Amyotrophic Lateral Sclerosis (ALS) is a neurodegenerative disease in which specialized nutritional support is essential. The objectives of our study were to describe nutritional support at the beginning of follow-up and its impact on anthropometry and survival.
MethodsAn interhospital registry was created for the hospitals of Castilla-León through a web platform designed for this purpose. An anamnesis was carried out on the evolution and nutritional history of the disease; and classical anthropometry was determined. The prescribed nutritional treatment was recorded. The parameters were measured at the beginning, at six and twelve months of nutritional follow-up.
ResultsA total of 93 patients [49 (52.7%) spinal; 44 (47.3%) bulbar)] were analyzed. The nutritional support route at the beginning was oral diet in 36 (38.7%) patients; oral nutritional supplementation (SON) in 46 (49.5%) patients; and in 11 (11.8%) patients percutaneous endoscopic gastrostomy (PEG). A decrease in the body mass index (BMI) was observed between the first and second visit [Start: 24.18 (3.29) kg/m2; 6 months: 23.69 (4.12) kg/m2; P < .05]. Less weight loss was observed at 6 months compared to the start of nutritional follow-up [Start: 8.09 (8.72)%; 6 months: 1.4 (6.29)%; P < .01]. 36 (38.7%) patients died but with no differences according to when nutritional support was started. Survival from the onset of symptoms was higher in the group of patients with artificial nutrition, although without reaching statistical significance [Oral: 28 (20.25) months; SON: 30 (16.75-48.25) months; PEG: 39 (27-52) months; P = .90].
ConclusionsPatients with ALS present a severe deterioration in nutritional status before the start of nutritional support. After the nutritional intervention, a slowdown in weight loss and nutritional deterioration was observed.
La esclerosis lateral amiotrófica es una enfermedad neurodegenerativa en la que el soporte nutricional especializado es básico. Los objetivos de nuestro estudio fueron describir el soporte nutricional al inicio del seguimiento y su impacto en la antropometría y supervivencia.
Material y métodosSe creó un registro interhospitalario para los hospitales de Castilla-León a través de una plataforma web diseñada a tal efecto. Se realizó una anamnesis sobre la evolución e historia nutricional de la enfermedad, y se determinó antropometría clásica. Se registró el tratamiento nutricional prescrito. Se midieron los parámetros al inicio, a los 6 y a los 12 meses del seguimiento nutricional.
ResultadosSe analizaron un total de 93 pacientes (49 [52,7%] espinal; 44 [47,3%] bulbar). La vía de soporte nutricional al inicio fue dieta oral en 36 (38,7%) pacientes; la suplementación oral nutricional en 46 (49,5%) pacientes y en 11(11,8%) pacientes gastrostomía endoscópica percutánea. Se observó un descenso del índice de masa corporal entre la primera y la segunda visita (inicio: 24,18 [3,29] kg/m2; 6 meses: 23,69 [4,12] kg/m2; p < 0,05). Se observó una menor pérdida de peso a los 6 meses respecto al inicio del seguimiento nutricional (inicio:8,09 [8,72%]; 6 meses: 1,4 [6,29%]; p < 0,01). Treinta y seis (38,7%) pacientes fallecieron sin diferencias en función del inicio del soporte. La supervivencia desde el inicio de los síntomas fue mayor en el grupo de pacientes con nutrición artificial, aunque sin alcanzar la significación estadística (oral: 28 [20,25] meses; suplementación oral nutricional: 30 [16,75-48,25] meses; gastrostomía endoscópica percutánea: 39 [27-52] meses; p = 0,90).
ConclusionesLos pacientes con ELA presentan un deterioro severo del estado nutricional antes del inicio del soporte nutricional. Tras la intervención nutricional se observó una ralentización en la pérdida ponderal y del deterioro nutricional.
Amyotrophic lateral sclerosis (ALS) is a degenerative neuromuscular disease caused by a gradual decrease in the functioning of motor neurons in the cerebral cortex, medulla oblongata and spinal cord. This generates muscle paralysis leading to gradual motor deterioration and potentially death.1
The incidence of ALS in Europe and North America is 1.5-2.5 people per 100,000 population per year, while the prevalence ranges from 2.7 to 7.4 cases per 100,000 population.2,3 The incidence is slightly higher in males (1.3-1.5:1) and increases with age, peaking around 70 years. The five-year survival rate of this disease is 20%.4
At present, there is no curative treatment and the prognosis for the disease is poor.4 However, there are effective measures for increasing patient quality of life and prolonging survival, including ensuring and maintaining good nutritional status starting in the early stages of the disease to prevent the development of medical complications related to malnutrition.
