Thanks to the advances in cancer treatment, the five-year survival rate after childhood cancer has increased up to 80%. Therefore 1/500 young adults will be a survivor. Endocrine sequelae are most common, affecting 40–60% of survivors. The most frequent sequelae include growth failure and gonadal and thyroid diseases. Sequelae occur more frequently in survivors from central nervous system tumors, leukemia, and lymphoma. Their development will depend on the type of cancer, its location, age at diagnosis, and treatment administered. Treatments associated to more endocrine sequels are cranial radiotherapy and hematopoietic cell transplantation. Because of the high prevalence of endocrine sequelae, international guidelines recommend endocrinologists to prospectively evaluate the survivors. As some of these endocrine changes will not develop until adult life, transition programs should be implemented, and active investigation should be made to decrease the endocrine consequences of cancer treatment.
La evolución en los tratamientos oncológicos ha supuesto un aumento de la supervivencia del cáncer infantil cercana al 80% a 5 años, por lo que 1/500 adultos jóvenes será un superviviente. Las secuelas endocrinas son las más comunes y afectan al 40–60%, siendo las más frecuentes las alteraciones del crecimiento y la disfunción gonadal y tiroidea. Los pacientes con tumores del sistema nervioso central, leucemias y linfomas son los que presentan más secuelas, y estas dependen del tipo de cáncer, su localización, la edad de diagnóstico y el protocolo de tratamiento; las terapias de mayor riesgo son la radioterapia craneal y el trasplante de progenitores hematopoyéticos. Dado este elevado riesgo, las guías internacionales recomiendan a los endocrinólogos evaluar prospectivamente a los supervivientes. Algunas de las alteraciones endocrinas no se manifestarán hasta la vida adulta, por lo que debemos crear programas de transición, así como ser activos en la investigación para reducir las secuelas endocrinas de los tratamientos del cáncer.
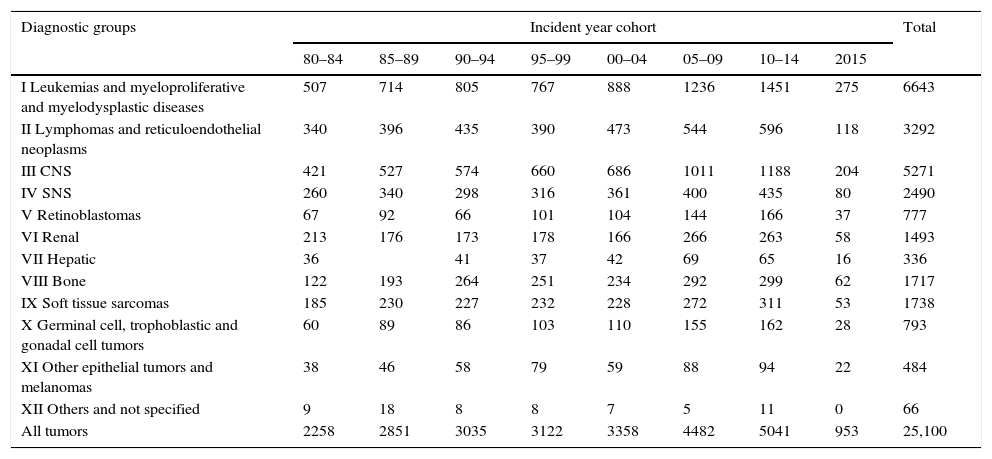
The evolution of cancer treatments has resulted in improvements in the 5-year survival rate, reaching 80% for all cancers globally, and 90% in the case of leukemias and lymphomas. Approximately one out of every 285 children is diagnosed with cancer, and one out of every 530 young adults is a cancer survivor.1 According to the Spanish Pediatric Tumor Registry (Registro Español de Tumores Pediátricos),2 a total of 25,100 cases of malignant disease were recorded in patients between 0 and 14 years of age in the period between 1980 and 2015. This gives us an idea of the number of young adults in this country that survive childhood cancer. It has also been reported that 62% of all adult survivors experience some medical sequelae, and that 40–60% suffer endocrine disease. This implies a risk far greater than that found in the general population.3,4
The present review offers a global view of the follow-up of pediatric cancer survivors, focusing on current knowledge regarding endocrine sequelae, with the aim of optimizing follow-up and improving long-term survival.
