During pregnancy, thyroid peroxidase (TPO) antibodies may increase the risk of developing subclinical hypothyroidism (SCH). Both conditions appear to be associated to maternal–fetal complications. The objectives of this study were to analyze if a relationship exists between TSH and TPO levels during pregnancy and the potential effects on gestational and perinatal complications, and to assess whether detectable, but not positive, TPO levels have an impact on development of gestational SCH.
MethodsA prospective study was conducted at the Leon Health Area (CAULE), where universal screening for gestational thyroid dysfunction is performed between weeks 7–13 of pregnancy. Data on TSH and TPO levels and gestational and perinatal complications were collected for all 2016 deliveries. Positive TPO antibodies were defined as values≥35IU/ml. In a previous study, a TSH level>3.72mU/l was established as the cut-off value for gestational SCH.
ResultsRecords corresponding to 1980 deliveries at CAULE, 21 abortions, and 18 deliveries outside the hospital were analyzed. Of the 1670 pregnant women screened (84.34%), 142 (8.50%) had positive TPO antibodies and their presence was associated to diagnosis of SCH (p<0.01) and to significantly higher mean TSH levels (3.51mU/l versus 2.46mU/l, p=0.03). There were no significant differences in gestational or neonatal complications. In the group with undetectable TPO antibodies (<10lU/ml), the mean TSH levels was slightly lower than in the group with TPO values ranging from 10 to 35IU/ml, but the difference was not significant (p=0.89).
ConclusionPresence of positive TPO antibodies is associated to higher TSH levels and higher risk of gestational SCH, but does not increase the rate of maternal–fetal complications.
Los anticuerpos antiperoxidasa tiroidea (ATPO) en la gestación pueden influir en el desarrollo de hipotiroidismo subclínico gestacional (HSG). Ambas entidades parecen asociarse a complicaciones maternas y fetales. Los objetivos de este estudio son analizar si existe relación entre los valores de TSH y ATPO durante el embarazo, los posibles efectos sobre complicaciones gestacionales y perinatales, y valorar si los ATPO detectables, pero no positivos, influyen en el desarrollo de HSG.
MetodologíaEstudio prospectivo realizado en el área sanitaria del Complejo Asistencial Universitario de León (CAULE), donde se realiza cribado universal para disfunción tiroidea gestacional entre la semana 7-13 de gestación. Se recogieron datos de TSH, ATPO, obstétricos y neonatales de los partos de 2016. Se considera ATPO positivo si≥35UI/ml. En estudio previo se estableció valor TSH>3,72 mU/L como corte para HSG.
ResultadosSe analizaron registros correspondientes a 1.980 partos en CAULE, 21 abortos y 18 partos fuera del centro. Se realizó cribado a 1.670 gestantes (84,34%): 142(8,50%) tuvieron ATPO positivos. La detección de ATPO positivo se asoció con el diagnóstico de HSG (p<0,01) y con media de TSH significativamente mayor (3,51 vs. 2,46mU/L; p=0,03). No encontramos diferencias significativas en las complicaciones gestacionales o neonatales. En el grupo con ATPO indetectable (<10Ul/ml) la media de TSH fue ligeramente inferior que en el grupo con valores de ATPO 10-35UI/ml, pero sin diferencias significativas (p=0,89).
ConclusiónLa presencia de ATPO positivo se asocia con valores de TSH más elevados y con mayor riesgo de HSG, pero no incrementa la tasa de complicaciones materno-fetales.
