Information on experience/management of severe hypoglycaemic events (SHEs) among people with insulin-treated diabetes (PWD) and caregivers (CGs) providing care to PWD was sought.
Materials and methodsAn online cross-sectional survey was conducted in eight countries. Inclusion criteria: PWD (aged≥18 years; self-reported type 1 [T1D] or insulin-treated type 2 [T2D] diabetes; experienced ≥1 SHE [hypoglycaemia requiring external assistance] in past 3 years); CGs (layperson aged ≥18 years; caring for PWD meeting all criteria above except age [≥4 years]). This descriptive analysis provides data from Spain. SHE-associated data relate to the most recent SHE.
ResultsAcross all groups (T1D PWD, n=106; T2D PWD, n=88, T1D CG, n=87; T2D CG, n=96), 76–89% reported that the SHE occurred at home; most common cause was eating less than planned (38–53%). Most usual action during the SHE was to intake carbohydrates (67–84%); glucagon use was low (9–36%). Discussion of the SHE with their healthcare provider (HCP) was reported by 70–75% of PWD. During the SHE, 35–69% of PWD/CGs reported feeling scared, unprepared and/or helpless.
ConclusionsMost SHEs occurred outside the healthcare setting; treatment therefore depends greatly on CGs. SHEs have a negative emotional impact on PWD/CGs, underscoring the need for HCPs to discuss SHEs with PWD/CGs, and to provide tools and strategies to prevent and effectively manage SHEs.
Se recopiló información sobre la experiencia y el manejo de episodios de hipoglucemia grave (EHG) entre personas con diabetes (PCD) tratadas con insulina y sus cuidadores.
Materiales y métodosSe realizó una encuesta transversal online en ocho países. Criterios de inclusión: PCD de ≥18 años con diabetes tipo 1 (DT1) o diabetes tipo 2 (DT2) tratada con insulina autoinformada y ≥ 1 EHG en los últimos tres años, que requirió asistencia externa; cuidador no profesional de ≥ 18 años que proporciona atención a PCD que cumplen los criterios anteriores excepto la edad (≥ cuatro años). Este análisis descriptivo proporciona información de España. Los datos de EHG se refieren al episodio más reciente.
ResultadosEn todos los grupos (PCD DT1, n = 106; PCD DT2, n = 88; cuidador DT1, n = 87; cuidador DT2, n = 96), un 76 a 89% refirió que el EHG ocurrió en casa; la causa más común fue comer menos de lo planeado (38 a 53%). La acción más habitual durante el EHG fue ingerir carbohidratos (67 a 84%); en pocos casos se usó glucagón (9 a 36%). El 70 a 75% de las PCD comentó el EHG con su profesional sanitario. Un 35 a 69% de las PCD/cuidadores refirieron que se sintieron asustadas, no preparadas y/o indefensas durante el EHG.
ConclusionesLa mayoría de los EHG no ocurrieron dentro del sistema sanitario y, por tanto, el tratamiento depende fundamentalmente de los cuidadores. Los EHG tienen un impacto emocional negativo en las PCD y en los cuidadores, destacando la necesidad de que los profesionales sanitarios hablen con ellos sobre este tema y de proporcionar herramientas y estrategias para prevenir y controlar eficazmente los EHG.
Hypoglycaemia is a common complication in people with type 1 (T1D) and type 2 diabetes (T2D).1,2 Severe hypoglycaemia is associated with an increased risk of morbidity and mortality1,3–5 and imposes a significant psychological burden on people with diabetes (PWD) and their caregivers (CGs). This may include extreme fear of hypoglycaemia, which can negatively impact quality of life and diabetes management.6–8 Hypoglycaemia and fear of hypoglycaemia are major challenges to be overcome in achieving optimal blood glucose control.2,6
Reported incidences of severe hypoglycaemia vary according to type of diabetes (e.g. T1D vs T2D), definition of severe hypoglycaemic events (SHEs) used, drugs/regimen employed, and study methodology. In a recent systematic review of studies conducted in Spain, the rate of SHEs ranged from 0.90 to 1.50/patient/year in people with T1D, and from 0.3 to 0.63/patient/year in those with T2D.8 Major risk factors for severe hypoglycaemia among people with T1D and T2D include mistimed/misdosed insulin, irregular/restricted food intake, history of severe hypoglycaemia and impaired awareness of hypoglycaemia.2,9–14
SHEs impose a significant financial burden on the healthcare system, with hospitalisation being a major contributing factor.15–17 The direct total cost of treating insulin-related severe hypoglycaemia in Spain has been estimated at €292.6 million, with average costs per episode of €716.82 and €680.49 in people with T1D and T2D, respectively.18 In a study comparing the cost of treating SHEs across nine European countries, the direct cost associated with an SHE per drug-treated patient was highest in Spain (€15.77).19
Preventing severe hypoglycaemia and optimising SHE management are important goals,1 and information on the experience and behaviours of PWD and CGs regarding SHEs in Spain could be helpful. The international CRASH (Conversations and Reactions Around Severe Hypoglycaemia) survey was developed to enhance our understanding of the experience and behaviours of PWD (T1D or insulin-treated T2D; referred to as T1D PWD and T2D PWD throughout) and CGs caring for people with insulin-treated diabetes (referred to as T1D CGs and T2D CGs throughout) regarding the management of SHEs in a population at high risk of such events. SHE-related areas investigated in the survey included social context, management strategies, conversations with healthcare providers (HCPs) and psychosocial impact. This publication focuses on CRASH data from PWD and CGs in Spain.
Materials and methodsStudy designCRASH was a cross-sectional survey study (conducted 9 October 2018–25 February 2019) in which T1D PWD, insulin-treated T2D PWD, T1D CGs and T2D CGs from eight countries (Canada, China, France, Germany, Japan, Spain, the UK and the USA) completed a 30-min online questionnaire. The Western Institutional Review Board (WIRB) provided ethics approval for the protocol and informed consent forms for each country.
