There is no agreement on the procedures to be used for diagnosis and treatment of gestational thyroid dysfunction. Controversy still exists on the normal range of thyroid-stimulating hormone (TSH) levels and use of gestational hypothyroidism (GH) screening. The aim of this study was to assess diagnosis and treatment of thyroid dysfunction during pregnancy in a group of Spanish hospitals.
Study designThis was a retrospective, multicenter study in pregnant females with GH attending Spanish healthcare centers from March 2013 to July 2014. Variables analyzed included diagnosis criteria for GH (availability of universal screening for gestational thyroid disorders and TSH reference values (RVs) by trimester of pregnancy): risk factors for GH, iodine intake from food or supplementation, gestational age (at diagnosis/treatment) and l-thyroxine treatment.
ResultsFourteen centers participated in the study. Universal screening was performed in only half of the centers, and only 14% had their own TSH RVs. Overall, 257 pregnant women were enrolled, 53.7% with hypothyroidism (HT) diagnosed before pregnancy (pre-GH) and 46.3% with HT diagnosed during pregnancy (intra-GH). A comparison of intra-GH and pre-GH women showed that intra-GH women made their first visit later (59.7% vs. 75.4% respectively before week 12, p=0.007) and had more frequently high TSH levels (>2.5μIU/ml) during the first trimester (94.4% vs. 67.0% respectively, p<0.001).
ConclusionsOur results suggest that GH may be underdiagnosed or inadequately diagnosed in most healthcare centers. These findings suggest the need of improving the current practice in Spain.
Los procedimientos a seguir para el diagnóstico y tratamiento de la disfunción tiroidea en la gestación no están del todo consensuados. Aún se discute el rango de normalidad de los valores de la hormona estimulante del tiroides (TSH) y el uso de screening para detectar hipotiroidismo gestacional (HG). El objetivo de este estudio es evaluar la forma de diagnóstico y tratamiento de la disfunción tiroidea durante la gestación en un grupo de hospitales de España.
Diseño del estudioEstudio retrospectivo, multicéntrico en mujeres embarazadas con HG atendidas en instituciones sanitarias españolas entre marzo de 2013 y julio de 2014. Las variables analizadas incluyeron criterios diagnósticos de HG (disponibilidad de screening universal para trastornos tiroideos gestacionales y valores de referencia de TSH según el trimestre gestacional); factores de riesgo de HG, ingesta de yodo mediante alimentos o suplementos, edad gestacional (al diagnóstico/tratamiento) y tratamiento con L-tiroxina.
ResultadosParticiparon un total de 14 centros. Únicamente la mitad de los centros empleaba el screening universal, y solo el 14% tenía valores de referencia de TSH propios. Se incluyeron un total de 257 embarazadas, 53,7% con diagnóstico de hipotiroidismo previo al embarazo (pre-HG) y 46,3% con hipotiroidismo diagnosticado durante el embarazo (intra-HG). Comparando los casos de pre-HG e intra-HG, las mujeres con intra-HG realizaban la primera visita más tarde (antes de la semana 12; 59,7% vs. 75,4% respectivamente, p=0,007) y tenían más frecuentemente valores elevados de TSH (>2,5μUI/ml) durante el primer trimestre (94,4% vs. 67,0% respectivamente, p<0,001).
ConclusionesNuestros resultados sugieren que el HG puede estar infradiagnosticado o diagnosticado indebidamente en la mayoría de los centros sanitarios. Estos hallazgos sugieren la necesidad de mejorar la práctica actual en España.
Hypothyroidism (HT) has been associated with adverse obstetric outcomes and deficiency in the psychoneurological development of the offspring.1 However, several aspects related to gestational hypothyroidism (GH) management2 lack overall consensus.