The presence of thyroid peroxidase (TPO) antibodies during pregnancy has been postulated as a risk factor for miscarriage and premature delivery.1 In general, the presence of autoimmunity, even with normal thyroid function, is relatively common in women of childbearing age. The prevalence of thyroid antibodies in a non-selected sample of women ranges from 6 to 20%, and increases to 17–33% in a population that has suffered miscarriages, fetal losses or premature deliveries.2
Autoimmunity is the most common cause of hypothyroidism in our setting. Clinical hypothyroidism, defined as elevated TSH and low FT4 or TSH>10mU/l independently of the FT4 value, if left untreated during pregnancy, is associated with obstetric complications, negative effects upon fetal neurocognitive development, an increased risk of premature delivery and miscarriage, as well as low birth weight and gestational hypertension.3
Although the mechanism by which TPO antibodies alter thyroid function during pregnancy is not known precisely, it is believed that the cause may be a mild deficiency in thyroid hormone availability when elevated TPO antibodies are present. On the other hand, it could be due to a lesser thyroid gland capacity to adapt to the increased demand for thyroid hormone synthesis during pregnancy.3
The initial general recommendations were that the upper TSH limit in pregnant women should be 2.5mU/l in the first trimester and then 3mU/l in the second and third trimesters.4 However, in recent years, various studies have shown that the reference limits need not be so strict. A study conducted in China involving almost 5000 patients showed TSH levels to be at their lowest between weeks 7–12 of pregnancy, though the concentrations only decreased from 5.31 (a normal value outside pregnancy) to 4.34mU/l in those weeks.5 Other studies have reported FT4 reductions when the TSH levels exceed 4.8mU/l.6 Consequently, the latest recommendation of the American Thyroid Association4 is to use the cut-off point of the reference population (i.e., that with no history of thyroid disease, negative autoimmunity, and adequate iodine intake), and that if proprietary reference levels cannot be established, use should be made of those provided by studies involving populations of similar characteristics. Based on the most recent literature, these guides conclude that in general, the upper limit of normal for TSH is 4mU/l in the first trimester of pregnancy. However, in pregnant women with TSH>2.5mU/l, they also recommend evaluation of the presence of TPO antibodies. Furthermore, in the event of TPO antibody positivity with TSH>2.5mU/l (though without exceeding the proprietary reference cut-off value), they recommend that treatment with levothyroxine should be considered.4