Participant recruitmentPurposive sampling was used to identify and recruit PWD and CGs separately (i.e. no PWD–CG dyads were actively recruited) from medical research panels comprising individuals who have agreed to take part in surveys of this type. As the study was descriptive only, no power calculation was performed. Sample size was based on anticipated access to participants via the medical research panels. Overall, CRASH aimed to recruit ∼400 individuals (100 T1D PWD, 100 T2D PWD, 100 T1D CGs, and 100 T2D CGs) from Spain.
Participant screeningParticipants were screened for eligibility using a series of questions based on the following inclusion and exclusion criteria. At the time of survey completion, PWD had to: be aged≥18 years; have self-reported T1D or insulin-treated T2D; have experienced ≥1 SHE in the past 3 years; be receiving insulin (via injections or pump). PWD also had to have been receiving insulin at the time of the most recent SHE. For T2D PWD, insulin could be administered in combination with oral diabetes drugs (except sulphonylureas) or injectable diabetes treatments.
All CGs were laypeople aged≥18 years who, at the time of survey completion, provided care for an individual with insulin-treated diabetes (not one who participated in the study, but one who met all the criteria listed above for PWD participants with the exception that PWD minimum age could be 4 years) and/or were relied upon in the event of an SHE. A CG could be living in the same household as the person with diabetes for whom they cared, or be a family member, relative, co-worker, teacher, roommate, or domestic helper.
PWD and CGs with schizophrenia or bipolar disorder, or those with≥10 prespecified health disorders, were excluded from the study.
Data collection: survey questionnaire and endpointsAll eligible participants (PWD and CGs) provided electronic consent through the web survey interface prior to administration of any study procedures or measures. The screening questions and main questionnaire were completed online.
The main questionnaire (which is included as Supplementary Material) comprised 48 questions with additional subquestions following predefined skip patterns. Questions were close ended with no free text fields. Most survey sections were programmed so that participants had to answer each question before moving on to the next. The questionnaire underwent pilot testing and translation into Spanish before implementation. This manuscript focuses on the main, clinically relevant findings of the survey.
Data collected included self-reported participant demographics plus general experience and management of severe hypoglycaemia (frequency; participant knowledge/recognition of symptoms; causes), focusing on the most recent SHE experienced by individuals with diabetes (location of the SHE; time of day; actions taken during/after the event; knowledge/use of management strategies; use of emergency services; conversations with HCPs; and psychosocial impact). An SHE was defined in the survey as: ‘A low blood sugar event that you cannot treat by yourself. This means a low blood sugar emergency during which you might have had seizures, coma or nearly lost consciousness. During such an event, you will need help from another person’.
The questionnaire was designed to elicit information from T1D PWD and T2D PWD on themselves and their own experiences, and from T1D CGs and T2D CGs on themselves as well as on the people with T1D or T2D for whom they cared. It should be noted that CGs may not have provided care, or even been present, during the most recent SHE, and that some PWD may have lost consciousness. Hence, participants’ responses to questions about the most recent SHE should be regarded as a report of what happened or what they were told had happened.
Awareness of hypoglycaemia was assessed in PWD using the Gold score, based on their responses to the question ‘Do you realise when a low blood sugar (hypoglycaemia) event is starting?’ using a 7-point Likert scale (1 representing ‘always aware’ and 7 representing ‘never aware’). Impaired awareness is defined as a score of ≥4.20
Statistical analysisAll data were analysed using descriptive statistics and are presented as n (%) for participants with specific responses to categorical questions or mean±standard deviation (SD), unless otherwise specified, for continuous variables. Incomplete surveys were not included in the analyses. The use of predefined skip patterns meant that not all respondents were asked to complete all questions; hence, denominators can vary by question. The results are presented by diabetes and participant type (T1D PWD, T2D PWD, T1D CG and T2D CG).
Statistical analyses were performed using Statistical Software package SAS V9.4 (The SAS Institute, Cary, NC, USA).
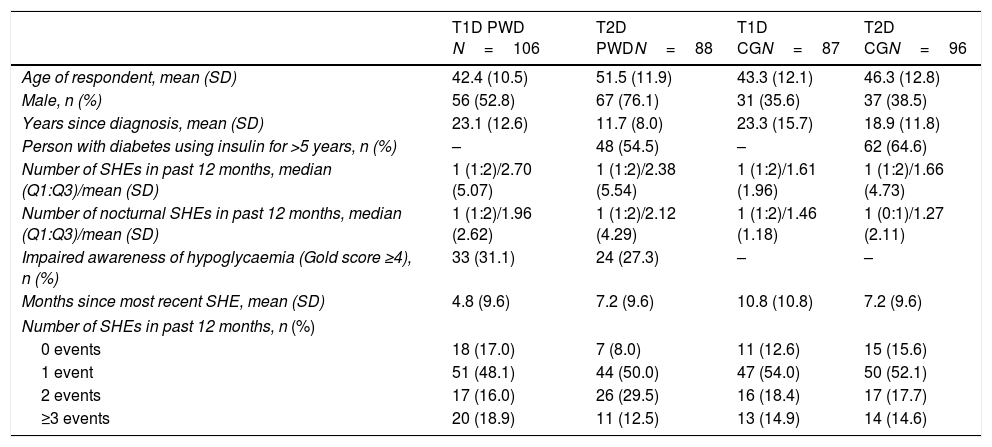
ResultsDemographics and clinical dataOf 9672 medical research panel members from Spain who were screened, 9366 completed the screening process. Of these, 8989 did not meet the inclusion criteria for the survey entry requirements (see Supplementary Fig. 1). Hence, a total of 377 respondents from Spain completed the survey (T1D PWD, n=106; T2D PWD, n=88; T1D CGs, n=87; T2D CGs, n=96).
Most PWD were men (63.4%) whereas most CGs (most often a family member/legal guardian [64.5%]) were female (62.8%). Respondents in the T2D PWD group were slightly older than those in the other groups (Table 1). Respective mean ages of the persons cared for by T1D and T2D CGs were 43.7 years and 70.9 years. Twenty (23.0%) T1D CGs cared for a child (aged 4–17 years) with diabetes; no T2D CG cared for a person aged<18 years. Most respondents were in full- or part-time employment (PWD 67.0%; CG 73.2%).