New cancer treatment perspectives in children and adolescentsThe most common cancers in the pediatric population are leukemias, lymphomas and tumors of the central nervous system (CNS) (Table 1). The sequelae among the survivors depend on the type of cancer, its location, patient age at the time of diagnosis, and the treatment protocol used (radiotherapy fractionation and dose; type and cumulative dose of chemotherapy). The changes in leukemia management include the identification of risk factors, the intensification of treatment and improvements in supportive measures, a lesser use of prophylactic radiotherapy, and the introduction of hematopoietic progenitor cell transplantation (HPCT) in cases of relapse, all of which have contributed to improve patient survival. In some diseases such as Hodgkin's lymphoma or Wilms tumor, where the survival rates are high, the current aim is to reduce their sequelae. Other diseases such as malignant astrocytomas or metastatic sarcomas continue to have a poor prognosis, and the aim in such cases is to develop new therapies capable of improving survival.5,6
Spanish Pediatric Tumor Registry (Registro Español de Tumores Infantiles [RETI-SEHOP]). Cases recorded according to diagnostic group, incident year cohorts, and microscopic verification (MV).
| Diagnostic groups | Incident year cohort | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 80–84 | 85–89 | 90–94 | 95–99 | 00–04 | 05–09 | 10–14 | 2015 | ||
| I Leukemias and myeloproliferative and myelodysplastic diseases | 507 | 714 | 805 | 767 | 888 | 1236 | 1451 | 275 | 6643 |
| II Lymphomas and reticuloendothelial neoplasms | 340 | 396 | 435 | 390 | 473 | 544 | 596 | 118 | 3292 |
| III CNS | 421 | 527 | 574 | 660 | 686 | 1011 | 1188 | 204 | 5271 |
| IV SNS | 260 | 340 | 298 | 316 | 361 | 400 | 435 | 80 | 2490 |
| V Retinoblastomas | 67 | 92 | 66 | 101 | 104 | 144 | 166 | 37 | 777 |
| VI Renal | 213 | 176 | 173 | 178 | 166 | 266 | 263 | 58 | 1493 |
| VII Hepatic | 36 | 41 | 37 | 42 | 69 | 65 | 16 | 336 | |
| VIII Bone | 122 | 193 | 264 | 251 | 234 | 292 | 299 | 62 | 1717 |
| IX Soft tissue sarcomas | 185 | 230 | 227 | 232 | 228 | 272 | 311 | 53 | 1738 |
| X Germinal cell, trophoblastic and gonadal cell tumors | 60 | 89 | 86 | 103 | 110 | 155 | 162 | 28 | 793 |
| XI Other epithelial tumors and melanomas | 38 | 46 | 58 | 79 | 59 | 88 | 94 | 22 | 484 |
| XII Others and not specified | 9 | 18 | 8 | 8 | 7 | 5 | 11 | 0 | 66 |
| All tumors | 2258 | 2851 | 3035 | 3122 | 3358 | 4482 | 5041 | 953 | 25,100 |
0–14 years, 1980–2015. Excluded, not classifiable in ICCC-3.
Cases excluded because not classifiable in ICCC-3: 2040.
Total recorded cases, including those not classifiable in ICCC-3: 27,140.