The prevalence of clinical hypothyroidism during pregnancy ranges from 0.25 to 0.91% in studies comprising more than 10 participants,6 while subclinical hypothyroidism (SCH) is diagnosed in 3–10% of all pregnancies, depending on the cut-off point used.3 However, in studies involving populations similar to that of the health area of León (Spain), which is iodine-deficient, the prevalence of SCH during pregnancy (TSH>2.5mU/l) reaches over 30%.7 It should be taken into account that such a large number of patients constitute a significant challenge both in terms of healthcare and as regards clinical management.
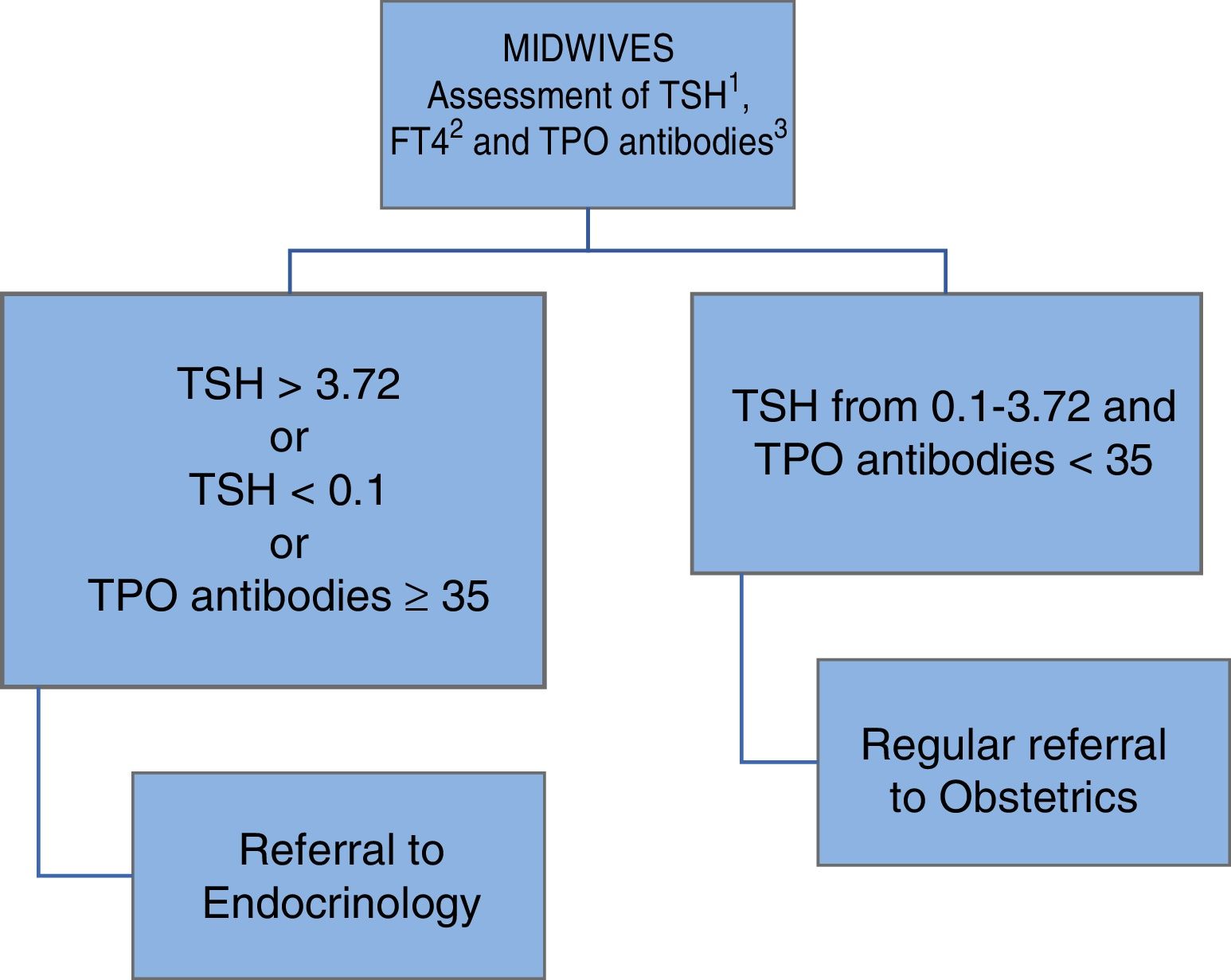
Universal screening for thyroid dysfunction in the pregnant population remains a subject of debate. In Spain, the Spanish Society of Endocrinology and Nutrition (Sociedad Española de Endocrinología y Nutrición, SEEN), in a joint document with the Spanish Society of Gynecology and Obstetrics (Sociedad Española de Ginecología y Obstetricia, SEGO), has recommended the early assessment (before the tenth week of pregnancy) of thyroid function in pregnant women.8 In the health area of León, universal screening for gestational thyroid dysfunction was implemented in May 2013 by the Endocrinology and Nutrition section, in collaboration with Obstetrics and primary care midwives. The American Thyroid Association does not position itself on this issue: it states that there is not enough evidence to recommend or not recommend universal screening, with the sole exception of women undergoing assisted reproduction processes or with a history of positive TPO antibodies. However, the Association does advise the study of pregnant women with risk factors for thyroid dysfunction4 i.e., those with a personal history of hypothyroidism/hyperthyroidism or with symptoms or signs of gestational thyroid dysfunction, positive autoimmunity or the presence of goiter, a history of neck irradiation or thyroid surgery, age>30 years, a personal history of fetal loss, miscarriages or prematurity, multiple pregnancies, a family history of thyroid dysfunction, morbid obesity (a body mass index [BMI]≥40kg/m2), amiodarone, lithium or iodinated contrast media use, or residence in an iodine-deficient area.
The primary objectives of our study were to describe the state of gestational thyroid dysfunction in the health area of León, and to assess the influence of TPO antibodies upon thyroid function during pregnancy and their potential association with maternal and fetal complications.
The secondary objective was to determine whether TPO antibody levels that are detectable (10–35IU/ml) but are not considered to represent positivity as such can influence the development of SCH.