Respondent characteristics (on survey completion).
| T1D PWD N=106 | T2D PWDN=88 | T1D CGN=87 | T2D CGN=96 | |
|---|---|---|---|---|
| Age of respondent, mean (SD) | 42.4 (10.5) | 51.5 (11.9) | 43.3 (12.1) | 46.3 (12.8) |
| Male, n (%) | 56 (52.8) | 67 (76.1) | 31 (35.6) | 37 (38.5) |
| Years since diagnosis, mean (SD) | 23.1 (12.6) | 11.7 (8.0) | 23.3 (15.7) | 18.9 (11.8) |
| Person with diabetes using insulin for >5 years, n (%) | – | 48 (54.5) | – | 62 (64.6) |
| Number of SHEs in past 12 months, median (Q1:Q3)/mean (SD) | 1 (1:2)/2.70 (5.07) | 1 (1:2)/2.38 (5.54) | 1 (1:2)/1.61 (1.96) | 1 (1:2)/1.66 (4.73) |
| Number of nocturnal SHEs in past 12 months, median (Q1:Q3)/mean (SD) | 1 (1:2)/1.96 (2.62) | 1 (1:2)/2.12 (4.29) | 1 (1:2)/1.46 (1.18) | 1 (0:1)/1.27 (2.11) |
| Impaired awareness of hypoglycaemia (Gold score ≥4), n (%) | 33 (31.1) | 24 (27.3) | – | – |
| Months since most recent SHE, mean (SD) | 4.8 (9.6) | 7.2 (9.6) | 10.8 (10.8) | 7.2 (9.6) |
| Number of SHEs in past 12 months, n (%) | ||||
| 0 events | 18 (17.0) | 7 (8.0) | 11 (12.6) | 15 (15.6) |
| 1 event | 51 (48.1) | 44 (50.0) | 47 (54.0) | 50 (52.1) |
| 2 events | 17 (16.0) | 26 (29.5) | 16 (18.4) | 17 (17.7) |
| ≥3 events | 20 (18.9) | 11 (12.5) | 13 (14.9) | 14 (14.6) |
CG, caregiver; PWD, people with diabetes; Q1, lower quartile; Q3, upper quartile; SD, standard deviation; SHE, severe hypoglycaemic event; T1D, type 1 diabetes; T2D, type 2 diabetes.
Among PWD, 29.4% exhibited impaired awareness of hypoglycaemia (Gold score ≥4) (Table 1). Insulin pump use at time of the most recent SHE was reported by 13 (12.3%) T1D PWD, 1 (1.1%) T2D PWD, 4 (4.6%) T1D CGs and 1 (1.0%) T2D CGs.
Previous SHEsThe mean time from the most recent SHE was 4.8 months in T1D PWD and 7.2 months in T2D PWD. Individuals with T1D and T2D experienced a median of one SHE in the past 12 months. The frequency of SHEs in the past 12 months is shown in Table 1. A total of 115 individuals with diabetes had experienced only one SHE during the past 3 years: 38 (35.8%) T1D PWD, 26 (29.5%) T2D PWD, 27 (31.0%) T1DM CGs and 24 (25.0%) T2D CGs.