The thyroid-stimulating hormone (TSH) is a sensitive marker of thyroid dysfunction (TD). Even so, the physiological changes throughout the gestation call for specific circulating ranges of TSH and free thyroxine (FT4) for the correct assessment of maternal thyroid function.3 Universal screening for TD is not recommended in the guidelines of international societies4–6 despite increasing evidence of the deleterious effects on the mother and fetus of even mild thyroid hormone deficiency.3 Screening is suggested only for pregnant women with TD risk factors, although selective TD screening may fail to identify up to 30–50% of pregnant women with clinical/subclinical HT.7
In the past decade, several studies8–15 assessed the GH management and the compliance with the guidelines recommendations (several international scientific societies), mainly using electronic questionnaires or database revision. Most studies showed deficiencies in HT diagnosis/treatment during gestation.
The TD Working Group (WG) of the Spanish Society of Endocrinology and Nutrition (SEEN) proposed (2012)16 universal screening for gestational TD and recommended the TSH reference ranges according to gestational trimester as outlined in the American Thyroid Association [ATA] guidelines (2011).6 This proposal was based on the high prevalence of this disease, its easy diagnosis, the availability of safe, effective treatment and the possibility of early therapeutic intervention capable of reducing complications.17
The objective of this study was to determine the use of universal screening for TD, the compliance with ATA-2011 guideline recommendations and GH management in pregnant women treated in 14 Spanish centers between March 2013 and July 2014.
MethodsObservational, non-interventional, multicenter study, approved by the Hospital Universitario Fundación Alcorcón Research Ethics Committee and carried out by 14 members of the TD WG (SEEN).
Study populationSample size estimation has been performed considering 234 patients with a 95% of trust level, 3.5% of accuracy, 8% of prevalence of gestational hypothyroidism and 5% of losses.
TD WG (SEEN) (32 members) were proposed to take part in a study about pregnant women with hypothyroidism. Each participant center should count with 20–40 pregnant woman/center with gestational hypothyroidism (diagnosed before or during pregnancy), who attended consecutively between March 2013 and July 2014 and who were treated with l-thyroxine. The study was not limited to any specific pregnancy period. A limitation to the number of patients per center was not previously established and, at the end, 2 centers with a number lower than 20 pregnant women were accepted. Data was extracted retrospectively from medical records and collected in a case report form (CRF) over a period of 6 months (May–November 2015).
Exclusion criteria were: twin pregnancy, hyperthyroidism, thyroid cancer, thyroid nodules (single nodule, multi-nodular) and some treatments (lithium salts, amiodarone, iodine-based contrast or therapeutic doses of I-131 [6 months prior to pregnancy]).
MeasurementsThe information collected included: patients age, iodized salt/iodine supplements consumption, use/type of TD screenings, availability of an established clinical approach to GH diagnosis/treatment (i.e. frequency of visits, measures in each visit, type of determination [TSH, FT4, anti-thyroid peroxidase (TPO) antibodies (Abs)], time to the first blood collection, etc.), reference values (RVs) according to gestational trimester and analytical methods.
Data from the five study visits (first prenatal visit, 3 follow-up visits and close-out visit [the one closest to labor]) included: gestational age, iodized salt/iodine supplements consumption, analytical parameters and l-Thyroxine treatment (prescriber, gestational age at onset and doses). Patient age, HT type, obstetrical history and GH risk factors (ATA-20116) were collected only from the first visit (neither type nor hypothyroidism etiology were required in pre-GH patients).
Statistical analysisConducted using SPSS (19.0, SPSS Inc., Chicago, IL/USA) (December 2015–March 2016). Descriptive statistics were used to present both qualitative (absolute values and percentages) and quantitative (mean, standard deviation, median, minimum/maximum, confidence intervals and number of valid cases) variables.
Subgroup comparisons were performed using a Chi-squared test or Fisher exact test (qualitative variables) and a Student's t-test or Mann–Whitney U-test (quantitative variables). Significance level: 5%.
ResultsFourteen Spanish centers participated in the study (10 hospitals, 2 specialty centers and 2 primary healthcare centers across 8 autonomous communities). The participating specialists were endocrinologists (n=12), primary care physicians (n=1) and midwives (n=1). Centers were from the Autonomous Communities of Madrid, Valencia, Andalucía, Cataluña, Aragón, Asturias, Navarra y Baleares, contributing with 20, 43, 21, 80, 20, 20, 20, 18 and 15 patients, respectively.