MethodologyA prospective study was carried out in those pregnant women who gave birth at the University Care Complex of León (Complejo Asistencial Universitario de León, CAULE) in 2016, with due registry in the deliveries database of the Department of Gynecology and Obstetrics of CAULE, and who had been monitored by the Endocrinology and Nutrition clinic during their pregnancy.
Bearing in mind the need for proprietary population reference values, in 2014 our group had conducted a cross-sectional study to determine the TSH levels during the gestation period in the CAULE. The inclusion criteria were healthy women with no previous disease, and who were between weeks 8 and 11 of pregnancy. The exclusion criteria were pregestational thyroid disease, visible or palpable thyroid morphological anomalies, no detectable TPO antibodies, hypothyroxinemia (FT4 normal interval 0.9–1.7ng/dl), a history of familial thyroid disease, pregestational or gestational diabetes, any chronic disease prior to pregnancy, assisted reproduction, and the use of any drug treatment capable of affecting thyroid function. The iodine nutritional status of the studied women was not taken into consideration. In order to establish the reference cut-off points, the distribution of TSH values was normalized through logarithmic transformation, followed by definition of the confidence intervals corresponding to percentiles 2.5 and 97.5.
For this purpose, between December 2013 and September 2014 we enrolled 1023 pregnant women in weeks 8–11 of pregnancy. A total of 289 pregnant women were excluded due to the abovementioned exclusion criteria. The final healthy pregnant population from which the reference values were calculated totalled 734 women. In these pregnant women the mean TSH concentration was 1.72 (0.83)mU/l, with a median of 1.69mU/l. In turn, TSH was 0.33mU/l for percentile 2.5 and 3.72mU/l for percentile 97.5, taken as reference for the first trimester of pregnancy (unpublished data).
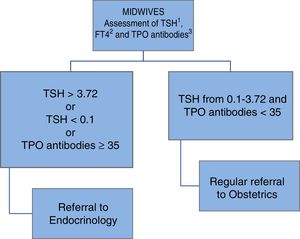
In May 2013 a universal thyroid function screening program was implemented in the health area of León, targeting all pregnant women, in collaboration with the Department of Gynecology and Obstetrics and primary care midwives, as shown in Fig. 1.
Women treated with thyroxine before pregnancy were excluded from the present study. Accordingly, we collected the following data from population screening performed between weeks 7–13 of pregnancy: TSH, FT4 and TPO antibodies (electrochemoluminescence immunoassay [ECLIA], Cobas®, Roche Diagnostics).
Patient age, weight and height were recorded from the endocrinology database, together with maternal smoking, a family or personal history of thyroid disease and diabetes mellitus, the use of vitamin supplements during pregnancy and their iodine contents, the consumption of iodinated salt, and the obstetric history.
With regard to the delivery data recorded by Obstetrics, we documented the gestational age, the type of delivery, the weight of the newborn infant and the Apgar test in minutes 1 and 5.
Thyroid peroxidase antibodies detected in our laboratory using the Roche Diagnostics Cobas® 6000 test are considered to be positive when ≥35IU/ml. However, our laboratory detects values between 10 and <35IU/ml, which are considered negative.
For comparison of the weights of the newborn infants we used the means of the infants born to term (gestational age ≥37 weeks) and the percentiles based on the Spanish growth study of 2010.9
The data were entered on an MS Excel version 2003 spreadsheet, and the statistical analysis was performed using the SPSS statistical package (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0, Armonk, NY, USA; IBM Corp.) Categorical variables were reported as absolute values and percentages, while quantitative variables were reported as the mean and standard deviation (SD).
Contingency tables were used to explore associations between qualitative data, and the Pearson chi-square test was used to assess the degree of association. Means in turn were compared using the Student's t-test.
The study was conducted in accordance with the guidelines of the Declaration of Helsinki, and all procedures performed in human subjects were approved by the local Clinical Research Ethics Committee.
ResultsIn 2016, we recorded a total of 1980 deliveries, 21 miscarriages in patients followed-up on by Endocrinology, and 18 patients with delivery outside the CAULE. Eighty-eight patients with prior thyroxine treatment were excluded from the statistical analysis. A total of 1931 naïve patients were thus included.