The review conducted by the Childhood Oncology Group (COG) revealed shortcomings in survivor control: 68% of the patients treated between 1970 and 1986 had not undergone regular controls, and 39% had had no contact with the center that had treated them.3 The different cohorts studied to date involve different methodological approaches, though they report a comparable risk of medical problems.7 In order to adequately estimate excess risk, some cohorts such as the Childhood Cancer Survivor Study or the Adult Life after Childhood Cancer in Scandinavia survey have compared the incidence with a cohort of siblings of the survivors or with a comparable healthy population.4,8 As a result of these studies, in 2003 the COG published a clinical guide (COG-LTFU Guidelines) with concrete recommendations regarding the follow-up of survivors, and with a specific section addressing endocrine sequelae.9
In this regard, endocrine problems are among the most frequently described sequelae, along with cardiovascular disease. Survivors of tumors of the CNS, leukemias and Hodgkin's lymphoma are the patients that experience most sequelae. The Adult Life after Childhood Cancer in Scandinavia cohort presented a relative risk (RR) for endocrine disease of 4.8 (95%CI 4.6–5.0), with higher values among those under 20 years of age. The Childhood Cancer Survivor Study in turn described the relative risks regarding hypothyroidism (RR 14.3; 95%CI 9.7–21.0), growth hormone (GH) deficiency (RR=277.8; 95%CI 111.1–694.9), the need for the induction of puberty (RR=86.1; 95%CI 31.1–238.2) and osteoporosis (RR=24.7; 95%CI 9.9–61.4). Endocrine disorders moreover have implications in terms of long-term cardiometabolic morbidity in the adult, which affects 18% of all survivors.3,4 Few studies have described the prevalence of endocrine disease with prospective application of the COG-LTFU guidelines. One of the largest series describes 519 patients with non-CNS tumors evaluated an average of 7.2 years after diagnosis, with a mean patient age of 12.1 years. Endocrine sequelae were present in 57.6% of the cases.10 Following the introduction of the cancer survivor follow-up unit at our center, we reviewed the endocrine sequelae of 194 patients (including tumors of the CNS) with a mean age of 10 years, 5.2 years after treatment. Sixty-three percent of the patients had some endocrine disorder. Furthermore, on their first visit almost 30% of the survivors presented endocrine-metabolic disease not previously diagnosed and/or treated.11
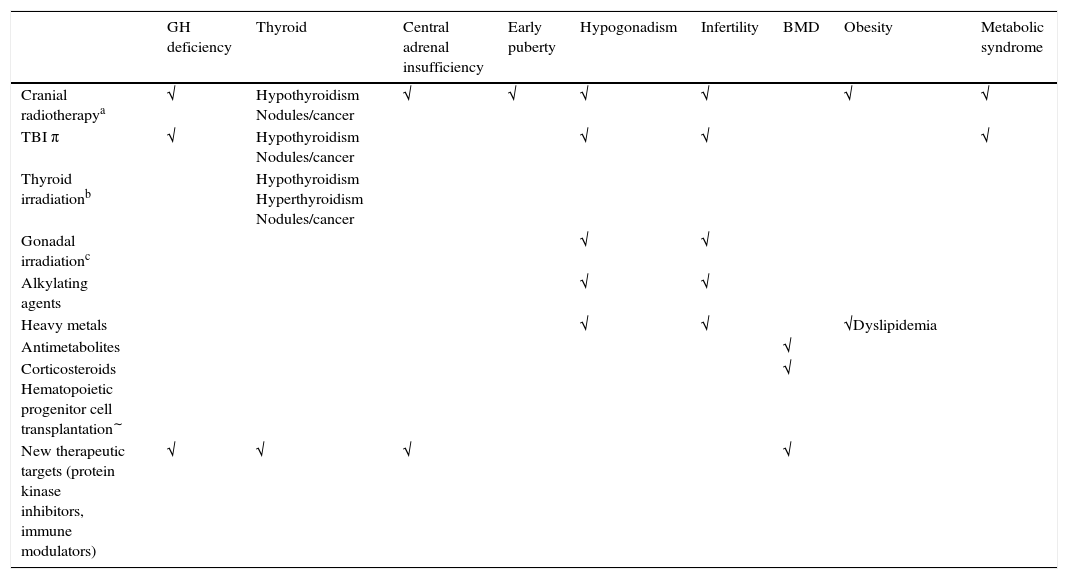
Endocrine sequelae in childhood cancer survivorsA description of the endocrine sequelae is provided below. In order to facilitate description, the sequelae are analyzed according to the reported risk corresponding to the different treatments received (Table 2).12
Endocrine sequelae in childhood cancer survivors: risk according to treatments received.
| GH deficiency | Thyroid | Central adrenal insufficiency | Early puberty | Hypogonadism | Infertility | BMD | Obesity | Metabolic syndrome | |
|---|---|---|---|---|---|---|---|---|---|
| Cranial radiotherapya | √ | Hypothyroidism Nodules/cancer | √ | √ | √ | √ | √ | √ | |
| TBI π | √ | Hypothyroidism Nodules/cancer | √ | √ | √ | ||||
| Thyroid irradiationb | Hypothyroidism Hyperthyroidism Nodules/cancer | ||||||||
| Gonadal irradiationc | √ | √ | |||||||
| Alkylating agents | √ | √ | |||||||
| Heavy metals | √ | √ | √Dyslipidemia | ||||||
| Antimetabolites | √ | ||||||||
| Corticosteroids Hematopoietic progenitor cell transplantation∼ | √ | ||||||||
| New therapeutic targets (protein kinase inhibitors, immune modulators) | √ | √ | √ | √ |
BMD, bone mineral density; TBI, total body irradiation.