Most patients diagnosed with ALS develop some degree of malnutrition in the course of the disease; it is seen in 16%-55% of patients at diagnosis.5 This nutritional decline is due to several conditions directly related to ALS pathophysiology: anorexia tied to the patient’s psychosocial situation and possible medication side effects; weakness of the abdomen and pelvic muscles causing constipation, which could indirectly reduce intake; abnormalities in chewing and swallowing related to abnormalities in the motor neurons of the medulla oblongata; “paradoxical” hypermetabolism, especially in early stages, leading to increased catabolism despite decreased mobility; and cognitive impairment in the form of frontotemporal dementia (20%-25% of patients).6
ALS is a disease with a highly variable course that depends on individual characteristics. However, nutritional status in this disease has been shown to underlie survival in these patients.7
One factor that promotes weight loss in patients with ALS and therefore compromises their survival is the onset of dysphagia. The prevalence of dysphagia varies by series and method of evaluation thereof (medical history-related methods [Eating Assessment Tool or EAT-10]), targeted clinical examinations (volume-viscosity method) or direct visualisation methods (videofluoroscopy or laryngoscopy). Clinical evaluation has detected dysphagia in 6%-15% of patients, while specific tests and examinations have detected 41.1%-70% of swallowing abnormalities in these patients. Endoscopic evaluation has shown prevalences of 47.8%-72.7% in patients depending on the form of onset, while videofluoroscopy has demonstrated some sort of dysphagia in up to 75% of cases.6 Often, dysphagia is the initial symptom and constitutes one of the most serious complications in these patients.8 Dietary counselling in dysphagia is done to facilitate swallowing, optimise nutritional intake and decrease the risk of aspiration.9 Cases in which intake capacity is clearly compromised require more invasive methods, such as placing a gastrostomy tube.
For this reason, early, suitable nutritional support, and achievement of weight and nutritional parameters, may improve the course of the disease.6
The objectives of this study were to evaluate the impact of specialised nutritional support on anthropometric parameters in patients with ALS; to report routes of nutrition and support techniques at the start of follow-up of patients with ALS at clinical nutrition practices; and to determine the impact on survival of the different types of specific nutritional support at the start of follow-up.
Material and methodsDesignA longitudinal, observational cohort study (cohorts: adapted oral diet, artificial supplementation and complete enteral nutrition) was designed to evaluate the patients’ nutritional situation at the start of follow-up on the nutrition unit and the effects of the patients’ nutritional status on their survival.
Patients referred to a clinical nutrition practice with a diagnosis of motor neuron disease at seven hospitals in the Spanish Autonomous Community of Castile-León between January 2015 and December 2017 were included.
ProceduresAfter the informed consent forms were signed and the patients were included in the study, the patients were registered in a web platform created for this purpose on the Centro de Investigación en Endocrinología y Nutrición [Endocrinology and Clinical Nutrition Research Center] website (www.ienva.org), in compliance with Spanish Organic Law 15/1999 on Protection of Personal Data (LOPD). This study was conducted in accordance with the Declaration of Helsinki, and all procedures were approved by the Hospital Clínico Universitario de Valladolid Medical Research Independent Ethics Committee (mIEC) with the code PI 17-543. The variables were recorded when nutritional support was started and then every six months during patient follow-up on the clinical nutrition unit.
With the data obtained, descriptive statistical analysis of prevalence and nutritional status in the patients was performed. Univariate and multivariate inferential statistical analysis was then performed to evaluate the course of the disease, as well as the effects of nutritional support on the proposed endpoints.
VariablesDisease characteristicsSex, date of birth and type of ALS characterised by form of onset were collected. Patients were classified as having spinal-onset ALS or bulbar-onset ALS. ALS and other motor neuron diseases were diagnosed according to the Airlie House and El Escorial criteria.10
To characterise the course of the disease, the following were collected: date of onset of symptoms, date of diagnosis of the disease in the neurology department and date on which the patient was first assessed in the endocrinology and nutrition department. Admissions associated with the disease were assessed as well. The rate of deaths and the dates on which they occurred were also analysed.
Type of nutritional treatmentThe patients were divided into three groups according to the specialised nutritional support that they started at the beginning of follow-up at the clinical nutrition practice.
Oral diet (ORAL): patients in whom nutritional treatment with an oral diet adapted to their requirements and capacity for swallowing was started with adjustments in the quality and texture of their diet.
Oral nutritional supplementation (ONS): patients who did not meet their nutritional requirements through an oral diet and therefore had oral artificial supplementation added to their diet with adjustments in quantity and/or texture.
Complete enteral nutrition by gastrostomy tube (PEG): patients with an alteration in their capacity for swallowing that prevented them from meeting their nutritional requirements through an oral diet. In these patients, treatment with complete enteral nutrition through a percutaneous endoscopy gastrostomy (PEG) was started.
Anthropometric evaluationAnthropometric evaluation of the subjects was performed with determination of weight, height and body mass index (BMI).
Weight was measured with accuracy within ±0.1 kg using scales rounding to the nearest 0.1 kg (SECA, Birmingham, United Kingdom). Height was measured with the patient standing using a stadiometer (SECA, Birmingham, United Kingdom). BMI was calculated using the following formula: weight (kg)/height × height (m2).
Patients in a wheelchair who could not move had their weight measured indirectly (the weight of the patient in the wheelchair minus the weight of the wheelchair). Height in these cases was determined by forearm length.
Percentage weight loss (%WL) was used to assess relative differences in weight.
Nutritional assessmentThe nutritional status of the patients was measured by means of subjective global assessment (SGA). This is a simple test for diagnosing and categorising malnutrition that includes variables from the medical history and physical examination. This test is subdivided into three categories: A: good nutritional status, B: moderate malnutrition and C: severe malnutrition.11
Data analysisThe data were stored in a database in the SPSS statistics software programme, version 23.0 (SPSS Inc., IL, United States), with an official licence belonging to the Universidad de Valladolid. The Kolmogorov–Smirnov test was used to analyse the normality of the distribution of the continuous variables.