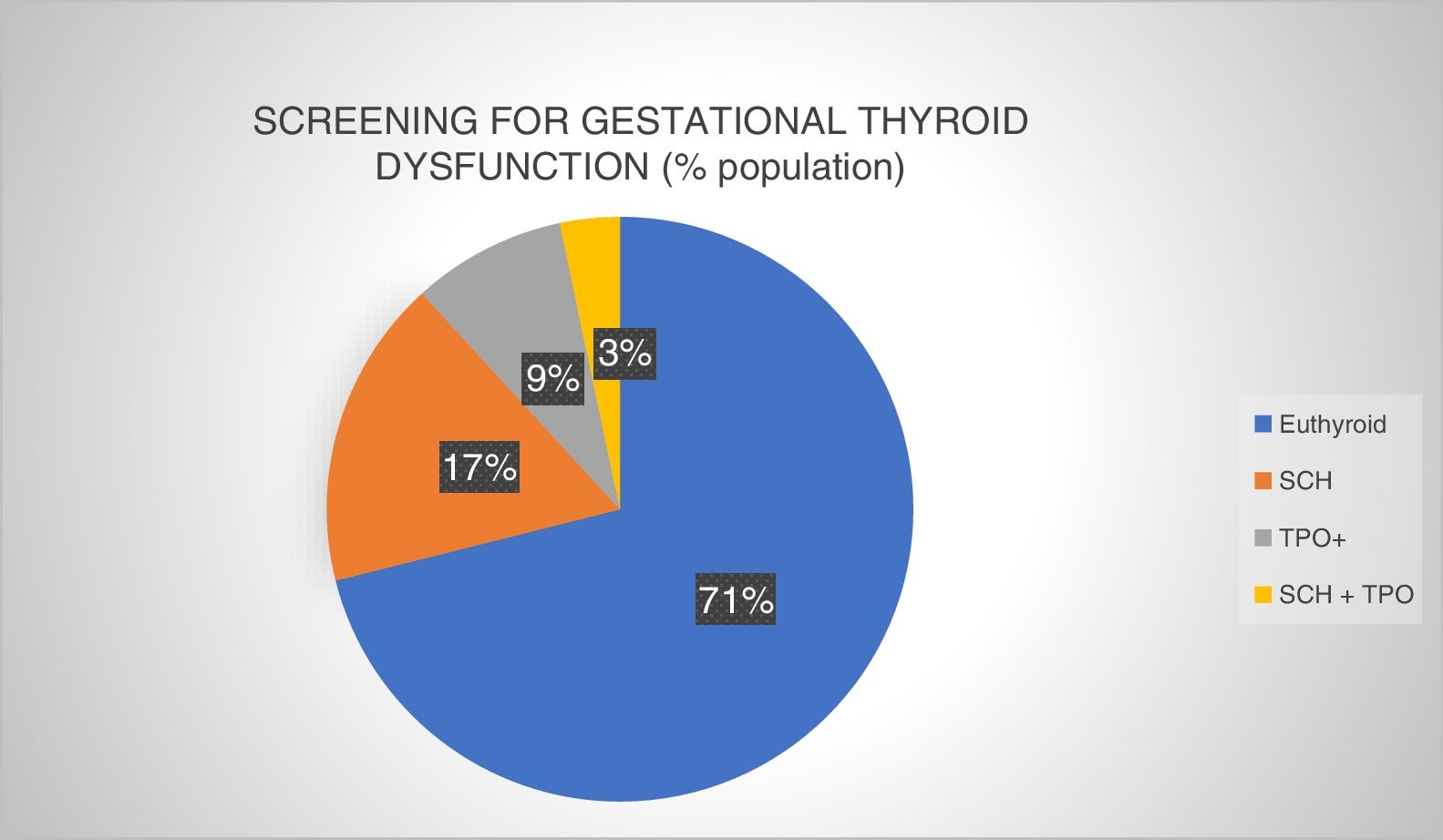
A total of 1670 patients were screened, meaning that 82.71% of all the pregnant women underwent testing at least for TSH in the first trimester of pregnancy. The screening results are described in Fig. 2.