Cranial radiotherapy (CRT) produces a significant increase in the risk of sequelae secondary to hypothalamic–hypophyseal involvement. The impact depends on the dose, fractionation and the irradiation field. The degree of involvement has been correlated to the so-called biological effective dose, which allows for the comparison of therapeutic schemes and prediction of the impact upon hypophyseal function.13
According to some studies, patient age is another important factor, younger age being associated with greater hypophyseal involvement. Furthermore, the risk of hypophyseal dysfunction increases over time; follow-up therefore should be prolonged for several years. It has been suggested that the use of other techniques such as proton radiotherapy could reduce the risk of sequelae, though further studies are needed to determine whether they can reduce the long-term endocrine sequelae.14
Somatotropic axis (GH)The most frequent and earliest manifesting type of hypophyseal involvement refers to GH. Alterations have been recorded after doses of 18Gy, and have been seen from as early as the first 12 months after treatment, likewise in relation to hypothalamic damage.15,16 In patients with a history of tumor disease of the CNS and CRT, a pathological test result is considered sufficient to establish the diagnosis.17 Studies have been made to determine whether there is an increased risk of recurrence in patients treated with recombinant growth hormone (rhGH), though the data do not appear to confirm this. Survivors are at an increased risk of suffering a second neoplasm independently of whether or not rhGH treatment is provided. Although some studies had reported an increased risk of second neoplasms (mainly meningiomas and gliomas), subsequent analyses appeared to correlate this risk to previous CRT.18,19 Despite the lack of studies, the consensus documents recommend a one year waiting period after the completion of cancer treatment, in order to ensure that there is no early recurrence.20
The protocolized follow-up of survivors has made it possible to optimize treatment with rhGH, with the observation of improvements in final body height, probably due to the early start of treatment, new therapeutic schemes, and the use of GnRH analogs in cases of early puberty.21 According to the literature, in only 60% of the adults treated with rhGH after childhood cancer is GH deficiency re-evaluated in the transition to adulthood.22 As has been pointed out by some authors, despite the demonstrated benefits of rhGH treatment in adults, the evidence of such benefits in the case of survivors may be limited by the negative effect of the treatments received. In this regard, prospective studies need to be expanded with endocrine-metabolic and quality of life assessments in adult life.23,24
Corticotropic axisAdrenocorticotropic hormone (ACTH) deficiency is less frequent and manifests later in time. Follow-up periods of over 10 years are needed to detect deficiencies in a significant group of patients in the studied cohorts. In view of the characteristics of oncological patients, the use of insulin hypoglycemia tests is restricted, and this complicates full evaluation of the hypothalamic–pituitary–adrenal axis. However, some authors have reported an acceptable correlation between insulin hypoglycemia testing and glucagon stimulation; the latter therefore could be used as an alternative.25 Involvement of the corticotropic axis over the long term has been described in 19% of all patients.26
Thyrotropic axisThe hypothalamic–pituitary–thyroid axis appears to be the least affected axis. Nevertheless, according to some authors the 6% incidence of central hypothyroidism reported in some studies may be due to underestimation in cases characterized by normal free T4 levels. They consequently recommend complementing such assays with the study of nocturnal thyroid stimulating hormone (TSH) elevation and the thyrotropin releasing hormone (TRH) test, which together yield a 36% prevalence of such alterations.27
Gonadotropic axisCranial radiotherapy at doses of over 18Gy predisposes to early puberty. The female gender or irradiation at a younger age increases the risk. Doses of 40Gy can result in delayed puberty or central hypogonadism in 11–15% of the patients.28
HyperprolactinemiaHigh doses (>40Gy) are likewise associated with hyperprolactinemia; prolactin measurement is therefore advised in the presence of suggestive clinical manifestations.
