LDL cholesterol, especially in patients with low LDL-c concentrations <70 mg/dL, Tg concentrations 150−400 mg/dL, and in non-fasting conditions. Direct LDL cholesterol assays should be used to determine LDL cholesterol if Tg ≥400 mg/dL.
In patients with significantly elevated Lp (a) concentrations, LDL cholesterol estimation should be corrected using the following formula:
Potential Lp (a) elevation should be especially considered in Sub Saharan patients, patients with the nephrotic syndrome, on peritoneal dialysis, or with a poor decrease of LDL cholesterol after having received a lipid-lowering therapy.
Post-analytical considerationsMarkers of "normality" and alertsThe clinical laboratory plays a crucial role in the assessment of cardiovascular risk in patients with dyslipidaemia. It is essential that specific reference values are established for the pediatric population.
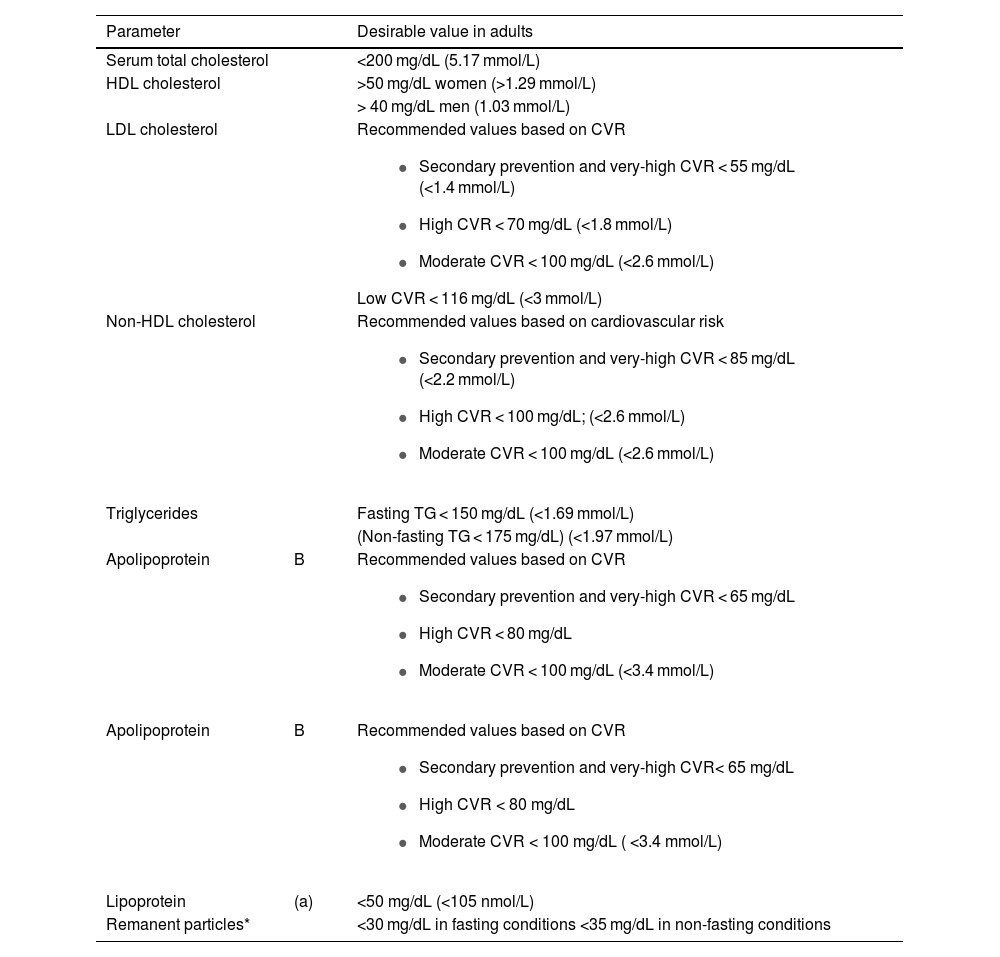
Desirable values should be provided in terms of cardiovascular risk and prevention on lipid profile reports.14–16Table 2 shows the desirable values for the main lipid parameters established for adults by the European Society of Cardiology, Arteriosclerosis and the Spanish Society of Laboratory Medicine (2019).17,18,36
Desirable lipid values in adults, according to the European Societies of Arteriosclerosis and Laboratory Medicine.17,18,36
| Parameter | Desirable value in adults | |
|---|---|---|
| Serum total cholesterol | <200 mg/dL (5.17 mmol/L) | |
| HDL cholesterol | >50 mg/dL women (>1.29 mmol/L) | |
| > 40 mg/dL men (1.03 mmol/L) | ||
| LDL cholesterol | Recommended values based on CVR
| |
| Non-HDL cholesterol | Recommended values based on cardiovascular risk
| |
| Triglycerides | Fasting TG < 150 mg/dL (<1.69 mmol/L) | |
| (Non-fasting TG < 175 mg/dL) (<1.97 mmol/L) | ||
| Apolipoprotein | B | Recommended values based on CVR
|
| Apolipoprotein | B | Recommended values based on CVR
|
| Lipoprotein | (a) | <50 mg/dL (<105 nmol/L) |
| Remanent particles* | <30 mg/dL in fasting conditions <35 mg/dL in non-fasting conditions | |
CVR, cardiovascular risk.