Continuous variables were expressed in terms of mean (standard deviation), and non-continuous variables were expressed in terms of median (p25-p75). Parametric variables were analysed using Student’s paired and unpaired t tests as well as analysis of variance (ANOVA), and non-parametric variables were analysed using the Friedman, Wilcoxon, Kruskal–Wallis K and Mann–Whitney U tests.
Qualitative variables were expressed in terms of percentage (%) and were analysed using the chi-squared test (with Fisher's correction and Yates's correction when necessary).
Survival was analysed based on type of nutritional support at the start of nutritional follow-up.
Multivariate analysis was performed using binary logistic regression to evaluate the effects of initial type of nutritional support on survival for more than 12 months from the start of nutritional follow-up, stratifying by age, baseline nutritional status and form of onset of ALS.
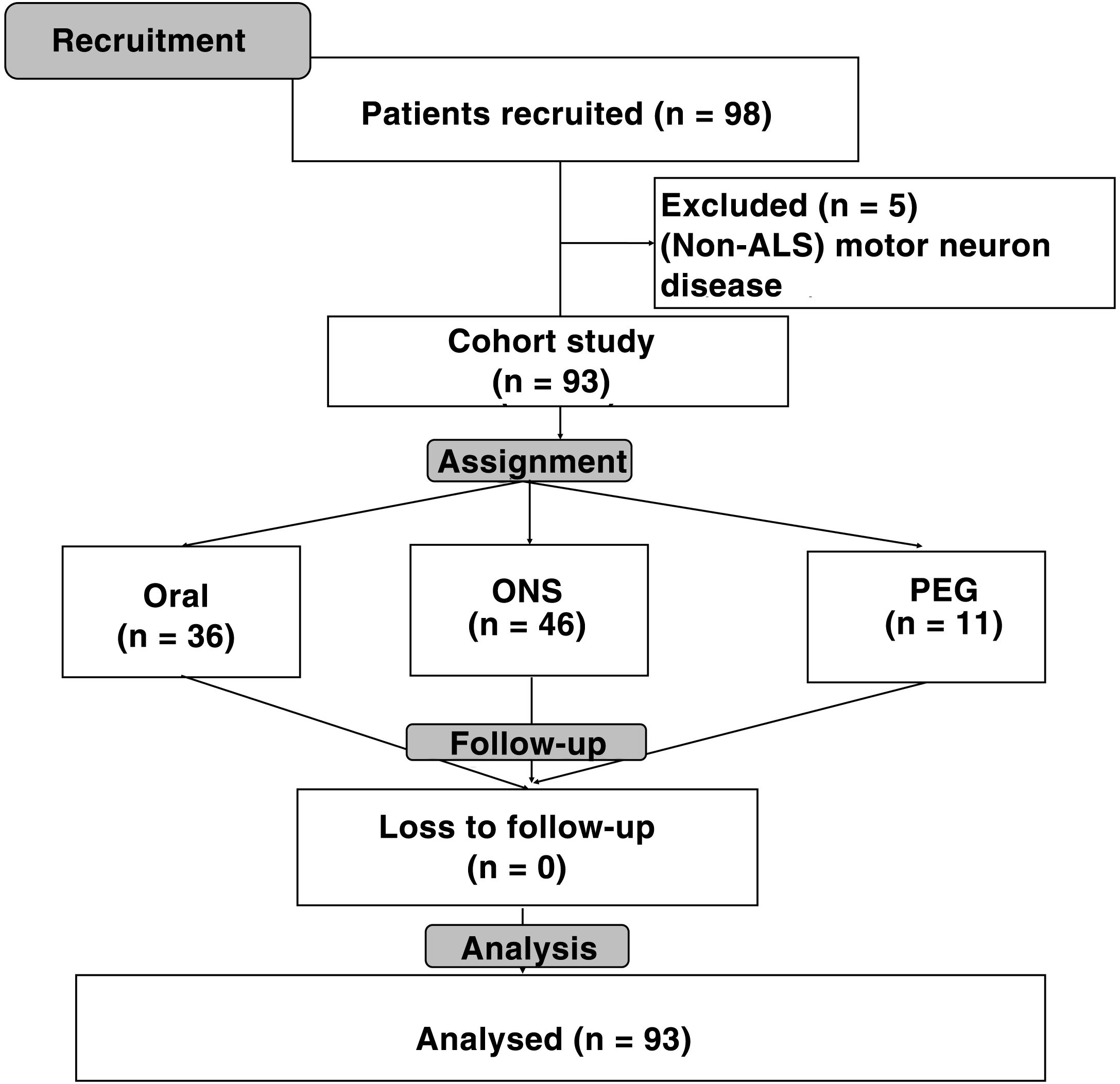
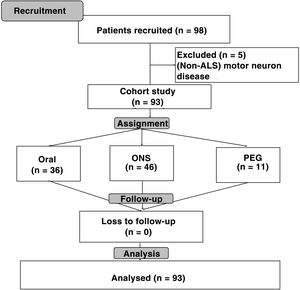
ResultsA total of 98 patients were recruited; of them, 93 (94.9%) had a diagnosis of amyotrophic lateral sclerosis (ALS), two (2%) had progressive bulbar palsy and three (3.1%) had primary lateral sclerosis. Among patients with ALS, 49 (52.7%) had spinal-onset symptoms and 44 (47.3%) had bulbar-onset symptoms (Fig. 1).