ChemotherapyGonadal involvementGonadal dysfunction is the main side effect of chemotherapy. High gonadotoxic potential fundamentally corresponds to the alkylating agents (mainly cyclophosphamide at doses of ≥7.5g/m2, ifosfamide ≥60mg/m2, the mechlorethamine, vincristine, procarbazine and prednisone (MOPP) protocol in ≥3 cycles or busulfan ≥600mg/m2).29 This risk is increased on adding radiotherapy at the gonadal or pelvic level, or total body irradiation (TBI). There appear to be no differences in the case of patients treated in prepubertal or pubertal stages.30 The testicles are particularly sensitive to the action of the alkylating agents. Specifically, the Sertoli cells and spermatogenesis are more often affected than the Leydig cells, which usually preserve their function. It has been reported that 70% of all patients treated for Hodgkin's lymphoma with the MOPP protocol, without radiotherapy, present azoospermia or oligospermia. This protocol has therefore been replaced by new and less toxic combinations.31 The evaluation of spermatogenesis therefore should be included in the follow-up programs. New biochemical markers such as inhibin B appear to have a closer correlation to spermatogenesis than follicle-stimulating hormone (FSH).32 Nevertheless, according to some authors the specificity of both markers is low. They consequently recommend testicular measurement and a seminogram among the survivors.33
The ovaries appear to be less sensitive than testicular tissue, though the risk increases on adding radiotherapy and/or hematopoietic progenitor cell transplantation (HPCT). Ovarian involvement can affect puberty, with an increased risk of premature ovarian failure (POF) or infertility over the long term. The cumulative incidence of POF among survivors is 8% at 40 years of age.34 No studies have compared the different therapeutic schemes with respect to the individual risk of each drug. It was generally believed that the ovaries of patients under 10 years of age are more resistant to the effects of chemotherapy. However, some authors have found the risk to be no different between patients treated before or after puberty.35,36 Even in the presence of regular menstrual cycles, survivors are at risk of lesser ovarian reserve and therefore may have a shorter fertile period. In addition to the gonadotropins, new promising markers such as anti-Müllerian hormone have been proposed. Patients treated with the MOPP protocol show a decrease in anti-Müllerian hormone with respect to other cancer survivors.37 However, the correlation of this observation to future fertility/fecundity has not been clearly established, and further studies are needed in this regard. The usefulness of the antral follicle count via transvaginal ultrasound has not been firmly established in childhood cancer survivors, and prospective studies need to be carried out in this respect.38
GrowthDifferent studies in leukemia patients have described a decrease in growth during chemotherapy and a lack of recovering growth until the end of intensive treatment. A discrete alteration in final body height (decrease of −0.59 standard deviations [SD]) has been observed in series of leukemia patients treated without radiotherapy. This observation is not correlated to alterations in the GH tests, since GH deficiency has been described in only 0.9–1.2% of the cases. Its systematic screening is therefore not advised.39
Neck radiotherapyThe use of neck radiotherapy, mainly in cases of lymphomas, implies an increased risk of thyroid gland involvement. Hypothyroidism has been described at doses above 10Gy, with less frequent cases of hyperthyroidism. The reported incidence of hypothyroidism reaches 75% in patients with lymphomas. In addition, there is an increased risk of thyroid nodules and/or thyroid cancer at doses of 20–29Gy. This risk decreases with doses of over 30Gy, in accord with their cell destructing effect. The risk is greater in children under 5 years of age. Latency periods of 94 months have been described for the development of hypothyroidism, and of up to 10–20 years for the appearance of nodules. This indicates that long-term follow-up is needed.40,41 The follow-up of these patients is currently recommended due to the risk of thyroid cancer, but there is no consensus regarding the screening technique to be used. A recent literature review has stated that the detection of early stage papillary thyroid cancer in children is associated with lesser recurrence and mortality. However, in contrast to the situation found in adults, there is no evidence of lesser morbidity over the long term.12,42–45
Abdominal radiotherapyThe testicles are very sensitive to radiotherapy; in adults, alterations have been described at doses of 0.2–0.7Gy, with increased FSH and seminogram alterations.24 Azoospermia has been reported at doses of 6–10Gy, and Leydig cell insufficiency at ≥20Gy.46 Although scarce, the series of survivors treated with chemotherapy and radiotherapy at doses of 30–45Gy in childhood have shown 83% to suffer azoospermia. However, 17% are able to recover spermatogenesis 12–15 years after treatment; the authors therefore recommend serial controls.25 In the case of the ovaries, it has been reported that close to 40% of the patients with Hodgkin's lymphoma subjected to radiotherapy suffer POF. In these cases the recovery of gonadal function is exceptional. In addition, irradiated patients have been reported to suffer an increased risk of miscarriage, low birth weight and postpartum bleeding.47