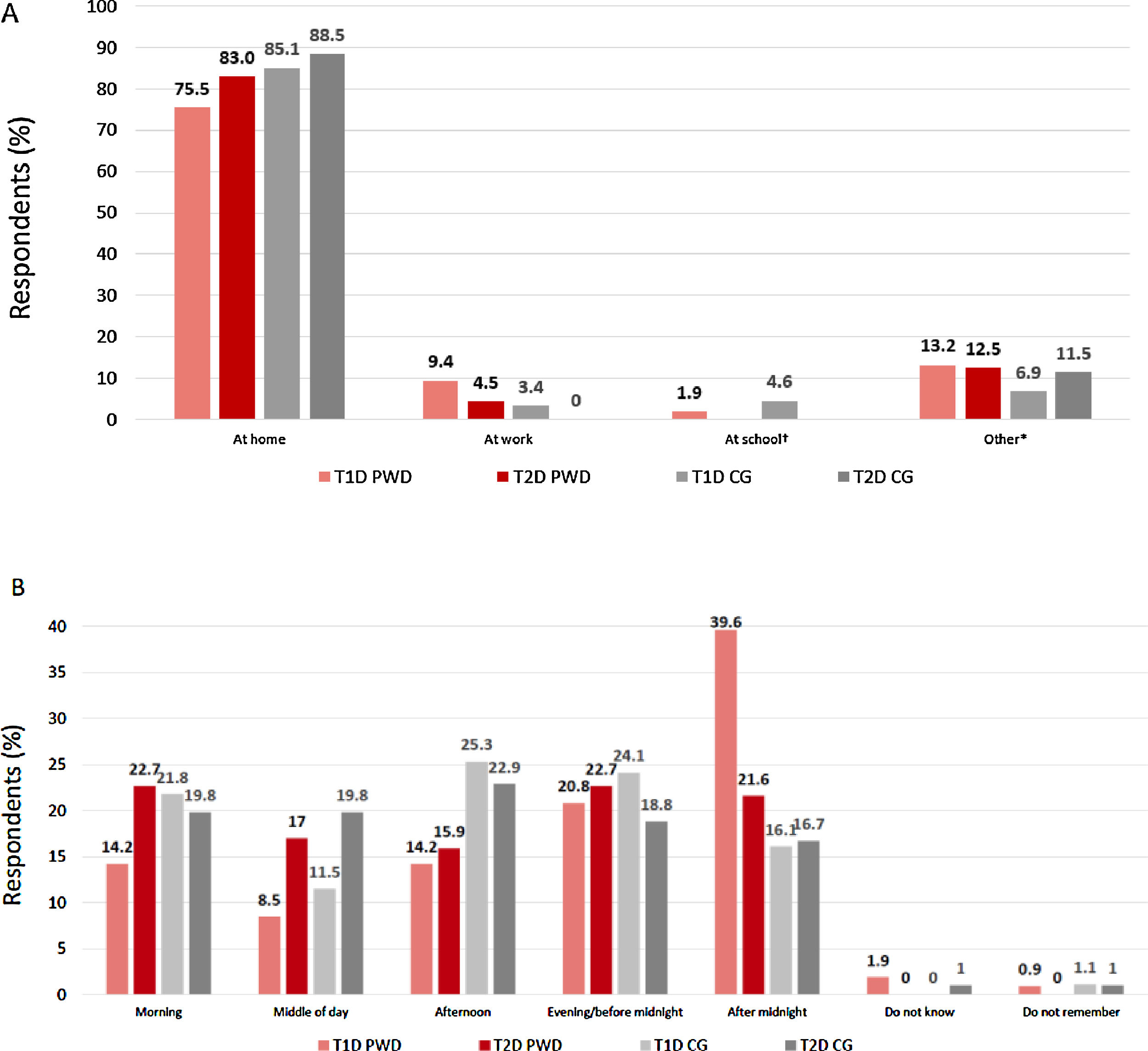
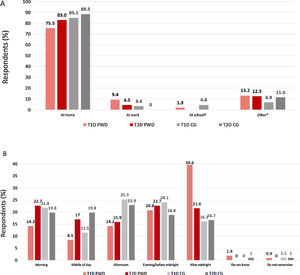
Where and when did the most recent SHE occur?The majority of PWD and CGs reported that the most recent SHE occurred at home (Fig. 1A). For T1D PWD, a substantially higher proportion of participants experienced their most recent SHE after midnight than at other times of day; for other participant groups, events were fairly evenly spread throughout the 24-hour period (Fig. 1B).
(A) Location and (B) timing of most recent severe hypoglycaemic event. *Includes at the mall/shopping centre/grocery store; on a boat/ship; in a car/bus/train; on a trip; walking on the street; at the gym; other. †Twenty CG were caring for children with T1D. CG, caregiver; PWD, people with diabetes; T1D, type 1 diabetes; T2D, type 2 diabetes.
In all groups, the most common reported cause of the most recent SHE was eating less than planned (T1D PWD 37.7%; T2D PWD 48.9%; T1D CGs 52.9%; T2D CGs 46.9%). Exercising more than planned/realised was also perceived as a common cause in individuals with T1D (reported by 25.5% T1D PWD and 18.4% T1D CGs; corresponding data for T2D PWD and T2D CGs: 9.1% and 9.4%).
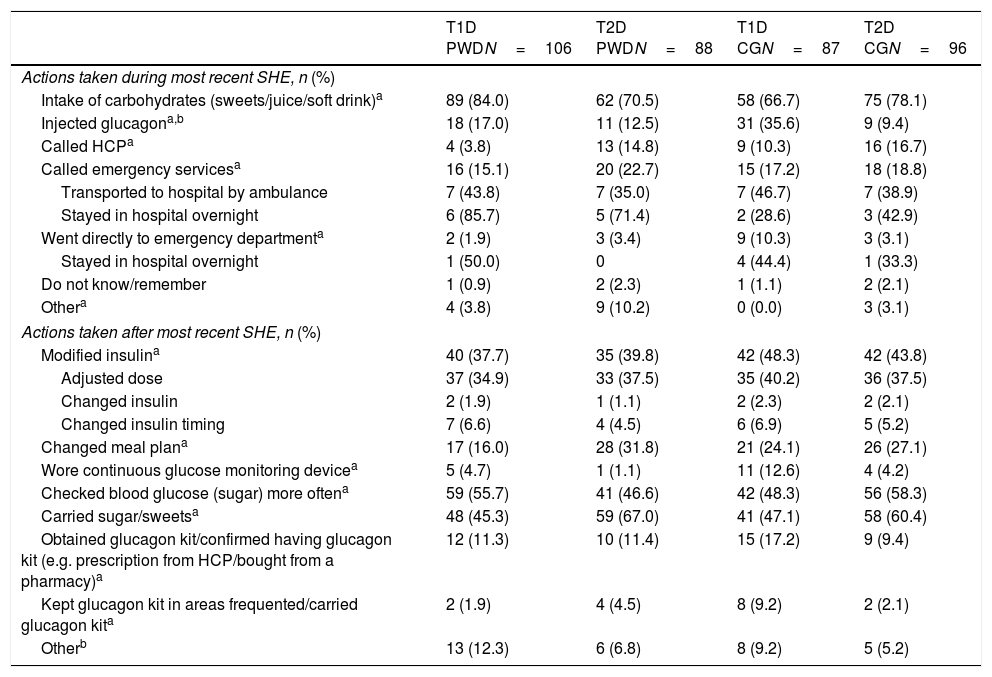
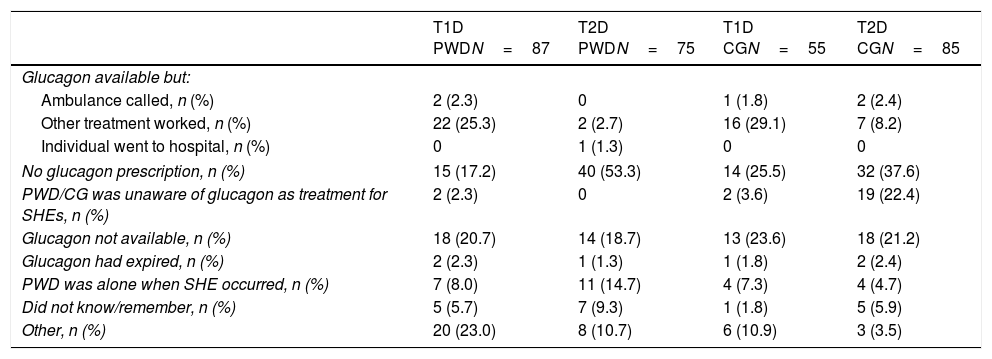
Actions during the most recent SHEDuring the most recent SHE, the action most often taken across all groups was to intake carbohydrates. Injection of glucagon was infrequent (Table 2). Among individuals with diabetes in whom glucagon was not used during the most recent SHE, one reason given consistently across all groups was that glucagon was not readily available; up to 53% of T2D PWD reported the lack of a prescription as a reason (Table 3). Moreover, among those respondents who reported that they had a glucagon kit, the expiration date of>50% (138/258) of kits was unknown. The minority of respondents (15.2–22.7%) reported that emergency services were called during the most recent SHE. Of the 19 PWD who went to hospital, 12 stayed overnight (Table 2).