TSH, FT4 and anti-TPO Abs were determined by chemiluminescence immunoassays. 6 centers used Abbot immunoassay (RV for general population: TSH 0.35–4.94μUI/ml; FT4 0.70–1.60ng/dl), 3 centers used Roche immunoassay (RV for general population: TSH 0.27–4.2μUI/ml; FT4 0.80–1.80ng/dl), 3 centers used Siemens immunoassay (RV for general population: TSH 0.55–4.78μUI/ml; FT4 0.80–1.76ng/dl), 1 center used Beckman immunoassay (RV for general population: TSH 0.38–5.33μUI/ml; FT4 0.58–1.64ng/dl) and 1 last center used Ortho Clinical Diagnostics immunoassay (RV for general population: TSH 0.41–4.87μUI/ml; FT4 0.80–1.95ng/dl).
2 centers had RV for pregnant women in their first trimester and were (p 2.5 and p 97.5): TSH 0.27–5.0μUI/ml; FT4 0.90–1.70ng/dl and TSH 0.17–4.15μUI/ml; FT4 0.99–1.67ng/dl, respectively.
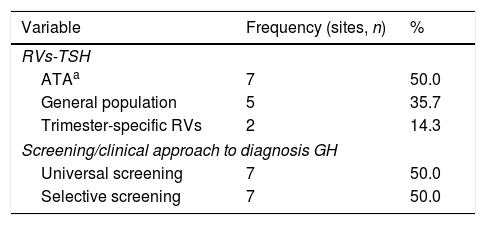
Seven centers (50%) used TSH RV (ATA-20116), 2 centers (14.3%) used their own trimester-specific TSH RV and 5 centers (35.7%) used general population TSH RV (Table 1).
Reference values (TSH) and screenings/clinical approach used to determine thyroid function and to confirm the diagnosis of HT in the first prenatal visit in the different participating centers (n=14).
| Variable | Frequency (sites, n) | % |
|---|---|---|
| RVs-TSH | ||
| ATAa | 7 | 50.0 |
| General population | 5 | 35.7 |
| Trimester-specific RVs | 2 | 14.3 |
| Screening/clinical approach to diagnosis GH | ||
| Universal screening | 7 | 50.0 |
| Selective screening | 7 | 50.0 |
Key: RVs: references values; ATA: American Thyroid Association; TSH: thyroid stimulating hormone.
Nine centers (64%) had a GH diagnosis protocol available and 12 centers (85.7%) performed screening TD at the first prenatal visit, mainly universal screening (Table 1).
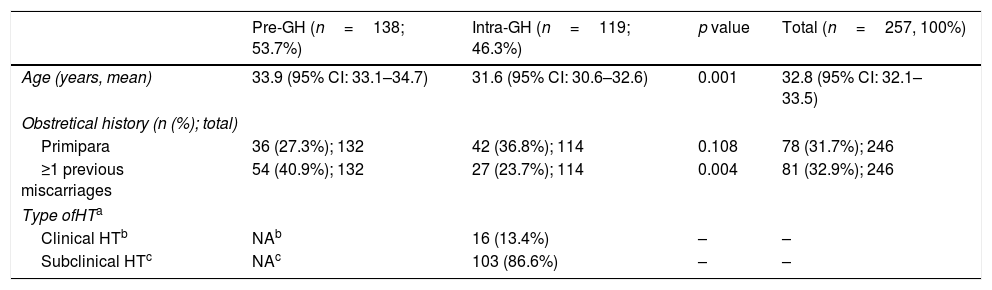
A total of 257 patients with a HT diagnosis were included; pre-GH patients (53.7%) were slightly older than intra-GH patients (Table 2). Most patients (68.3%) had prior pregnancies, with previous miscarriages in one third of the patients (Table 2).
