Iodine deficiency is linked to thyroid dysfunction, particularly in pregnant women. The objective of this study was to ascertain the iodine levels of women in the second trimester of pregnancy, analysing the influence of iodine ingestion on urinary iodine concentration (UIC) and maternal thyroid function.
MethodsA prospective observational study of pregnant women from Health Area IV of Asturias (northern Spain) recruited before 13 weeks of gestation between May and June 2017. A questionnaire on iodine intake was completed at the first visit, and urine and serum samples were collected at baseline and again during the second trimester. UIC, thyroid stimulating hormone (TSH) and free thyroxine (FT4) obtained in the second trimester of gestation were analysed and related to iodine intake. Thyroid autoimmunity was also analysed in half of the pregnant women at baseline.
ResultsA total of 241 pregnant women were studied. Of these, 56.7% used iodised salt, 46.7% consumed ≥2 servings of dairy products daily and 88.1% took iodine supplements. Median UIC was 191μg/l (135.3–294μg/l), with 68.1% of the women having UIC ≥150μg/l. Only iodised salt consumption provided protection against iodine deficiency (odds ratio 0.35 [0.20–0.63], p=0.001). In women with no autoimmune thyroid disease (n=88), mean levels of TSH were lower in those that consumed iodised salt than in those that did not (respectively, 2.08±0.89mIU/l vs. 2.56±1.02mIU/l, p=0.025). In women with autoimmune thyroid disease (n=30), mean levels of TSH were higher in those that took iodine supplements than in those that did not (respectively, 2.97±1.25mIU/l vs. 1.16±0.41mIU/l, p=0.002).
ConclusionsThe pregnant women studied from Health Area IV in Asturias maintain adequate nutritional iodine status in the second trimester of gestation. In our sample, only the consumption of iodised salt was associated with adequate iodine nutrition, without affecting maternal thyroid function. Most of the women used iodine supplements, which was linked to higher levels of TSH in pregnant women with autoimmune thyroid disease.
La deficiencia de yodo se relaciona con disfunción tiroidea, especialmente en las gestantes. El objetivo del estudio fue conocer los niveles de yodo de las mujeres en el segundo trimestre de gestación, analizando la influencia de la ingesta de yodo sobre la concentración urinaria de yodo (urinary iodine concentration [UIC]) y la función tiroidea materna.
MétodosEstudio observacional prospectivo realizado en gestantes del Área Sanitaria IV de Asturias (norte de España) reclutadas antes de la semana 13 de gestación entre mayo y junio de 2017. Se completó un cuestionario sobre la ingesta de yodo en la primera visita y se recogieron muestras de orina y de suero al inicio del embarazo y durante el segundo trimestre. Se analizaron la UIC, la hormona estimulante del tiroides (thyroid stimulating hormone [TSH]) y la tiroxina libre (FT4) obtenidas en el segundo trimestre de gestación y se relacionaron con la ingesta de yodo. También se analizó la autoinmunidad tiroidea en la mitad de las gestantes al inicio del embarazo.
ResultadosSe estudiaron un total de 241 mujeres embarazadas. De ellas, el 56,7% utilizaban sal yodada, el 46,7% consumían ≥2 raciones de productos lácteos al día y el 88,1% tomaban suplementos yodados. La mediana de UIC fue de 191μg/l (135,3-294μg/l), y el 68,1% de las mujeres tenían una UIC ≥150μg/l. Solo el consumo de sal yodada proporcionó protección contra la deficiencia de yodo (odds ratio: 0,35 [IC 95%: 0,20-0,63], p=0,001). En las mujeres sin enfermedad tiroidea autoinmune (n=88) la TSH media fue más baja en las que consumían sal yodada frente a las que no la consumían (respectivamente, 2,08±0,89mIU/l frente a 2,56±1,02mIU/l, p=0,025). En las mujeres con enfermedad tiroidea autoinmune (n=30) la TSH media fue mayor en las que tomaron suplementos de yodo frente a las que no lo hicieron (respectivamente, 2,97±1,25mIU/l frente a 1,16±0,41mIU/l, p=0,002).
ConclusionesLas gestantes del Área Sanitaria IV de Asturias mantienen un adecuado estado nutricional de yodo en el segundo trimestre de la gestación. En nuestra muestra solo el consumo de sal yodada se relacionó con una adecuada nutrición de yodo, sin afectar a la función tiroidea materna. La mayoría de las mujeres utilizaron suplementos de yodo, lo que se relacionó con niveles más altos de TSH en gestantes con enfermedad tiroidea autoinmune.
Iodine is an essential micronutrient for the formation of the thyroid hormones. This element is only acquired through the diet and iodine deficiency is associated with a group of adverse effects, known as iodine deficiency disorder (IDD), which include goitre, thyroid hormone changes and increased incidence of miscarriage, perinatal mortality and congenital abnormalities.1
Pregnant women constitute one of the most vulnerable groups due to their need for increased ingestion of iodine and because of the serious consequences that iodine deficiency may have on the foetus, principally disorders related to growth and neurodevelopment.2 Indeed, this is the biggest cause of preventable neurological damage in the world.