Actions taken during and after most recent SHE.
| T1D PWDN=106 | T2D PWDN=88 | T1D CGN=87 | T2D CGN=96 | |
|---|---|---|---|---|
| Actions taken during most recent SHE, n (%) | ||||
| Intake of carbohydrates (sweets/juice/soft drink)a | 89 (84.0) | 62 (70.5) | 58 (66.7) | 75 (78.1) |
| Injected glucagona,b | 18 (17.0) | 11 (12.5) | 31 (35.6) | 9 (9.4) |
| Called HCPa | 4 (3.8) | 13 (14.8) | 9 (10.3) | 16 (16.7) |
| Called emergency servicesa | 16 (15.1) | 20 (22.7) | 15 (17.2) | 18 (18.8) |
| Transported to hospital by ambulance | 7 (43.8) | 7 (35.0) | 7 (46.7) | 7 (38.9) |
| Stayed in hospital overnight | 6 (85.7) | 5 (71.4) | 2 (28.6) | 3 (42.9) |
| Went directly to emergency departmenta | 2 (1.9) | 3 (3.4) | 9 (10.3) | 3 (3.1) |
| Stayed in hospital overnight | 1 (50.0) | 0 | 4 (44.4) | 1 (33.3) |
| Do not know/remember | 1 (0.9) | 2 (2.3) | 1 (1.1) | 2 (2.1) |
| Othera | 4 (3.8) | 9 (10.2) | 0 (0.0) | 3 (3.1) |
| Actions taken after most recent SHE, n (%) | ||||
| Modified insulina | 40 (37.7) | 35 (39.8) | 42 (48.3) | 42 (43.8) |
| Adjusted dose | 37 (34.9) | 33 (37.5) | 35 (40.2) | 36 (37.5) |
| Changed insulin | 2 (1.9) | 1 (1.1) | 2 (2.3) | 2 (2.1) |
| Changed insulin timing | 7 (6.6) | 4 (4.5) | 6 (6.9) | 5 (5.2) |
| Changed meal plana | 17 (16.0) | 28 (31.8) | 21 (24.1) | 26 (27.1) |
| Wore continuous glucose monitoring devicea | 5 (4.7) | 1 (1.1) | 11 (12.6) | 4 (4.2) |
| Checked blood glucose (sugar) more oftena | 59 (55.7) | 41 (46.6) | 42 (48.3) | 56 (58.3) |
| Carried sugar/sweetsa | 48 (45.3) | 59 (67.0) | 41 (47.1) | 58 (60.4) |
| Obtained glucagon kit/confirmed having glucagon kit (e.g. prescription from HCP/bought from a pharmacy)a | 12 (11.3) | 10 (11.4) | 15 (17.2) | 9 (9.4) |
| Kept glucagon kit in areas frequented/carried glucagon kita | 2 (1.9) | 4 (4.5) | 8 (9.2) | 2 (2.1) |
| Otherb | 13 (12.3) | 6 (6.8) | 8 (9.2) | 5 (5.2) |
CG, caregiver; HCP, healthcare professional; PWD, people with diabetes; T1D, type 1 diabetes; T2D, type 2 diabetes.
Reasons for not using glucagon during the most recent SHE.a
| T1D PWDN=87 | T2D PWDN=75 | T1D CGN=55 | T2D CGN=85 | |
|---|---|---|---|---|
| Glucagon available but: | ||||
| Ambulance called, n (%) | 2 (2.3) | 0 | 1 (1.8) | 2 (2.4) |
| Other treatment worked, n (%) | 22 (25.3) | 2 (2.7) | 16 (29.1) | 7 (8.2) |
| Individual went to hospital, n (%) | 0 | 1 (1.3) | 0 | 0 |
| No glucagon prescription, n (%) | 15 (17.2) | 40 (53.3) | 14 (25.5) | 32 (37.6) |
| PWD/CG was unaware of glucagon as treatment for SHEs, n (%) | 2 (2.3) | 0 | 2 (3.6) | 19 (22.4) |
| Glucagon not available, n (%) | 18 (20.7) | 14 (18.7) | 13 (23.6) | 18 (21.2) |
| Glucagon had expired, n (%) | 2 (2.3) | 1 (1.3) | 1 (1.8) | 2 (2.4) |
| PWD was alone when SHE occurred, n (%) | 7 (8.0) | 11 (14.7) | 4 (7.3) | 4 (4.7) |
| Did not know/remember, n (%) | 5 (5.7) | 7 (9.3) | 1 (1.8) | 5 (5.9) |
| Other, n (%) | 20 (23.0) | 8 (10.7) | 6 (10.9) | 3 (3.5) |
CG, caregiver; PWD, people with diabetes; SHE, severe hypoglycaemic event; T1D, type 1 diabetes; T2D, type 2 diabetes.
After the most recent SHE, the most frequent actions taken by individuals with diabetes were prevention focused: carrying sugar/sweets, checking blood glucose more frequently and modifying their insulin regimen mostly via dose adjustment (Table 2). Fewer than 20% of individuals with diabetes expanded their treatment options by obtaining a glucagon kit (Table 2).