Prevalence of gestational thyroid dysfunction at screening in the first trimester of pregnancy (University Care Complex of León [Complejo Asistencial Universitario de León, CAULE], 2016). Euthyroid: normal thyroid function and negative autoimmunity; SCH: subclinical hypothyroidism; SCH+TPO: subclinical hypothyroidism with positive autoimmunity; TPO+: positive thyroid peroxidase antibodies.
All of the patients were taking oral iodine supplements at doses ranging from 150 to 300μg/day.
TSH and thyroid peroxidase antibodiesOf the 1670 patients screened, 1551 had data with reference to TPO antibodies (92.87%). We found that 38.73% of the patients with positive TPO antibodies developed SCH, versus only 16.25% of those without autoimmunity (p<0.01).
The mean TSH concentration in the TPO antibody-positive group was 3.51mU/l (2.14) versus 2.46mU/l (2.72) in the TPO antibody-negative group (p=0.03).
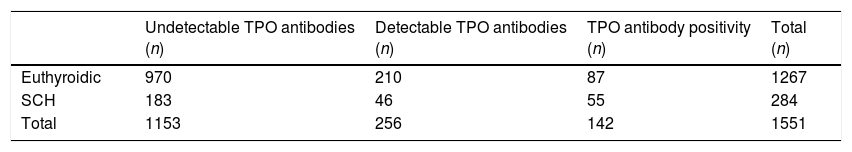
In 1153 of the patients (69.04%), TPO antibodies proved undetectable (<10IU/ml), while in 256 patients (15.33%) TPO antibody values ranged from 10 to 35IU/ml. In the latter group, 17.97% (n=46) presented TSH>3.72mU/l. In pregnant women with undetectable autoimmunity, SCH was diagnosed in 15.87% of the cases (p=0.40) (Table 1).
Contingency table of thyroid peroxidase antibody levels and gestational subclinical hypothyroidism.
| Undetectable TPO antibodies (n) | Detectable TPO antibodies (n) | TPO antibody positivity (n) | Total (n) | |
|---|---|---|---|---|
| Euthyroidic | 970 | 210 | 87 | 1267 |
| SCH | 183 | 46 | 55 | 284 |
| Total | 1153 | 256 | 142 | 1551 |
SCH: gestational subclinical hypothyroidism.
Undetectable TPO antibodies: <10IU/ml. Detectable TPO antibodies: 10–35IU/ml. TPO antibody positivity: ≥35IU/ml.
In the case of undetectable TPO antibodies, the mean TSH levels were 2.44mU/l (2.89) versus 2.53mU/l (1.69) in the detectable antibody group (TPO antibodies 10–35IU/ml), though the differences were not significant (p=0.89).
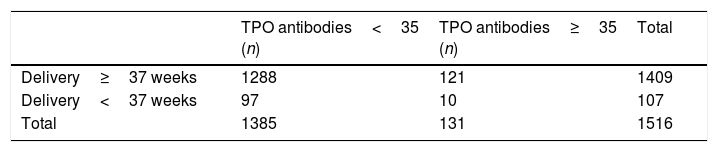
Maternal–fetal complications in the presence of autoimmunityIn 2016, a total of 107 preterm deliveries (<37 weeks) were recorded in CAULE, representing a preterm birth rate of 5.40%.
There were no differences in prematurity according to positive or negative TPO antibody status (7.63% versus 7%; p=0.79) (Table 2).
With regard to the type of delivery, 420 were assisted (21.21%), while the prevalence of cesarean section in 2016 was 16.70% (n=390). In pregnant women with positive TPO antibodies, cesarean section was performed in 19.08% of the cases, which was virtually the same as in the case of antibody-negative women (19.49%) (p=0.99).