Hematopoietic progenitor cell transplantationEndocrine alterations are the most common sequelae following HPCT. Such alterations are of a multifactorial origin, secondary to rescue therapy added to the cumulative effects of chemotherapy, high-dose corticosteroids and the use of total body irradiation. These patients have a two- to three-fold increased risk of endocrine disease.4 It is very common to observe hypogonadism (83%), hypothyroidism (56%) and GH deficiency (50%). This frequency is even higher than that seen in patients subjected to prophylactic cranial radiotherapy, possibly because of lesser dose fractionation. HPCT is moreover a risk factor for growth both in patients with and without radiotherapeutic conditioning regimens. On the other hand, HPCT predisposes not only to overweight, but also to increased central adiposity and metabolic risk.48
New therapeutic targetsNovel therapies have also been introduced in recent years in pediatric oncology, though their impact in terms of endocrine sequelae requires further studies.49,50 Protein kinase inhibitors, interferon-alpha and monoclonal antibodies have been related to thyroid gland alterations (thyroiditis and hypothyroidism).51 Alterations of the corticotropic axis have been described (sometimes of a subclinical nature) with the use of protein kinase inhibitors; systematic controls of this axis are therefore advised.52 Ipilimumab has been reported to cause hypophysitis, resulting in panhypopituitarism.53 Decelerated growth has been described in patients treated with imatinib, this problem being more severe in smaller children.54 Likewise, there have been reports of calcium–phosphate metabolic alterations in adults treated with protein kinase inhibitors.55
Combination therapies—multifactorial sequelaeCalcium–phosphate metabolismA decrease in bone mineral density (BMD) has been observed in childhood cancer survivors, the underlying cause being of a multifactorial nature. An increased risk has been associated with treatment with hematopoietic progenitor cells, methotrexate or prolonged corticosteroid therapy, and with older patients. Since the best approach for measuring BMD in pediatric patients is subject to debate, the screening method of choice is controversial. The COG-LTFU guidelines recommend densitometry or quantitative computed axial tomography in high risk patients upon admission to the follow-up program, and the repetition of such measures if clinically indicated. The Z-score associated with increased fracture risk in the pediatric population likewise has not been defined. As a result, there is no consensus regarding the values indicating the treatment in such cases.4 In terms of efficacy in reducing fractures over the long term, some authors recommend the promotion of physical activity, with the optimization of calcium intake and vitamin D levels among the survivors.56,57
ObesitySome authors do not include obesity among the sequelae, due to its multifactorial nature and the difficulty of establishing a cause–effect relationship with the treatments. The prevalence of obesity is variable; some studies document an overweight/obesity rate of 25%/18%, which is even lower than that of the general pediatric population in the same geographical setting. The risk is greater in patients subjected to cranial radiotherapy at doses of >20Gy, in women, and in irradiated patients <4 years of age. However, an increase in metabolic risk has been observed, 26% of the patients presenting insulin resistance and 23% suffering from dyslipidemia in relation to an increase in abdominal adiposity or certain therapeutic modalities such as HPCT and/or total body irradiation.58,59
ConclusionIn the last decade progress has been made in improving the health of childhood cancer survivors. In this regard, the creation of international survivor registries has been an inflexion point. There is still a long way to go, however. In effect, we need more multidisciplinary units for the care of childhood cancer survivors; we must be proactive in our approach to patient follow-up and in the recording of sequelae; and we must ensure continuity between pediatric and adult units, since many of the sequelae will not become apparent until the patients reach adulthood. Scientific bodies have a crucial role to play here, by promoting the follow-up of survivors, encouraging ties between pediatric and adult units, and contributing to general strategies designed to encourage a healthy lifestyle. In other words, our goal is no longer limited to just healing the patient.
Conflicts of interestThe author states that she has no conflicts of interest.
Thanks are due to the Hematological Oncology team of Hospital Sant Joan de Déu for its support, and particularly to Dr. Ofelia Cruz, Dr. Albert Catalá and Dr. Ibañez for their valuable contributions to this review.
Please cite this article as: Casano Sancho P. Secuelas endocrinológicas en supervivientes de cáncer infantil. Endocrinol Diabetes Nutr. 2017;64:498–505.