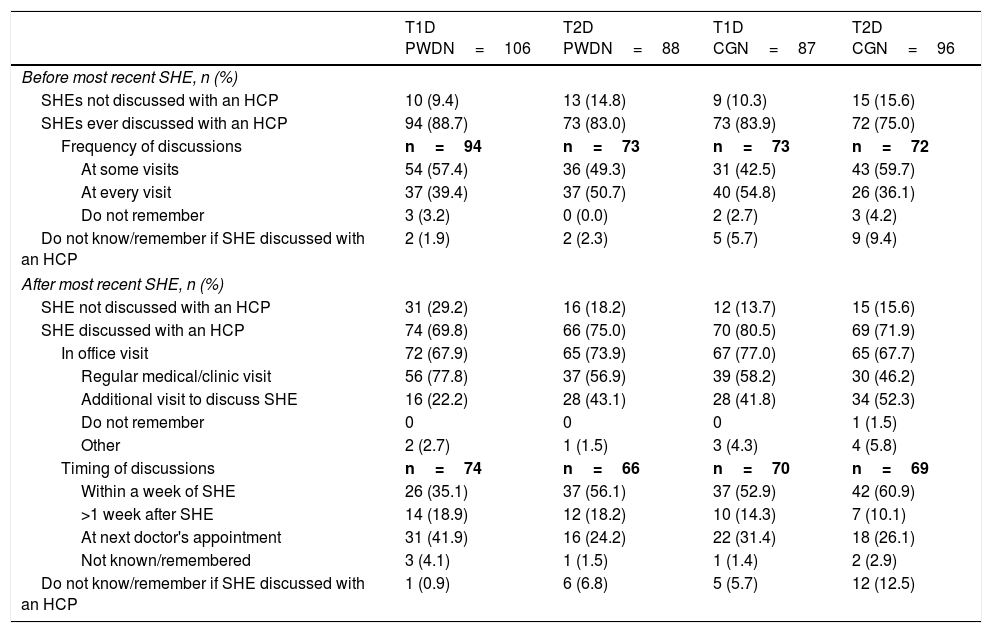
Patient–HCP conversations around SHEsAmong the T1D PWD who had ever discussed severe hypoglycaemia with an HCP, most reported that, prior to their most recent SHE, such discussions took place at some visits, with fewer reporting discussions at every visit. Roughly half of T2D PWD reported discussions on this subject at every visit, and half at some visits (Table 4).
Patient–HCP conversations about SHEs before and after most recent SHE.
| T1D PWDN=106 | T2D PWDN=88 | T1D CGN=87 | T2D CGN=96 | |
|---|---|---|---|---|
| Before most recent SHE, n (%) | ||||
| SHEs not discussed with an HCP | 10 (9.4) | 13 (14.8) | 9 (10.3) | 15 (15.6) |
| SHEs ever discussed with an HCP | 94 (88.7) | 73 (83.0) | 73 (83.9) | 72 (75.0) |
| Frequency of discussions | n=94 | n=73 | n=73 | n=72 |
| At some visits | 54 (57.4) | 36 (49.3) | 31 (42.5) | 43 (59.7) |
| At every visit | 37 (39.4) | 37 (50.7) | 40 (54.8) | 26 (36.1) |
| Do not remember | 3 (3.2) | 0 (0.0) | 2 (2.7) | 3 (4.2) |
| Do not know/remember if SHE discussed with an HCP | 2 (1.9) | 2 (2.3) | 5 (5.7) | 9 (9.4) |
| After most recent SHE, n (%) | ||||
| SHE not discussed with an HCP | 31 (29.2) | 16 (18.2) | 12 (13.7) | 15 (15.6) |
| SHE discussed with an HCP | 74 (69.8) | 66 (75.0) | 70 (80.5) | 69 (71.9) |
| In office visit | 72 (67.9) | 65 (73.9) | 67 (77.0) | 65 (67.7) |
| Regular medical/clinic visit | 56 (77.8) | 37 (56.9) | 39 (58.2) | 30 (46.2) |
| Additional visit to discuss SHE | 16 (22.2) | 28 (43.1) | 28 (41.8) | 34 (52.3) |
| Do not remember | 0 | 0 | 0 | 1 (1.5) |
| Other | 2 (2.7) | 1 (1.5) | 3 (4.3) | 4 (5.8) |
| Timing of discussions | n=74 | n=66 | n=70 | n=69 |
| Within a week of SHE | 26 (35.1) | 37 (56.1) | 37 (52.9) | 42 (60.9) |
| >1 week after SHE | 14 (18.9) | 12 (18.2) | 10 (14.3) | 7 (10.1) |
| At next doctor's appointment | 31 (41.9) | 16 (24.2) | 22 (31.4) | 18 (26.1) |
| Not known/remembered | 3 (4.1) | 1 (1.5) | 1 (1.4) | 2 (2.9) |
| Do not know/remember if SHE discussed with an HCP | 1 (0.9) | 6 (6.8) | 5 (5.7) | 12 (12.5) |
CG, caregiver; HCP, healthcare professional; PWD, people with diabetes; SHE, severe hypoglycaemic event; T1D, type 1 diabetes; T2D, type 2 diabetes.
Following the most recent SHE, 31 (29.2%) T1D PWD and 16 (18.2%) T2D PWD did not discuss the event with their HCP (Table 4). Of those who did, a notable proportion of each group waited until their next appointment (Table 4). This occurred within a month in fewer T1D PWD/CGs (26.4%) than in T2D PWD/CGs (52.9%).