Out of all the patients analysed, 53 (57%) were men and 40 (43%) were women. The mean age of the patients was 64.63 (17.67) years (spinal-onset: 61 [17.48] years; bulbar-onset: 67.5 [18.07] years; p = 0.08).
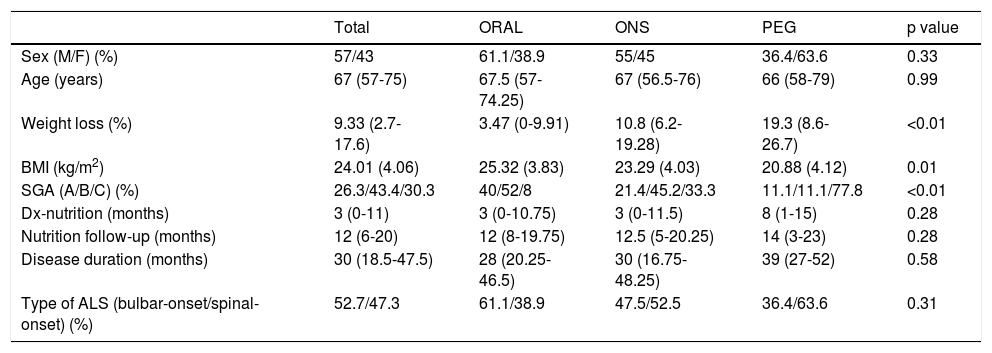
Initial type of nutritional support was oral diet in 36 (38.7%) of the patients; ONS in 46 (49.5%) and complete enteral nutrition (PEG) in 11 (11.8%). Median follow-up on the clinical nutrition unit was 12 (6-20) months. No significant differences were seen with regard to age, sex, delay from diagnosis to the patient's first visit on the nutrition unit, disease duration since onset of symptoms or form of onset of ALS. A higher percentage weight loss and a lower BMI at the start of nutritional follow-up were seen in patients with ONS and PEG (Table 1).
Differences in variables at baseline and in follow-up according to initial type of nutritional support.
| Total | ORAL | ONS | PEG | p value | |
|---|---|---|---|---|---|
| Sex (M/F) (%) | 57/43 | 61.1/38.9 | 55/45 | 36.4/63.6 | 0.33 |
| Age (years) | 67 (57-75) | 67.5 (57-74.25) | 67 (56.5-76) | 66 (58-79) | 0.99 |
| Weight loss (%) | 9.33 (2.7-17.6) | 3.47 (0-9.91) | 10.8 (6.2-19.28) | 19.3 (8.6-26.7) | <0.01 |
| BMI (kg/m2) | 24.01 (4.06) | 25.32 (3.83) | 23.29 (4.03) | 20.88 (4.12) | 0.01 |
| SGA (A/B/C) (%) | 26.3/43.4/30.3 | 40/52/8 | 21.4/45.2/33.3 | 11.1/11.1/77.8 | <0.01 |
| Dx-nutrition (months) | 3 (0-11) | 3 (0-10.75) | 3 (0-11.5) | 8 (1-15) | 0.28 |
| Nutrition follow-up (months) | 12 (6-20) | 12 (8-19.75) | 12.5 (5-20.25) | 14 (3-23) | 0.28 |
| Disease duration (months) | 30 (18.5-47.5) | 28 (20.25-46.5) | 30 (16.75-48.25) | 39 (27-52) | 0.58 |
| Type of ALS (bulbar-onset/spinal-onset) (%) | 52.7/47.3 | 61.1/38.9 | 47.5/52.5 | 36.4/63.6 | 0.31 |
ALS: amyotrophic lateral sclerosis; BMI: body mass index; Dx: diagnosis; F: female; M: male; ONS: oral nutritional supplementation; PEG: percutaneous endoscopic gastrostomy; SGA: subjective global assessment; A: good nutritional status; B: risk of malnutrition; C: severe malnutrition.