The mean weight of the full-term infants of TPO antibody-positive mothers was 3305.53 (463.69)g (p41; −0.24 SD), which proved slightly greater than that of the infants of mothers with negative autoimmunity: 3264.92 (457.74)g (p37; −0.34 SD). The difference was not statistically significant (p=0.35). The prevalence of full-term infants considered small for gestational age (<2500g) was greater among mothers with positive autoimmunity, though without statistically significant differences: 3.02% in those with negative TPO antibodies versus 4.10% in those with positive antibodies (p=0.51).
DiscussionMost previous studies show that TSH levels in pregnant women with positive TPO antibodies, even in the absence of known prior thyroid dysfunction, are higher than in pregnant women without autoimmunity. In the study published by Negro et al.,10 the TSH level was 1.25mU/l in TPO antibody-positive women versus 0.82mU/l in women without antibody positivity (p<0.01). In our series, the difference was statistically relevant, and the mean TSH levels were found to be elevated in both groups, this possibly being related to the iodine deficiency that characterizes our population.11 In the study conducted in Bierzo, a region in the province of León, the mean urinary iodine concentration in pregnant women in the first trimester was 71.40μg/l, while the urinary iodine concentration recommended by the World Health Organization is between 149 and 249μg/l.
In pregnancy, the prevalence of antithyroid antibodies is 10–20%, depending on the cut-off point used by each individual laboratory.12 In our study, the prevalence of TPO antibody-positivity was 8.50% among pregnant women screened in the first trimester. This figure is slightly lower than in other studies, though it should be taken into account that we excluded those patients previously treated with thyroxine (4.44% of all deliveries in 2016).
The relationship between TPO antibodies and obstetric complications is complicated and has generated a considerable amount of controversy. As mentioned above, thyroid autoimmunity appears to increase TSH and the risk of developing gestational SCH. Increased TSH alone has been shown to increase the risk of maternal and neonatal negative effects such as premature delivery, and may therefore represent a confounding factor when complications are being evaluated.13 In Texas, thyroid function was studied in a cohort of 17,298 pregnant women. The incidence of SCH (defined as TSH≥percentile 97.5 with normal FT4 levels) was found to be 2.3%, with an increased preterm delivery rate as compared to the control group (4% versus 2.50%; p<0.05).14
Our results showed no association between prematurity and positive autoimmunity. Nevertheless, this is a highly controversial issue, and studies can be found that support both hypotheses. In this regard, Glinoer et al.15 recorded twice as many preterm deliveries in pregnant women with autoimmunity (16% versus 8%; p<0.05). Another study carried out by Ghafoor et al. in 1500 Pakistani women also revealed this association (26.8% versus 8%; p<0.01).16 In turn, Negro et al. found a strong correlation between prematurity and positive TPO antibodies (22.4% versus 8.2%) in pregnant women from Italy.17
By contrast, an Australian study published by Tierney et al.18 recorded no differences with respect to preterm birth in women with positive thyroid antibodies versus those without. Likewise, in New Jersey, Stagnaro-Green et al. found no association in this respect between women with or without TPO antibodies.19
Another aspect to be considered when complications are being evaluated is the possible impact of treatment with thyroxine. In our case, a high proportion of the pregnant women with autoimmunity received replacement therapy because they were also diagnosed with SCH. In fact, some studies have shown the prematurity rate to decrease in TPO antibody-positive pregnant women when thyroxine is administered.20 By contrast, thyroxine administration in patients negative for TPO antibodies and with TSH<10mU/l appears to offer no benefit in this sense.21
Fewer data are available on other obstetric complications. Negro et al.10 studied the cesarean section rates and found them to be similar in pregnant women with or without TPO antibodies (20.90% in antibody-negative cases versus 22.40% in antibody-positive women; p=0.56). There were also no differences in newborn infant weight according to maternal autoimmune status (weight<2500g: 4.80% versus 6.50%; p=0.23), these results being similar to those of our own study.