The most important measure for avoiding iodine deficiency in the general population is the universal addition of iodine to salt.3 It is estimated that 88% of the world population use iodised salt, resulting in the populations of 118 of 152 countries studied having adequate nutritional iodine status. However, many of these countries have studies in the pregnant population that show iodine deficiency and, in many others, there are no studies available.4 Since the use of iodised salt is not universal (used in over 90% of homes) and iodine sufficiency has not been achieved in the general population, the World Health Organization (WHO) and multiple scientific societies, such as the American Thyroid Association (ATA), the Endocrine Society and the European Thyroid Association, recommend the use of iodine supplements for pregnant women.
The use of such supplements has proved to be an effective measure in reducing the incidence of IDD, particularly in areas with a severe deficit of iodine. That said, their generalised use can lead to iodine excess, which has been linked to deterioration in thyroid function. Excessive ingestion of iodine temporarily inhibits the synthesis of thyroid hormones as a result of a mechanism known as the Wolff-Chaikoff effect.5 In healthy individuals, the thyroid ‘escapes’ from this effect and recovers normal function after a few days. However, in individuals with thyroid disorders, especially Hashimoto thyroiditis, there is a fault in this ‘escape’ system which may set in motion iodine-induced hypothyroidism.6 An increased tendency to maternal hypothyroidism and hypothyroxinaemia in pregnant women has been reported, even when ingestion of iodine is only slightly above the required levels.7 In addition, the foetal thyroid may also be affected by an excess of iodine since it is not mature enough to have a fully developed Wolff-Chaikoff effect escape mechanism until the 36th week of gestation.8
Asturias (in northern Spain) is a region without iodine deficiency in the general population since 2000, and adequate iodine nutritional status in pregnant women in Health Area IV of Asturias was demonstrated in 2014. Based on these results, a technical report from the Regional Health Department of Asturias was published in 2015 that recommended the prescription of individualised iodine supplementation in pregnant women, according to iodine intake. Thus, if pregnant women have a regular intake of iodised salt and consume three or more servings of dairy products per day, iodine supplementation is not indicated. Otherwise, the midwife indicates the supplementation from the first visit. Data from the 2017 update have been published, which indicates an optimal nutritional status of iodine in the pregnant population in the first trimester of gestation.9
The aim of this study was to find out whether pregnant women in Health Area IV maintain adequate nutritional iodine levels in their second trimester. The influence of iodine ingestion on UIC and maternal thyroid function, as well as the relationship between them, was also investigated. Finally, an additional objective of the study was to compare the results of ioduria and thyroid function in the first and second trimester of gestation.