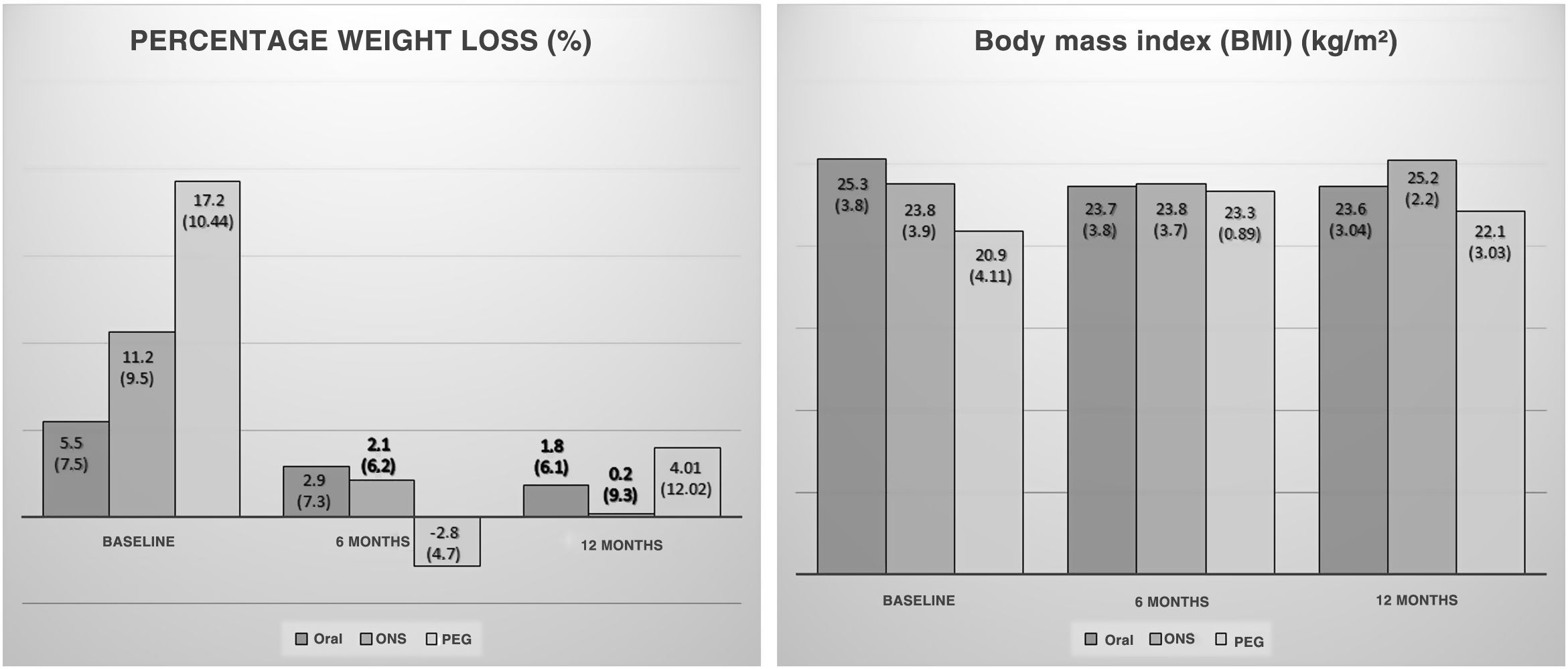
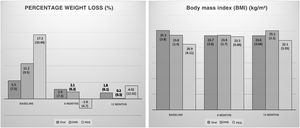
A drop in BMI was seen between the first and the second visit at six months (baseline: 24.18 [3.29] kg/m2; 6 months: 23.69 [4.12] kg/m2; p < 0.05). Patients who continued follow-up also showed no differences in BMI at 12 months (6 months: 24.48 [2.81] kg/m2; 12 months: 24.01 [2.69] kg/m2; p = 0.31). No significant differences were seen when the groups were stratified by initial nutritional support (Fig. 2).
Rates of weight loss were lower at six months of follow-up compared to those observed at the start of nutritional follow-up (baseline: 8.09 [8.72%]; 6 months: 1.4 [6.29%]; p < 0.01). There were no changes in speed of weight loss at 12 months (6 months: 1.8 [6.67%]; 12 months: 1.43 [8.81%]; p = 0.87). A significant slowdown in weight loss was seen in patients with oral nutritional supplementation (Fig. 2).
No significant differences were seen between the groups in changes in BMI (kg/m2) (6 months (ORAL: –0.74 [1.7]; ONS: –0.5 [1.48]; PEG: 0.76 [1.1]; p = 0.24); 12 months (ORAL: –0.5 [1.59]; ONS: –0.21 [2.4]; PEG: –1.2 [3.2]; p = 0.76)) and %WL (%) (6 months (ORAL: 0.15 [–1.8-7.6]; ONS: 0.66 [–2.65-6.9]; PEG: –2.81 [–7.47-0.45]; p = 0.28); 12 months (ORAL: 0 [–1.96-8.6]; ONS: 0 [–4.26-2.48]; PEG: 4.01 [–2.67-12.69]; p = 0.22)) at either six or 12 months.