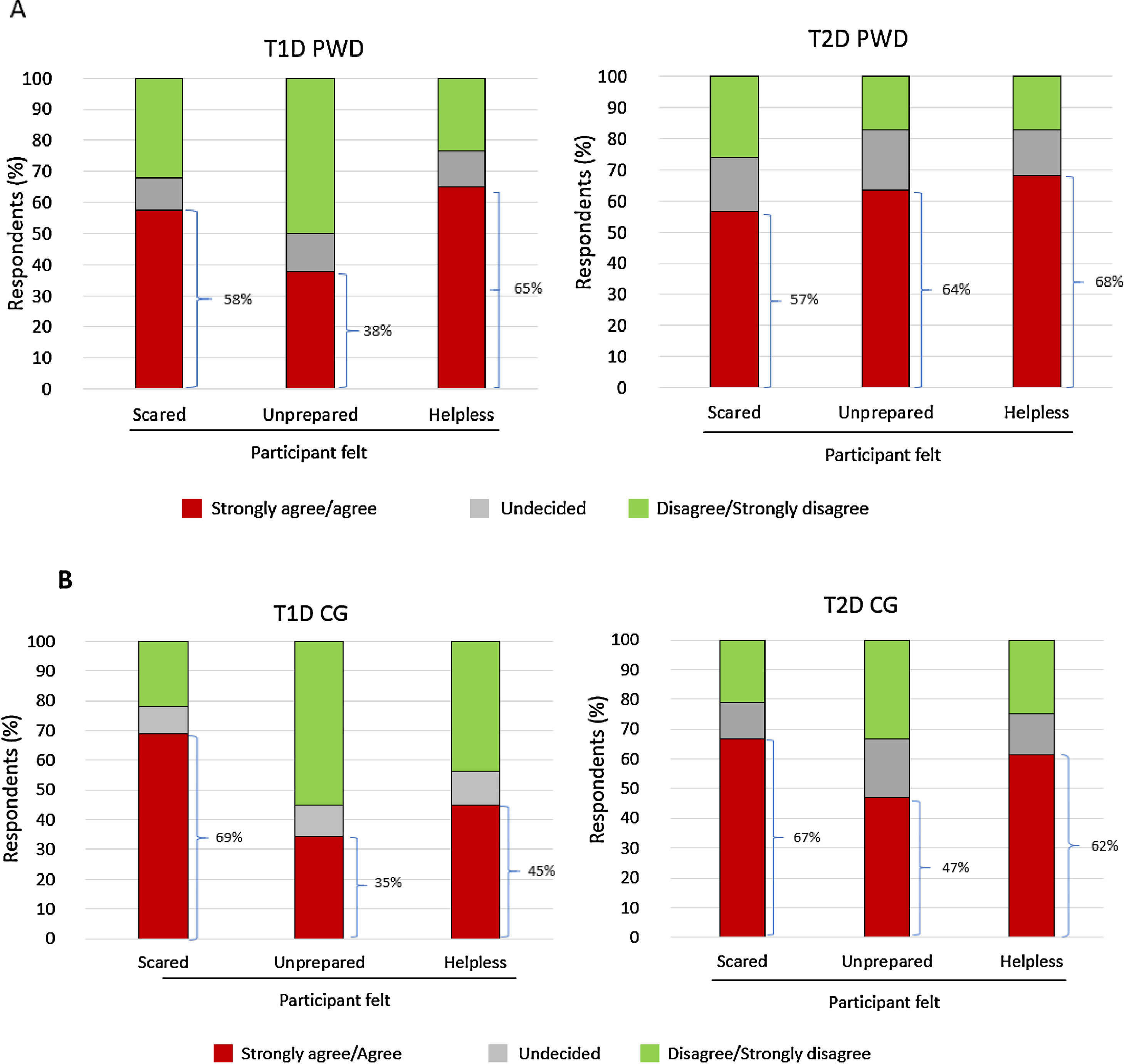
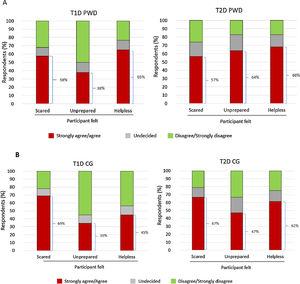
Emotional impactSubstantial proportions of PWD and CGs agreed/strongly agreed that experiencing/witnessing the most recent SHE made them feel scared, unprepared, and/or helpless (Fig. 2A and B). Between 17.9% and 23.9% of PWD and 20.7% and 24.0% of CGs reported that the most recent SHE impacted the mood/emotional status of the individual with diabetes; 9.1–10.4% of PWD and 27.6–32.3% of CGs reported that the most recent SHE impacted the mood/emotional status of the CG. Corresponding data for the impact on daily activities of PWD were 10.4–22.7% (reported by PWD) and 12.6–18.8% (CGs); corresponding data for impact on daily activities of CGs were 5.7–8.0% (PWD) and 14.9–20.8% (CGs).
DiscussionThe CRASH survey has provided valuable data about SHEs in a sample of Spanish individuals with T1D or insulin-treated T2D who are at risk of SHEs (i.e. ≥1 SHE within the past 3 years) from both the PWD and CG perspective.
In CRASH, the median number of SHEs in the past 12 months reported by Spanish T1D PWD/CGs was similar to that reported by T2D PWD/CGs, suggesting that persons who have had an SHE are at high risk of additional SHEs, regardless of whether they have T1D or T2D. This contrasts with a recent systematic review of observational Spanish studies,8 which reported a higher incidence of SHEs in people with T1D (0.90–1.50/person/year) than in those with T2D (0.30–0.63/person/year). Likewise, a cross-sectional survey conducted in 2014 reported the annual frequency for self-reported SHEs to be higher in Spanish people with T1D than in those with T2D (0.90 vs 0.30–0.40).21 The discrepancy between our results and those from observational studies may reflect the fact that CRASH specifically collected information on individuals with diabetes who were considered at high risk of SHEs. This contention is supported by data from the Spanish cohort of another cross-sectional survey conducted in individuals who had experienced ≥1 SHE in the past year (published by Lammert et al.22 in 2009) which reported similar median numbers of SHEs in the past 12 months in people with T1D and T2D.
The Spanish data from the CRASH study indicate that SHEs occurred throughout the day in T2D PWD/CG, but T1D PWD reported that the highest proportion of events occurred after midnight. A similar finding of a greater incidence of SHEs at night in patients with T1D from Spain, Germany and the UK was reported by Lammert et al.22 As expected, we found that SHEs mostly occurred in the home, a finding also consistent with the survey by Lammert et al.22 Both the CRASH and the Lammert et al. surveys found that <10% of respondents experienced their most recent SHE at work. This information, taken together with the finding in CRASH that 20–30% of individuals with diabetes (as reported by PWD and CGs) did not subsequently discuss their most recent SHE with an HCP, leads us to conclude that many SHEs are occurring outside and are unknown to the healthcare system.