Characteristics of the GH patients included in the study (n=257).
| Pre-GH (n=138; 53.7%) | Intra-GH (n=119; 46.3%) | p value | Total (n=257, 100%) | |
|---|---|---|---|---|
| Age (years, mean) | 33.9 (95% CI: 33.1–34.7) | 31.6 (95% CI: 30.6–32.6) | 0.001 | 32.8 (95% CI: 32.1–33.5) |
| Obstretical history (n (%); total) | ||||
| Primipara | 36 (27.3%); 132 | 42 (36.8%); 114 | 0.108 | 78 (31.7%); 246 |
| ≥1 previous miscarriages | 54 (40.9%); 132 | 27 (23.7%); 114 | 0.004 | 81 (32.9%); 246 |
| Type ofHTa | ||||
| Clinical HTb | NAb | 16 (13.4%) | – | – |
| Subclinical HTc | NAc | 103 (86.6%) | – | – |
Abbreviations: NA: no available.
In GH patients, diagnosis was determined mainly during the first visit (mean 9.2 weeks [0.7–21.9 weeks; 95% CI 8.5–9.9]); subclinical-HT was the most common (86.6%) type (Table 2). Subclinical-HT was defined when pregnant women had TSH values > to the upper limit of TSH RV (ATA-20116) (48 patients), > to the upper limit of trimester-specific range (20 patients), or > to the upper limit of general population (35 patients) with normal FT4 values.
Most GH patients (85.7%) had GH risk factors (ATA-20116): >1 (40.3%) or >2 (30.3%); the most frequent (66.4%) was “age older than 30 years” followed by “family history of TD” (27.5%).
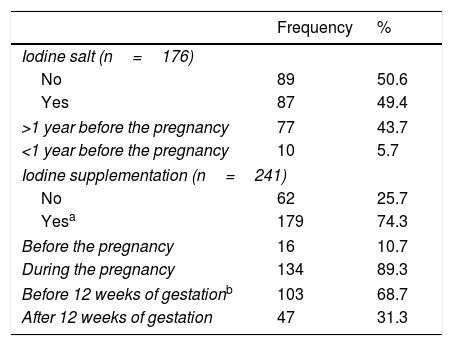
Overall, half of the patients took iodized salt, mainly for more than 1 year before pregnancy (Table 3). Iodine supplements (median dose, 204.1μg/day; range 100–400μg/day; 95% CI 199.2–209.0) were consumed by 74.3% patients; beginning at 11.4 weeks (mean) of gestation (median, 11 weeks; range 3–28 weeks; 95% CI: 10.6–12.2). Few patients started them before pregnancy (Table 3).
Iodine salt/supplementation consumption at the time of pregnancy across participating women (n=257).
| Frequency | % | |
|---|---|---|
| Iodine salt (n=176) | ||
| No | 89 | 50.6 |
| Yes | 87 | 49.4 |
| >1 year before the pregnancy | 77 | 43.7 |
| <1 year before the pregnancy | 10 | 5.7 |
| Iodine supplementation (n=241) | ||
| No | 62 | 25.7 |
| Yesa | 179 | 74.3 |
| Before the pregnancy | 16 | 10.7 |
| During the pregnancy | 134 | 89.3 |
| Before 12 weeks of gestationb | 103 | 68.7 |
| After 12 weeks of gestation | 47 | 31.3 |
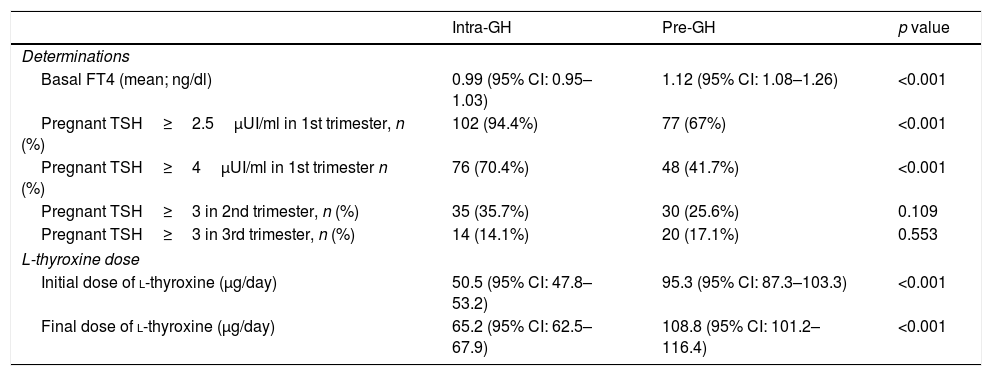
The pre-GH patients were older than intra-GH patients (33.9 vs 31.6 years, p=0.001) (Table 2) Pre-GH and intra-GH patients performed similar number of visits, but the visit before week 12 was more frequent in the pre-GH group than intra-GH group (75.4% vs 59.7%, p=0.007).