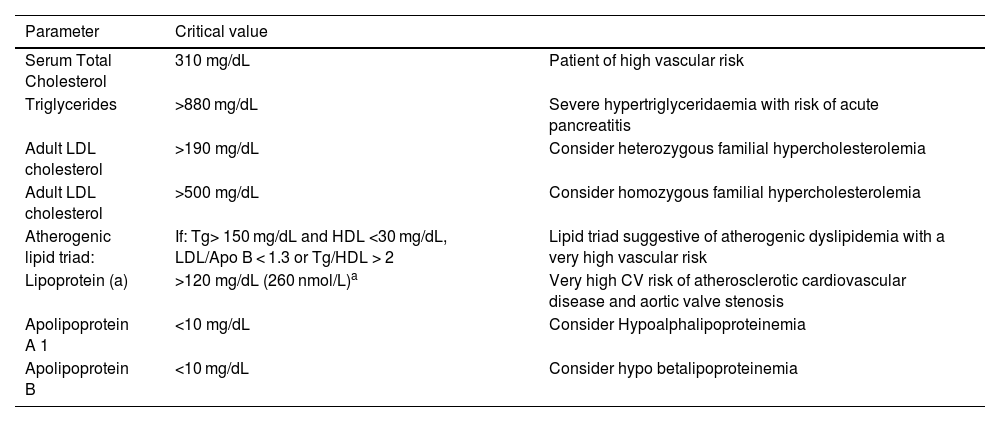
Critical values should be flagged and reported as such to the requesting physician, as shown in Table 3.
Recommended alerts of the information system/laboratory report.
| Parameter | Critical value | |
|---|---|---|
| Serum Total Cholesterol | 310 mg/dL | Patient of high vascular risk |
| Triglycerides | >880 mg/dL | Severe hypertriglyceridaemia with risk of acute pancreatitis |
| Adult LDL cholesterol | >190 mg/dL | Consider heterozygous familial hypercholesterolemia |
| Adult LDL cholesterol | >500 mg/dL | Consider homozygous familial hypercholesterolemia |
| Atherogenic lipid triad: | If: Tg> 150 mg/dL and HDL <30 mg/dL, LDL/Apo B < 1.3 or Tg/HDL > 2 | Lipid triad suggestive of atherogenic dyslipidemia with a very high vascular risk |
| Lipoprotein (a) | >120 mg/dL (260 nmol/L)a | Very high CV risk of atherosclerotic cardiovascular disease and aortic valve stenosis |
| Apolipoprotein A 1 | <10 mg/dL | Consider Hypoalphalipoproteinemia |
| Apolipoprotein B | <10 mg/dL | Consider hypo betalipoproteinemia |
LDL, low density lipoprotein; Apo, apolipoprotein; Tg, triglycerides; CV, cardiovascular.
Recommendation 5: On laboratory reports, the reference values for lipid parameters should always be referred to to the cardiovascular risk of the patient, rather than to normal reference values for the general population. The use of asterisks marking values outside the population normality range is discouraged. The use of flagging systems is recommended for extreme lipid concentrations suggestive of severe dyslipidaemias. Specific values should be established for the pediatric population.