Finally, a study was made of the presence of possible differences in thyroid function in patients with TPO antibody titers that were detectable but below the normal range according to our laboratory reference cut-off value. Our sample showed a slight TSH increase with TPO antibody titers ranging from 10 to 35IU/ml, though without statistically significant differences. Few studies have addressed this issue to date. In a recent study, Korevaar et al.22 evaluated a pregnant population (n=11,212) in an attempt to improve the specificity of the TPO antibody cut-off point. To this effect they conducted a meta-analysis of three large population-based studies in the Netherlands. As mentioned above, the general recommendation is to apply the proprietary cut-off values for TSH and FT4 in gestational thyroid dysfunction, and to use the cut-off values of the manufacturer of the assay kit for the TPO antibodies. These values have been tested in the general population and not specifically in pregnancy. Hence, it is often difficult to establish comparisons between different trials, since variability in defining TPO antibody positivity of between 15 and 143IU/ml has been observed. Korevaar et al. found a positive association between TSH levels and a negative correlation to the FT4 values (p<0.01). They established a cut-off point from which the TSH levels increased significantly (percentile 92) while the FT4 levels decreased (percentile 94). The authors found a great difference with respect to this cut-off value and that of the reference laboratory: Generation R study23 25.7 versus 60IU/ml of the laboratory; ABCD study24 30.7 versus 80IU/ml of the reference. In the HAPPY trial,25 however, the percentile and laboratory values were similar: 35 versus 34IU/ml.
In addition, it was confirmed that there is a positive association between premature delivery and positive TPO antibodies as established according to the reference value. Further studies are therefore required to determine the TPO antibody cut-off point during pregnancy, as this can lead to severe alterations in thyroid function and the need for thyroxine treatment.22
In conclusion, the present study provides additional evidence of an association between thyroid antibody positivity and TSH elevation, and therefore a greater tendency toward the development of subclinical hypothyroidism in pregnancy. The strengths of this study include the total number of women investigated and the large percentage of patients included in the universal screening protocol using a cut-off point inherent to the study area. As a weakness of the study, mention must be made of the uniformity of the population, comprising women from the area of León, which is an iodine-deficient area. In this case, iodine nutritional status was not specifically studied, and pregnant women were generally supplemented; they consequently might not have been iodine deficient. No association was found with maternal–fetal complications in autoimmune-positive pregnant women, though this issue is subject to controversy, and there are theories both in favor and against such an association. All this supports the need for prospective evaluations, including another important factor: levothyroxine therapy in euthyroid women with positive autoimmunity to thyroid peroxidase.
Author contributionsPaula Fernández-Martínez and Rocío Aguado-García conceived and designed the study.
Paula Fernández-Martínez analyzed the data and drafted the manuscript. All the authors contributed to data acquisition, the drafting of the manuscript, and approved the final version of the article.
Financial supportThis study received no funding from public or commercial sources.
Conflicts of interestAll authors declare that they have no conflicts of interest.
Please cite this article as: Fernández Martínez P, Aguado García R, Barajas Galindo DE, Hernández Moreno A, Alejo Ramos M, García Arias S, et al. Influencia de los anticuerpos antiperoxidasa tiroidea en los valores de TSH de gestantes y en las complicaciones materno-fetales. Endocrinol Diabetes Nutr. 2018;65:444–450.






![Prevalence of gestational thyroid dysfunction at screening in the first trimester of pregnancy (University Care Complex of León [Complejo Asistencial Universitario de León, CAULE], 2016). Euthyroid: normal thyroid function and negative autoimmunity; SCH: subclinical hypothyroidism; SCH+TPO: subclinical hypothyroidism with positive autoimmunity; TPO+: positive thyroid peroxidase antibodies. Prevalence of gestational thyroid dysfunction at screening in the first trimester of pregnancy (University Care Complex of León [Complejo Asistencial Universitario de León, CAULE], 2016). Euthyroid: normal thyroid function and negative autoimmunity; SCH: subclinical hypothyroidism; SCH+TPO: subclinical hypothyroidism with positive autoimmunity; TPO+: positive thyroid peroxidase antibodies.](https://static.elsevier.es/multimedia/25300180/0000006500000008/v1_201810270629/S2530018018301239/v1_201810270629/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