The mean waiting time between samples collection for all determinations and the first visit was 2.5 weeks (maximum delays: 16 weeks for TSH and FT4; 13 weeks for TPO Abs).
Overall, basal FT4 value (mean) was 1.06ng/dL (higher in pre-GH patients, Table 4). TSH<4μUI/ml (ATA-2017)4: 40.2% patients; TSH<2.5μUI/ml (ATA-20116): 17.1% patients; 60.8% of patients were positive for anti-TPO Abs.
Differential characteristics among Intra-GH and Pre-GH patients.
| Intra-GH | Pre-GH | p value | |
|---|---|---|---|
| Determinations | |||
| Basal FT4 (mean; ng/dl) | 0.99 (95% CI: 0.95–1.03) | 1.12 (95% CI: 1.08–1.26) | <0.001 |
| Pregnant TSH≥2.5μUI/ml in 1st trimester, n (%) | 102 (94.4%) | 77 (67%) | <0.001 |
| Pregnant TSH≥4μUI/ml in 1st trimester n (%) | 76 (70.4%) | 48 (41.7%) | <0.001 |
| Pregnant TSH≥3 in 2nd trimester, n (%) | 35 (35.7%) | 30 (25.6%) | 0.109 |
| Pregnant TSH≥3 in 3rd trimester, n (%) | 14 (14.1%) | 20 (17.1%) | 0.553 |
| L-thyroxine dose | |||
| Initial dose of l-thyroxine (μg/day) | 50.5 (95% CI: 47.8–53.2) | 95.3 (95% CI: 87.3–103.3) | <0.001 |
| Final dose of l-thyroxine (μg/day) | 65.2 (95% CI: 62.5–67.9) | 108.8 (95% CI: 101.2–116.4) | <0.001 |
Abbreviations: GH: gestational hypothyroidism; FT4: free thyroxine; Pre-GH: HT determined before pregnancy; Intra-GH: HT determined during pregnancy; TSH: thyroid-stimulating hormone.
l-Thyroxine treatment was mainly prescribed by endocrinologist (pre-GH: 60.7%; intra-GH: 76.6%). Most pre-GH patients (92.0%) were receiving l-thyroxine before pregnancy (dose range: 12.5–200μg/day; 79% of patients fell into the of range 26–100μg/day). Throughout gestation, doses were reduced, maintained or increased in 0.8%, 31.1% and 68.2% of pre-GH patients, respectively. In intra-GH patients, the mean delay between diagnosis and l-thyroxine onset was 2.8 weeks (range 0.1–15.7 weeks). Initial and final dose of l-thyroxine in pre-GH/intra-GH groups are showed in Table 4.
The mean gestational ages at first, second and third follow-up visits were 18.1 weeks (95% CI 17.4–18.8), 23.4 weeks (95% CI 22.6–24.2), and 28.1 weeks (95% CI 27.1–29.1), respectively.
We detected high TSH levels in 82.9%, 40.3% and 25.3% of patients in the first, second and third trimester, respectively (normal limits THS; first trimester<2.5μUI/ml; second/third trimester<3μUI/ml; ATA-20116). Both in pre-GH and intra-GH women, the percentage of women out of range (TSH) was falling over time. Overall, the percentage of patients with TSH<3μUI/ml (ATA-2011)6 was increasing: 59.7% (first follow-up), 77.6% (second follow-up) and 88.7% (third follow-up).