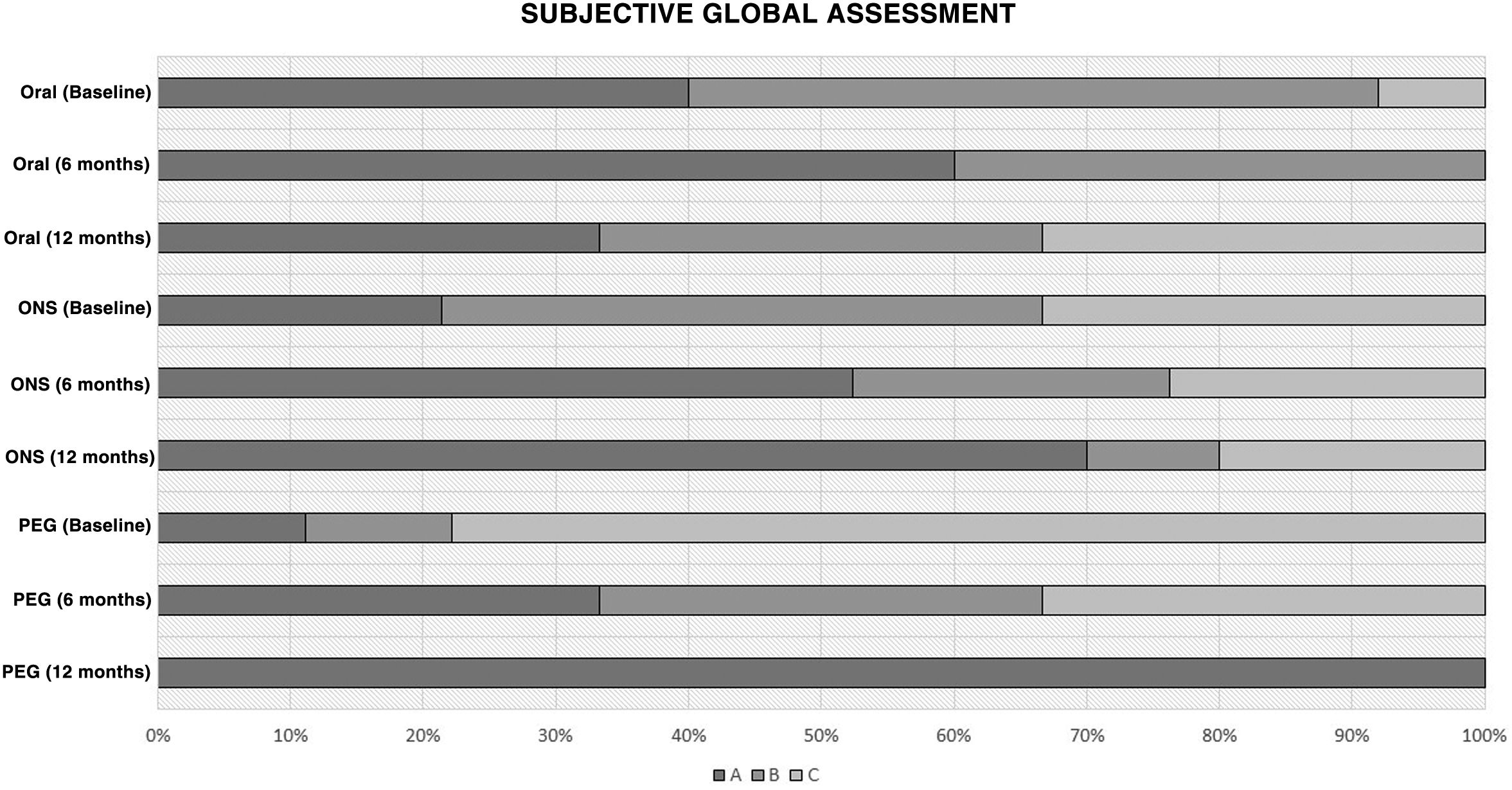
Nutritional assessmentAn increase in the percentage of patients with good nutritional status measured by SGA, and an improvement in nutritional status measured by SGA, were observed at six and 12 months in all study groups (Fig. 3).
Differences in course of nutritional assessment according to subjective global assessment (SGA).
A: good nutritional status; B: risk of malnutrition; C: severe malnutrition (according to nutritional support at the start of nutritional follow-up).
PEG: percutaneous endoscopic gastrostomy; Oral: oral diet; ONS: oral nutritional supplementation.
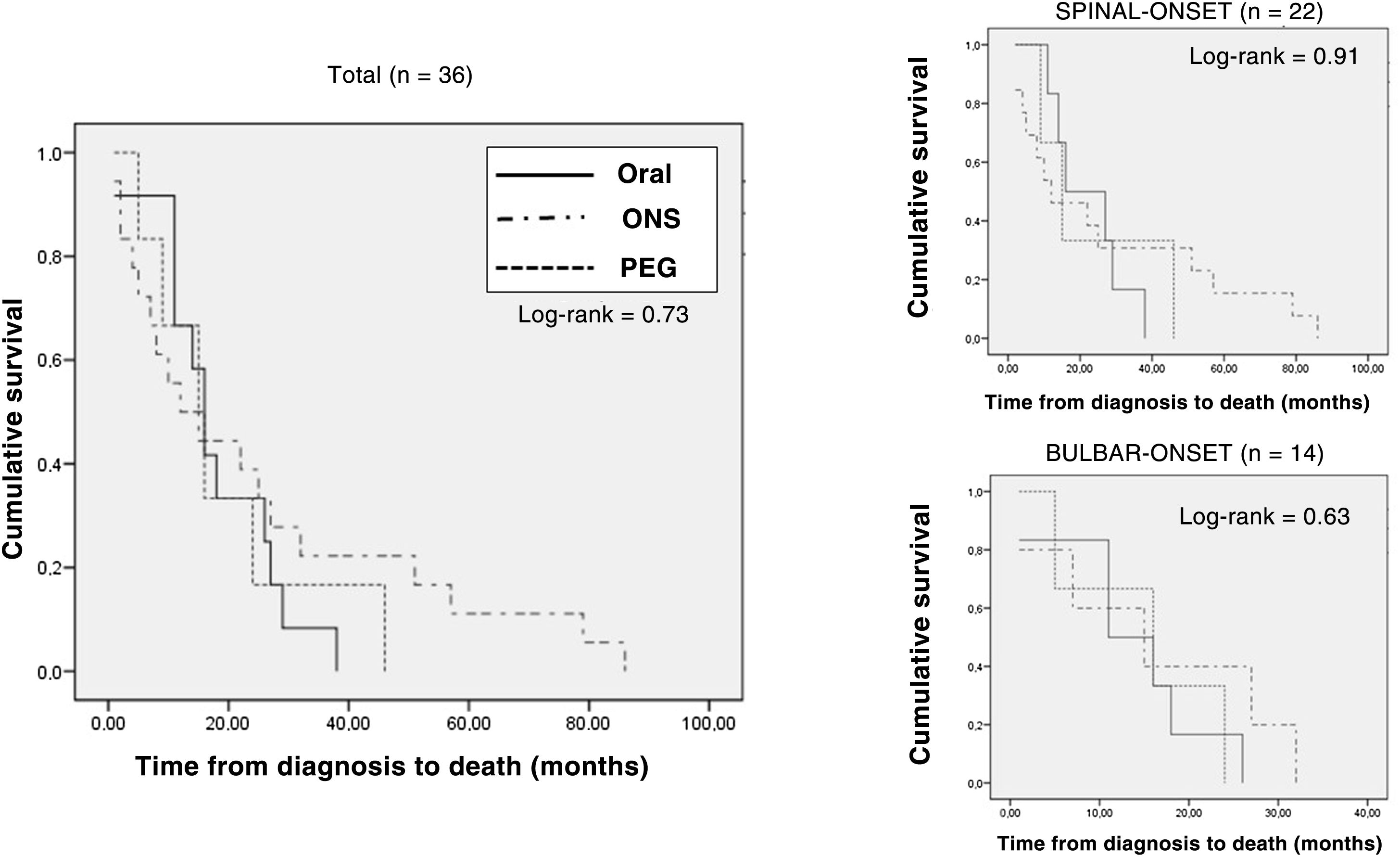
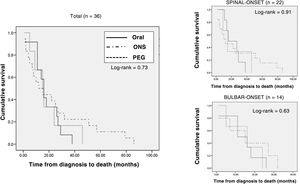
The median follow-up of the patients from diagnosis was 30 (18.5-47.5) months. Out of all patients, 36 (38.7%) died during the follow-up period (spinal-onset: 22 patients [44.9%]; bulbar-onset: 14 patients [31.8%]). The median time between diagnosis and death was 15.50 (8.25-27) months (spinal-onset: 15.50 [8.75-15.50] months; bulbar-onset: 15.50 [6.50-24.50] months).
Analysis of type of nutritional support prescribed at the start of follow-up at the nutrition practice (adapted oral diet, adapted oral diet plus oral nutritional supplementation, and enteral nutrition through a gastrostomy tube) found no differences in terms of rate of deaths (ORAL: 12 [33%]; ONS: 18 [39.1%]; PEG: 6 [54.5%]; p = 0.45). Survival from the onset of symptoms was slightly higher, though not significantly so, in the group of patients with PEG and artificial supplementation (ORAL: 28 [20.25-46.5] months; ONS: 30 [16.75-48.25] months; PEG: 39 [27-52] months; p = 0.90). Kaplan–Meier survival curves are shown in Fig. 4.
Kaplan–Meier survival curves according to type of nutrition at the start of nutritional follow-up in the entire sample of patients who died, stratified according to form of onset (spinal-onset/bulbar-onset).
PEG: percutaneous endoscopic gastrostomy; Oral: oral diet; ONS: oral nutritional supplementation.