CRASH results also show that some SHEs can require immediate medical assistance for resolution. The use of emergency services was reported in up to 23% of individuals with diabetes, with hospitalisation required in ∼10%. Other studies from Spain have reported higher levels of hospitalisation (∼20%).3,22
As SHEs may remain unrecognised by healthcare resource utilisation studies, the burden posed by this condition on patients, CGs and the healthcare system may be currently underestimated. Our findings, and those of Orozco-Beltrán et al.21 also suggest that a notable proportion of individuals with T1D or T2D are not routinely questioned about hypoglycaemic events during HCP consultations. This is contrary to current Spanish guidelines, which recommend regular evaluation by HCPs of patients’ abilities to detect and treat hypoglycaemia.1 Hence, further efforts are needed to ensure that current guidelines, including discussions about prevention, emergency preparedness for, and treatment of severe hypoglycaemia, are followed.
Across all T1D/T2D PWD or CG groups, the most common perceived cause of the most recent SHE was eating less than planned. This finding is in agreement with that of Lammert et al.,22 who found the most frequent cause of SHEs to be irregular/insufficient food intake in patients with T1D and T2D from Spain. Additionally, in CRASH, T1D PWD and CGs highlighted exercising more than planned/realised as a common cause – a finding also reported by Lammert et al.22 in Spanish T1D patients. Hence, in line with other studies, the CRASH data indicate that SHEs can occur simply as a consequence of carrying out normal daily activities.
Hypoglycaemia negatively impacts quality of life in patients with T1D and T2D and their CGs.6,7 The Spanish CRASH data support these findings, with many PWD and CGs reporting feeling scared, unprepared and/or helpless when experiencing/witnessing the most recent SHE. It is important for PWD to be able to trust their CGs to take, and for CGs to be confident of taking, appropriate action(s) during an SHE. This requires individuals with diabetes and their CGs to be well prepared if an SHE occurs, and our survey data suggest that, generally, improvements in the preparedness of both groups are required. The finding that most recent SHEs occurred at home emphasises the need for recommended treatments for severe hypoglycaemia to be readily to hand at all times.
In CRASH, the action most often taken across all groups at the time of the most recent SHE was to intake carbohydrates, without reference to HCPs or emergency services, as per current Spanish guidelines.1 For patients who are unconscious and unable to swallow, these guidelines recommend the subcutaneous or intramuscular injection of glucagon for SHEs. However, many CRASH respondents reported that PWD did not have a glucagon prescription or that glucagon was not readily available during the most recent SHE. Moreover, for over 50% of the glucagon kits reported on in CRASH, respondents indicated that they did not know the expiration date. Furthermore, even after the most recent SHE, only a few (12.2%) expanded their treatment options by obtaining a glucagon kit. Ensuring that individuals with diabetes have access to glucagon, as per current recommendations,1 may help to prevent the negative emotions that surround severe hypoglycaemia.17 Therefore, overall, CRASH survey data suggest a need for greater education about severe hypoglycaemia among patients, particularly those with impaired hypoglycaemia awareness. Impaired awareness rates found in CRASH (T1D PWD 31%, T2D PWD 27%) are similar to those reported in the 2009 cross-sectional survey from Spain published by Lammert et al.22 (T1D, 33%; T2D, 27%).
Limitations of this study include the use of medical research panels, which may have led to selection bias. The demographics of the CRASH participant groups may therefore not reflect those of the general populations of T1D and T2D PWD in Spain. Data were self-reported and this carries the potential for misclassification of hypoglycaemia (minimised by using the accepted standard SHE definition)1,23 and recall bias, which is an inherent limitation of retrospective surveys. In this instance, recall will be affected not only by the time since the most recent SHE, but also if multiple events had occurred in the past 12 months. However, the fact that patient recall of SHEs has been shown to be robust over a 12-month period24 makes this less likely. With regard to PWD awareness of hypoglycaemia, the Gold score20 is not currently validated for use in Spanish. However, it has previously been used to evaluate hypoglycaemia awareness in studies that include Spanish populations, such as the previously mentioned cross-sectional survey by Lammert et al.22 As data are descriptive only, no formal between-group comparisons can be made. Finally, participants’ responses to questions about the most recent SHE should be regarded as a report of what happened or what they were told had happened.
ConclusionCRASH survey data from Spain found that most SHEs occurred outside the healthcare setting, with patient treatment heavily dependent on CGs. The negative emotional impact of SHEs on PWD and CGs underscores the need for an ongoing conversation about SHEs between HCPs and PWD/CGs, who should consistently query the hypoglycaemia knowledge and experiences of PWD/CGs, and provide education, tools and support, as well as strategies to prevent and effectively manage these distressing events.
FundingThis work was supported by Eli Lilly and Company.
Data statementData available upon request.
Conflic of interestsF. Javier Ampudia-Blasco has received fees for acting as a scientific advisor and speaker for Eli Lilly and Company.
Esther Artime, Silvia Díaz, Miriam Rubio and Beth Mitchell are employees of and minor shareholders in Eli Lilly and Company. Jesús Reviriego is a former employee and minor shareholder in Eli Lilly and Company. Beatrice Osumili is an employee of Eli Lilly and Company.
Mark Peyrot has received personal fees and non-financial support from Eli Lilly during the conduct of this study. He also reports personal fees and non-financial support from Calibra and Novo Nordisk.
Robin Pokrzywinski is an employee of Evidera, which received funding for this study from Eli Lilly and Company.
Erik Spaepen is a consultant to Eli Lilly and Company. Frank Snoek has received fees from Eli Lilly and Company, Abbott, the Dutch Diabetes Research Foundation, Novo Nordisk, Roche Diabetes Care and Sanofi. All fees are paid to the institute.
The authors would like to acknowledge Gill Gummer and Janet Douglas (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Eli Lilly and Company.