At the close-out visit (96.9% of patients; overall mean gestational age: 32.5 weeks [7–41 weeks; 95% CI: 31.8–33.2]) TSH was <4μUI/ml (ATA-20174) in 93.2% of patients; and <3μUI/ml (ATA-20116) in 82.1% of patients. Throughout gestation, 3.5% of patients miscarried at a mean gestational age of 11.4 weeks (range 7–19 weeks).
Overall, the mean gestational age at labor was 39 weeks (95% CI 38.8–39.2), without statistical difference between intra-GH and pre-GH patients. Recommendations for l-thyroxine dose modifications after labor were reported for 80% of patients: same pre-gestational dose (28%; ATA-20116), same gestational dose (12.7%), new dose (39.4%). Discontinuation until the three-month postpartum checkup was indicated in 21% of patients.
DiscussionThe results of this retrospective study showed a large portion (64%) of spanish centers with an established diagnostic approach to GH, contrary to most previous studies.8,9,11–14 Only Granfors et al.10 reported written guidelines for the GH diagnosis/management in most centers (97%).
Thyroid function in pregnant women is mainly assessed in the first trimester with universal/selective screening. Despite all participants belonging to the SEEN TD WG that proposed universal screening for gestational TD (201216), this screening was only performed in half of the centers. The relatively short time between the publication of SEEN's proposal and the patients’ assessment period could explain this moderate impact.
The universal/selective screening for TD in pregnant women had been previously highlighted in Europe and Latin America8,14,15; however, it remains controversial. In the absence of strong evidence for treatment efficacy in subclinical-HT, international guidelines,5,6 including ATA-2017,4 recommend targeted TSH testing for high-risk groups. Nevertheless, some authors,5,17–19 recommend routine thyroid function screening in pregnant woman based on the superiority of universal screening.
In our study, the most common HT risk factors in pregnant woman (age>30 years; family history of TD) are defined in the ATA-2011 guidelines,6 although being over 30 years old has been questioned as a gestational TD risk factor.17,18 Previously,8,15 personal/family history of TD and goiters were the main risk factors.
The adequate risk factors assessment in the pregnant population, described mainly in non-pregnant population,6 will allow their predictive value for TD during pregnancy to be definitively stablished.
We observed iodine salt intake in less than half the pregnant women, similar to the general Spanish population.20 The World Health Organization (WHO) and United Nations International Children's Emergency Fund (UNICEF) recommend iodine supplementation to pregnant woman if <90% of households consume iodized salt and with urinary iodine concentration (UIC) in school-aged children (median)<100μg/L.21 During pregnancy, the daily requirement for iodine increases from 150μg to 250μg to cover the needs of the fetus,22 to guarantee the increase of around 50% of thyroxine production by mother thyroid and to equilibrate mother iodine pool considering the increased loss of iodine by mother kidney.23 In Spain, despite UIC in school-aged children is >170μg/L,24 iodine deficiency still exist in most pregnant women.25 Therefore, SEEN's TD WG25 recommends universal iodine supplementation during pregnancy and lactation despite the weak recommendation 8 of ATA guidelines (2017) indicating that there is no need to initiate iodine supplementation in pregnant women receiving thyroxine. This decision could be explained because the input of iodine supplied by the thyroxine intake is not enough for reach the recommended amount (150–250μg). Our results show that almost 75% of pregnant women consumed iodine supplements (median 200μg/day); only 10% before gestation and the remaining women after week 11, not complying with the guidelines’ recommendations5,6 (before week 11 or in the early stages of gestation).
The mean gestational age observed at first TSH determination was slightly lower than in other studies (8.7 vs. 9–14 weeks14 or 11–1312 weeks). However, the GH patients started the treatment at gestational week 13.3 because of the delay between the analytical sample collection and the first visit. This reflects the need for better organizational measures.
Other thyroid function tests are only recommended in the case of abnormal TSH values.4–6 However, this recommendation is not being implemented, and FT4 and anti-TPO Ab determinations are also performed in the first visits8,13; in our results FT4 (87.9%) and anti-TPO Ab (48.6%) .It could suggest an intention to amplify the diagnosis possibilities in pregnant woman at this visit; a cost-benefit analysis has not yet been performed.