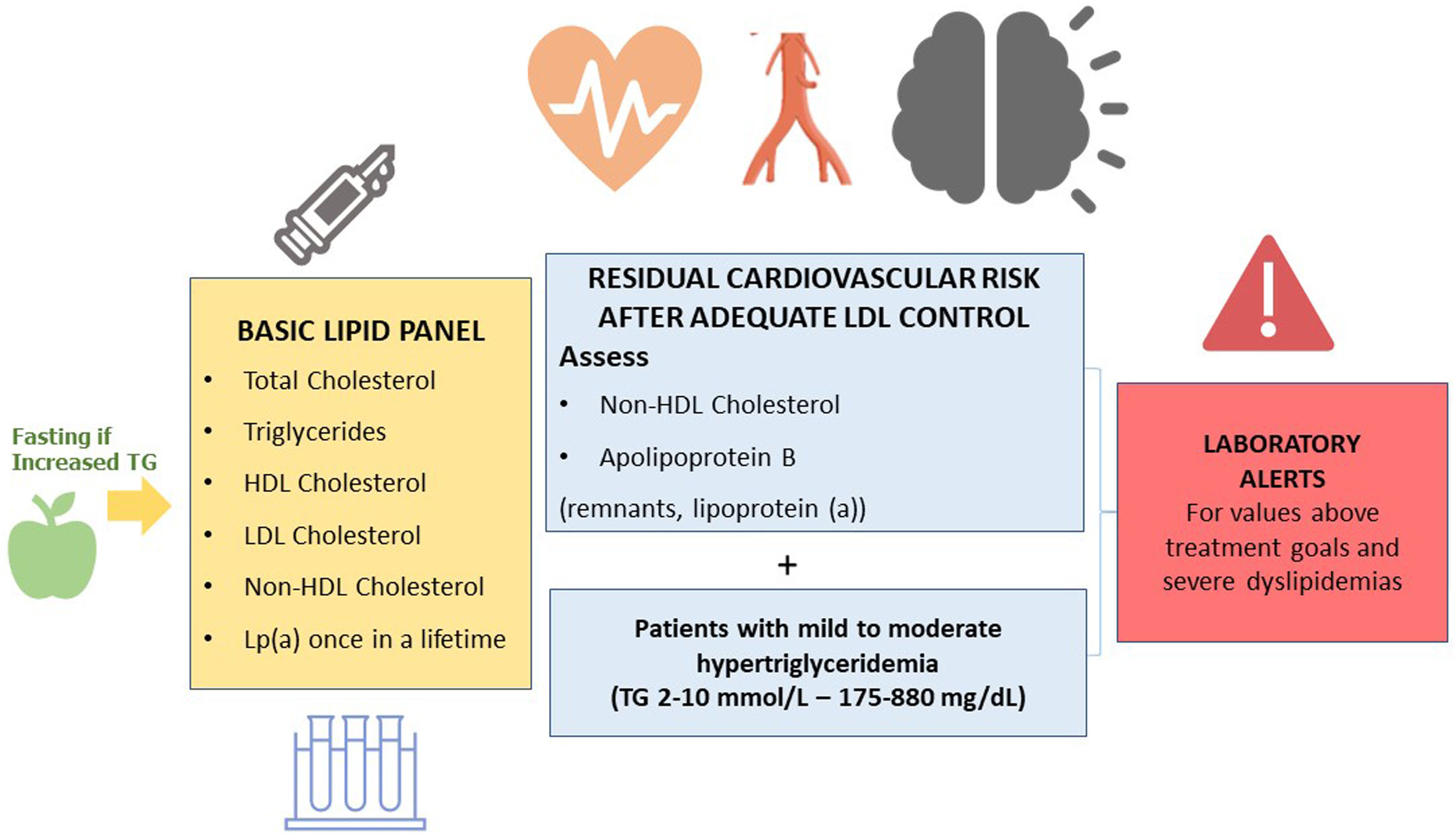
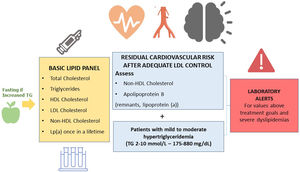
What parameters should a basic lipid profile include?The basic lipid profile should include the determination of total cholesterol, HDL cholesterol, triglycerides, non-HDL cholesterol, and LDL cholesterol.9,36–39 (Fig. 1).