Multivariate analysis was performed to evaluate the relationship between initial type of nutritional support and survival for more than 12 months from the start of nutritional follow-up, stratifying by age, baseline nutritional status and form of onset of ALS; no significant differences were found (OR: 0.97 [95% CI: 0.35-2.71]; p = 0.95).
DiscussionThis study set out to assess the effects of the different specialised nutritional support methods at the start of follow-up at the clinical nutrition practice in patients with ALS. The main results seen were that most of the patients started nutritional support with an adapted diet and ONS. Patients with artificial supplementation and gastrostomy showed stability or even improvement in their anthropometric parameters. Patients in whom artificial nutrition therapy (supplementation or gastrostomy) was initiated, despite starting follow-up at the practice with a poorer nutritional status, showed a trend towards improvement in survival, though not a significant one.
Patients with ALS usually present some degree of malnutrition when they are diagnosed with the disease. Systematic screening for malnutrition, both at diagnosis and in follow-up, and referral to a specialised nutrition department are very important in these patients.6 This assessment should be performed with at least one basic anthropometric evaluation with weight, height and BMI, in addition to a measurement of %WL. In the study conducted, all patients underwent determination of these parameters as well as a nutritional assessment by means of SGA. A decline in nutritional status was seen regardless of form of onset of ALS; this decline was more striking in patients who were started on artificial nutritional support. The indication for this type of nutritional support was probably related to the nutritional status detected. Other forms of motor neuron disease were excluded from analysis to arrive at a certain homogeneity in terms of the onset of disease and symptoms.