We detected high TSH levels in 82.9%, 40.3% and 25.3% of patients in the first, second and third trimester, respectively (normal limits THS; first trimester<2.5μUI/ml; second/third trimester<3μUI/ml; ATA-20116). To avoid over-diagnosis of subclinical-GH using TSH limit<2.5μUI/ml,11 and considering the significant differences in the upper limits of the different populations, ATA-2017 guidelines4 proposed 4.0μUI/ml as upper limit in the absence of TSH-specific values, which represents an upper limit reduction of 0.5μUI/ml in the non-pregnant population of most trials. Considering these criteria, 24.2% of our patients, with TSH>4.0μUI/ml, would be out of range in the first gestational trimester.
Differentiating our study form most of the others on GH, in which HT was established prior to gestation,10,12,14 we included women with HT diagnosis during the pregnancy (46%). Previous HT diagnosis involves l-thyroxine treatment before the study, but even so 43–55% of patients showed TSH>2.5μUI/ml in the first trimester.12,14 Despite the increase of l-thyroxine doses after knowledge of the pregnancy, up to 40% of patients had TSH>2.5μUI/ml.26 This suggests the need for careful handling of these patients to assure euthyroidism throughout the entire gestation period.
Despite the high percentage of patients with normal TSH at the close-out visit, only 21% made the 4 visits before labor recommended for maternal thyroid function monitoring (ATA-2011).6 Increased compliance with this recommendation could have reduced these elevated TSH rates.
Most patients received a recommendation for l-thyroxine doses modification following labor The reasons for these modifications were not reported, but the need for the pre-gestational dose increase in patients with postpartum Hashimoto's thyroiditis (50%)27 may have been a factor. Further studies are needed to identify predictive factors for an adequate postpartum l-thyroxine dosage in GH.
To the best of our knowledge, there are studies with HT diagnosed prior to10,12,14 or during11,28 gestation; but none comparing pre-GH and intra-GH results. We have detected significant differences between these groups. First visit is performed before week 12 mainly in pre-GH patients, probably because the knowledge/implementation of the guideline recommendations to adjust the l-thyroxine dose in treated patients as soon as possible after the confirmation of pregnancy. The higher FT4 values observed in the pre-GH patients support this explanation.
Considering TSH<2.5μUI/ml (ATA-2011)6 and <4μUI/ml (ATA-2017)4 as normal limits in the first trimester, GH is insufficiently treated mainly in intra-GH patients. The fact that dose adjustment in pre-GH patients occurs earlier than therapy onset in intra-GH patients confirms this results. The l-thyroxine dose escalation for patients treated before pregnancy is well established,4–6 in contrast to the appropriate initial dose in intra-GH patients, with different fixed doses (initial/final) or varying doses based on initial TSH values and patient's body weight.29 The higher initial/final doses observed in pre-GH women would support this idea.
TSH values (normal ranges, ATA-20116) were similar in both groups during the second and third trimesters, but the differences in the early stages of gestation should be emphasized. At this stage, adequate maternal thyroid function is essential for fetal development.30 Our results show the need for better organizational measures in health centers to achieve early l-thyroxine treatment onset or adjustment in GH patients.
The main limitation of this study is its retrospective observational design; considering also the heterogeneity of the study population (HT diagnosis before and after gestation, wide range of TSH RV among centers). The absence of a control group (pregnant women without HT) did not allow further analysis, but it was not needed in order to meet our research objectives. Furthermore, the fact that some participants belong to SEEN's TD WG could show an approach to GH management different from that of other healthcare providers. Considering providers overall, the results could suggest even more underdiagnoses in the first trimester.
In conclusion, the present study shows that GH may be under-diagnosed in most Spanish health centers. Organizational improvements are needed to allow the earlier initiation or adjustment of l-thyroxine treatment in these patients.
Conflict of interestsThe authors declare that they have no competing interests regarding the publication of this paper.
We thank Merck, S.L.U. for their collaboration in the writing and publication of this article.