The Consensus Statements of the European Society of Arteriosclerosis and the European Society of Laboratory Medicine also recommend the estimation of remnant particles.9–17 Elevated lipoprotein (a) confers an increased vascular risk. Therefore, it is recommended that it is measured at least once in life, since levels are substantially determined by genetic factors.9–17
In patients with Tg > 400 mg/dL, direct determination of LCL cholesterol is recommended in order to obtain more reliable values.40 If available, Apo B is a marker of special interest, since it is the best marker of the number of atherogenic lipoproteins.41 When direct determination of LDL cholesterol or Apo B is not available, non-HDL cholesterol can be used as an approximation.
Recommendation 6: The basic lipid profile should include the determination of total cholesterol, HDL cholesterol, triglycerides, non-HDL cholesterol, and LDL cholesterol estimation. In patients with mild/moderate hypertriglyceridaemia, testing non-HDL cholesterol and Apo B is recommended for assessing residual cardiovascular risk.
What is non-HDL cholesterol tested for?The estimation of non-HDL cholesterol involves a simple calculation (total cholesterol - HDL cholesterol) that reflects the cholesterol of atherogenic lipoproteins. Non-HDL cholesterol strongly correlates with Apo B concentrations. Also, it is the parameter of reference in the vascular risk assessment formulas SCORE2 (Systematic Coronary Risk Evaluation) and SCOREOP (Systematic Coronary Risk Evaluation old people).9,42,43 An additional advantage of this parameter is that it is not influenced by fasting. Moreover, it can be determined in patients with Tg > 400 mg/dL or be useful as a guideline in laboratories where direct LDL or Apo B determination is not available.44
When should Apolipoprotein B used?Apo B is an excellent predictor of cardiovascular events, since this apoprotein is found in the main atherogenic lipoproteins, namely: LDL, lipoprotein (a), VLDL and IDL.41–45 Testing Apo B is equivalent to measuring the amount of atherogenic lipoproteins, since each one contains a single molecule of Apo B. Apo B values are not affected by fasting. The amount of lipoparticles can also be measured by MRI (magnetic resonance imaging). However, this technique is not available in routine clinical practice.46
Apo B is especially relevant in patients with elevated triglycerides, diabetes mellitus, obesity, metabolic syndrome, or very low LDL cholesterol. In these cases, measurement or estimation of LDL cholesterol may be inaccurate and not consider the atherogenic component of other lipoproteins.
Apo B test is rarely included in standard lipid profiles or ASCVD risk assessment tests. Monogenic disorders such as familial hypercholesterolemia (FH) can be easily recognized using a standard panel of lipids. In these cases, measuring Apo B is not necessary (Annex. Supplementary Material, Table S4).47 On another note, Apo B concentration helps classify severe dyslipidaemias, such as combined familial hyperlipidaemia and familial dysbetalipoproteinemia48 (Annex. Supplementary Material. Figure).
Recommendation 7: Testing Apo B is recommended for assessing vascular risk; classify dyslipidaemias and characterize particle size. It is also preferable to non-HDL cholesterol testing in patients with mild-to-moderate hypertriglyceridaemia (175−880 mg/dL), diabetes, obesity, metabolic syndrome, or very low LDL cholesterol (<70 mg/dL).
When should lipoprotein (a) be determined?Testing Lp (a) is recommended at least once in life to estimate vascular risk.9,49–52 This determination is especially relevant in patients with early-onset cardiovascular disease, familial hypercholesterolemia, poor response to statin therapy, aortic stenosis, or recurrent ischemic events, and in relatives to patients with elevated Lp (a). The cardiovascular risk of patients with very elevated Lp (a) (>180 mg/dL/>430 nmol/L) is similar to that of patients with heterozygous familial hypercholesterolemia.53,54 One of the challenges of Lp(a) determination is the variability of results across the different detection techniques. Another disadvantage is the unavailability of a direct equivalence between values reported in mg/dL and in nmol/L, according to the different apoprotein (a) isoforms.
Lp (a) should only be measured once in life, given that it is substantially determined by genetics and there are no specific drug therapies available as yet. Exceptions to this rule include transition to menopause, pregnancy, use of oral contraceptives, chronic kidney disease/nephrotic syndrome, or when a specific treatment is used to reduce Lp (a) or modulate the recommended therapeutic options, such as PCSK9 inhibitors.55
Recommendation 8: Lp (a) should be determined only once in life, except when its levels may be affected by significant changes, as the development of nephrotic syndrome or the use of a therapy to reduce Lp (a). The most appropriate units of measurement are nmol/L. (Annex. Supplementary Material. Comment).