In this study, the median time to referral to the nutrition department was three months. Paradoxically, in the patients with PEG, who were the ones with the poorest nutritional status, this referral came later with the gastrostomy already placed. Nutritional support should be started as early as possible regardless of degree of malnutrition. In fact, in the context of multidisciplinary teams, earlier referral to a nutrition department has been linked to a lower rate of malnutrition.12
Specialised nutritional support starting from diagnosis is associated with improvements in parameters related to disease course. In the sample analysed, patients in follow-up were seen to achieve a decrease in weight loss progression, with stabilisation of weight; weight gain in the first six months was even achieved in the PEG group. These results were consistent with data such as those from Morassuti et al., who found that starting a specific nutritional protocol led to lower rates of weight loss and higher rates of one-year survival.13
Initial type of nutritional support also is a matter of debate, due to the limited existing evidence in this regard. Our study found that the most commonly used specialised nutritional support technique was an oral diet with adjustments in type and texture, plus oral artificial supplementation. This might have been due to the nutritional status of the patient and to the striking decrease in intake observed. Regarding the use of oral artificial supplementation in ALS, Dorst et al. compared two types of hypercaloric nutritional supplements: one with a high fat content and one with a high carbohydrate content. Both of them showed improvements in weight.14 Another study, by Körner et al., also found that hypercaloric oral supplementation could improve quality-of-life tests, achieving weight stabilisation or even weight gain.15 In summary, in situations in which oral artificial supplementation is indicated, hypercaloric and high-protein products could be advisable for weight stabilisation.
Regardless of use of nutritional supplementation, dietary counselling based on patient capacity for oral intake is key. In fact, whether patients continue to enjoy eating influences the decision to place a gastrostomy tube, because if patients have their oral diet properly adjusted, they will achieve nutrient intake suited to their disease state and their nutritional status can be optimised.16 For this reason, in the study conducted, even in patients with an adapted oral diet (with adjustments in food texture or type), weight stabilisation was achieved, though not to as striking a degree as in patients in whom artificial nutrition was used.
Disease progression leads to different degrees of dysphagia and difficulty eating, which may render patients unable to meet their energy requirements with an oral diet. Therefore, alternative routes of nutrition that duly meet nutritional requirements and prevent complications associated with dysphagia, such as bronchial aspiration, must be sought. A study by Stavroulakis et al. found that the factors that most heavily influenced both patients and their family members with respect to gastrostomy placement were intake difficulty (long meals requiring effort), choking and weight loss.17 In general, PEG placement is associated with initial improvements in anthropometric parameters, as well as certain benefits in terms of survival, as could be seen in our data.
The influence on survival of initial type of nutrition has not been considered as such in the literature. A study by López-Gómez et al. showed that patients who started follow-up with a specialised nutrition team had higher rates of survival than patients who did not,18 but found no differences based on initial nutritional support. A study by Luchesi et al. found an indication for non-oral feeding to be related to a shorter disease duration,19 but this might have been linked to advanced disease stages. Our study found no significant differences in relation to initial type of nutritional support, although it did find a trend towards increased survival in patients who started artificial nutritional support (oral supplementation or complete enteral nutrition) compared to those with an adapted oral diet only. This could have been related to improvements associated with increased calorie and protein intake achieved with artificial nutrition in themselves, and to a better underlying disease course. In fact, Dorst et al. demonstrated increased survival in patients with a high calorie intake, although their study did not evaluate any particular formula.20
The main limitations of this study were the variations in signs and symptoms and in disease progression, which hampered comparison and extrapolation of survival. These variations may have affected statistical significance, as larger sample sizes would be required in a disease with a low prevalence. Moreover, as ours was a multicentre study in a highly complex disease, there were different protocols for action and referral of patients to a nutrition practice. Despite this, follow-up and treatment on the different nutrition units were done in a uniform manner.
Evidence on nutritional support in this disease must be generated with prospective multicentre studies to monitor the endpoints in larger samples. It would also be very interesting to evaluate the effects of nutritional support on body composition beyond “classic” anthropometric variables.
ConclusionsThe main route of nutrition at the start of specialised nutritional support in patients with ALS was the oral route with associated dietary adjustments and artificial supplementation. Patients with ALS showed a severe decline in their nutritional status associated with moderate to severe weight loss before they started nutritional support. After they started specialised nutritional support, their weight loss was seen to slow down and their nutritional status was seen to improve within the first few months of follow-up.
FundingThis study was funded with two competitive science grants: a research grant from the Gerencia Regional de Salud de Castilla y León [Castile-León Regional Health Directorate] (SACyL), with code GRS 1238/A/16, and a research grant from the Sociedad Castellano-Leonesa de Endocrinología, Diabetes y Nutrición [Castile-León Society of Endocrinology, Diabetes and Nutrition] (SCLEDyN) in 2017.
Conflicts of interestNone of the authors of the study had any conflicts of interest in relation to the conduct thereof.
Please cite this article as: López-Gómez JJ, Ballesteros-Pomar MD, Gómez-Hoyos E, Pintor de la Maza B, Penacho-Lázaro MA, Palacio-Mures JM, et al. Efecto del tipo de soporte nutricional especializado sobre la evolución del paciente con esclerosis lateral amiotrófica (ELA). Registro interhospitalario SCLEDyN. Endocrinol Diabetes Nutr. 2021;68:699–707.