Should inflammation be assessed in patients with arteriosclerosis?Chronic inflammatory processes are associated with an increased cardiovascular risk, regardless of the risk attributable to conventional risk factors.56 High-sensitivity C-reactive protein is the analytical parameter most frequently used as a maker of low-intensity inflammation. It has a high variability, and there is no defined consensus on the values that should be considered ‘elevated’ for the estimation for vascular risk assessment.36
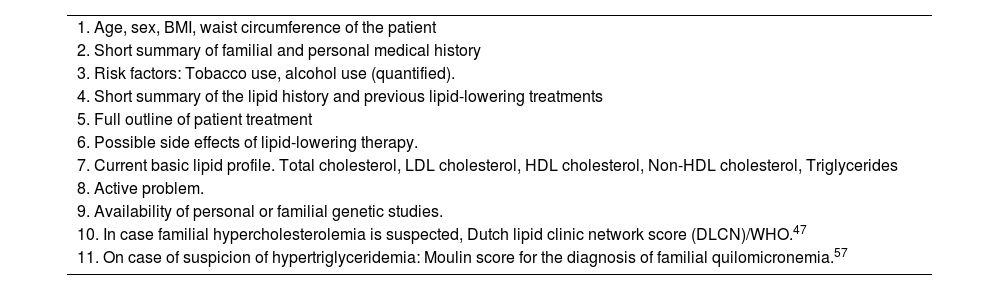
Innovations in the diagnosis of dyslipidaemias: parameters for an e-consultationFor an e-consultation to be rapid and effective, the basic parameters to be included for the diagnosis of dyslipidaemias are shown in Table 4.
Reference data required for assessing cardiovascular riks in an e-consultation.
| 1. Age, sex, BMI, waist circumference of the patient |
| 2. Short summary of familial and personal medical history |
| 3. Risk factors: Tobacco use, alcohol use (quantified). |
| 4. Short summary of the lipid history and previous lipid-lowering treatments |
| 5. Full outline of patient treatment |
| 6. Possible side effects of lipid-lowering therapy. |
| 7. Current basic lipid profile. Total cholesterol, LDL cholesterol, HDL cholesterol, Non-HDL cholesterol, Triglycerides |
| 8. Active problem. |
| 9. Availability of personal or familial genetic studies. |
| 10. In case familial hypercholesterolemia is suspected, Dutch lipid clinic network score (DLCN)/WHO.47 |
| 11. On case of suspicion of hypertriglyceridemia: Moulin score for the diagnosis of familial quilomicronemia.57 |
BMI, body mas index; LDL, low density lipoprotein; HDL, high density lipoprotein, WHO, World Health Organization.
This study did not receive any funds.
Competing interestsNone.
Committee of Lipids and Vascular Diseases of the Spanish Society of Laboratory Medicine and Task Force for Cardiac Rehabilitation of the Spanish Society of Cardiology.
Nuria Amigó Grau. Biosfer Teslab, IISPV, CIBERDEM, Universidad Rovira i Virgili, Tarragona, España.
Pilar Calmarza Calmarza. Departamento de Bioquímica.Hospital Universitario Miguel Servet. Zaragoza, España.
Silvia Camòs Anguila. Servicio de Bioquímica Clínica, Hospital Hospital Universitari de Girona Josep Trueta.
Beatriz Candás Estebanez. Laboratorio Clínico. Hospital de Barcelona.
María José Castro Castro. Laboratorio Clínico Hospital Universitario de Bellvitge, Barcelona, España.
Carla Fernández Prendes. Laboratorio de Bioquímica Hospital Universitario Germans Trias i Pujol, Barcelona, España.
Irene González Martínez. Servicio de análisis clínicos. Hospital 12 de Octubre. España.
María Martín Palencia. Hospital Universitario de Burgos. España
Carlos Romero Román. Laboratory of Clinical Biochemistry. Hospital de Albacete, España.
José Puzo Foncillas. Laboratorio Hospital General San Jorge, Huesca, España.
Almudena Castro Conde. Unidad de Rehabilitación Cardiaca. Servicio de Cardiología. Hospital Universitario La Paz. Madrid, España.
Rosa Fernández Olmo. Servicio Cardiología Hospital Universitario de Jaén, España.