Materials and methodsStudy populationA prospective, observational, descriptive and analytical study was conducted in pregnant women. Recruitment was carried out among all the pregnant women that attended their initial visit to a midwife between May and June 2017 at their health centre in Health Area IV, the central region of Asturias, with a population of over 330,000 inhabitants.
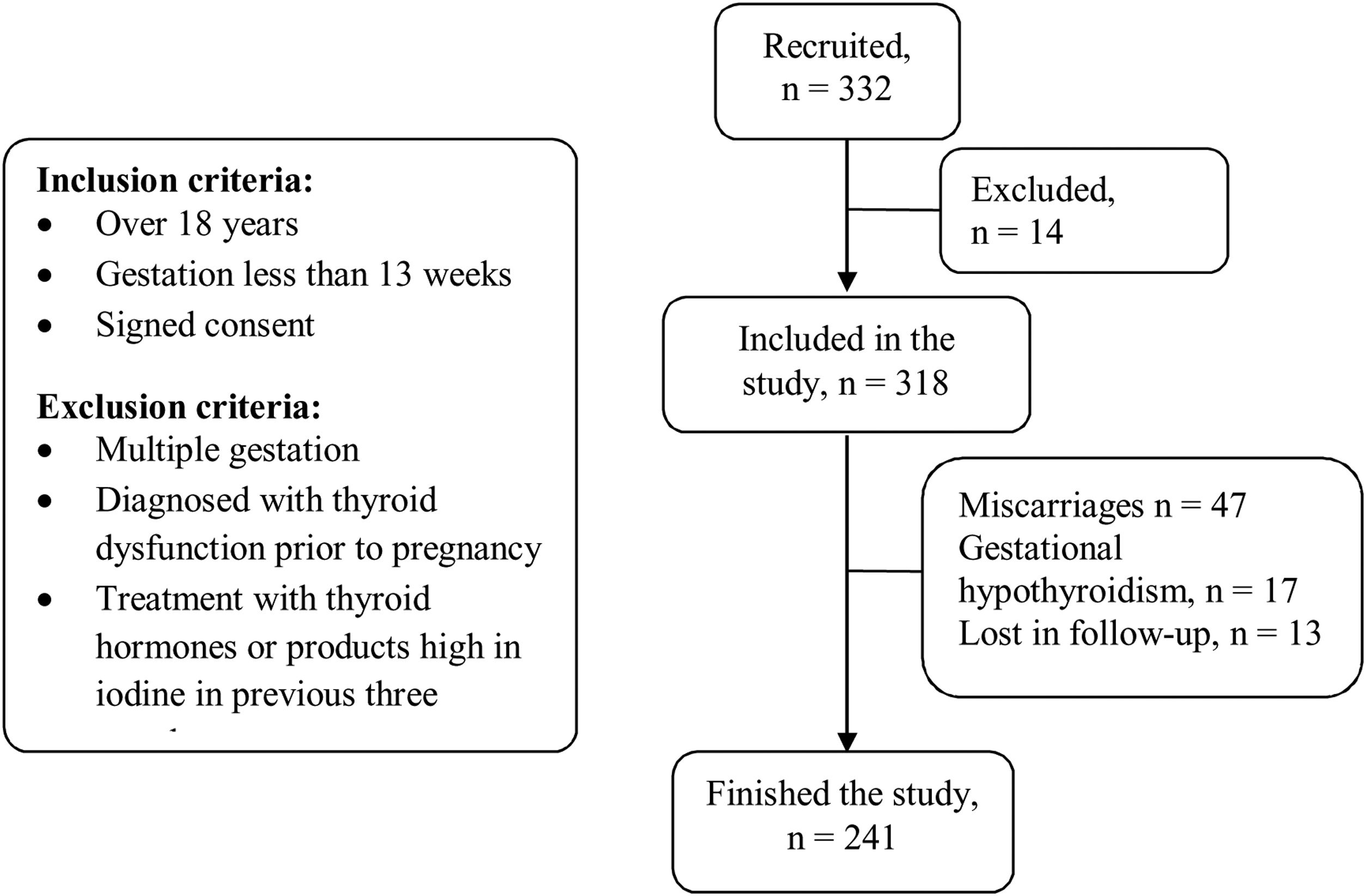
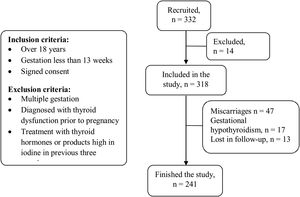
The inclusion criteria for the study were: pregnant women over 18 years old, gestation of less than 13 weeks and signed informed consent. The following exclusion criteria were applied: multiple gestation, diagnosis of thyroid dysfunction before the pregnancy, treatment within the last three months with thyroid hormones or any product containing high iodine levels. Pregnant women with levels of thyroid stimulating hormone (TSH) greater than 4.5mIU/l during the first trimester were diagnosed with gestational hypothyroidism, according to the normal values calculated for our health area, and were excluded from this study. A flow diagram is included in Fig. 1.
The study was approved by the Clinical Studies Ethics Committee of the Principality of Asturias. All participants signed informed consent forms.
Variables studiedOn the women's first visit to the midwife they completed a questionnaire to evaluate their ingestion of iodine which included questions related to their habitual consumption of iodised salt and dairy products (daily servings of milk, yoghurt and cheese), as well as whether they took iodine supplements.
UIC, TSH and free thyroxine (FT4) levels were measured in the first trimester and again in the second trimester. To determine UIC, a random urine sample was collected and submitted to inductively coupled plasma mass spectrometry (ICP-MS) using an ICP-MS 7700x system (from Agilent Technologies, Santa Clara, CA, USA). Good linearity was achieved between 10 and 450μg/l (R2>0.99), with an intra-laboratory imprecision of ≤2.9% and a total error of ≤7.3%.
At the same time, a blood sample was taken to determine levels of TSH, FT4 and thyroid autoimmunity (antiperoxidase antibodies [TPOAb] and anti-thyroglobulin antibodies [TgAb]). These variables were analysed with electrochemiluminescence (Roche Diagnostics, Basel, Switzerland). The coefficient of variation of TSH was 0.8–2.9% and that for FT4 was 1.8–3.2%. For the second trimester, the reference interval for TSH in Health Area IV was 0.80–5.80mIU/l and that for FT4 was 0.70–1.20ng/dl. The assessment of thyroid autoimmunity was performed only in the first trimester of gestation. The presence of positive TPOAb (≥34IU/ml) and/or TgAb (≥18IU/ml) without thyroid dysfunction was termed “autoimmune thyroid disease”.
Statistical analysisA descriptive analysis was performed to obtain relative and absolute frequencies for qualitative variables, and position and dispersion measurements for quantitative variables. The differences between numerical variables were examined using the Student's t-test or Wilcoxon test for independent samples, depending on whether or not the hypothesis of equality was verified. In the case of groups of three or more variables, either an ANOVA or a Kruskal–Wallis test was used, depending on whether the hypothesis of normality and/or homoscedasticity was met. The relationship between two qualitative variables was evaluated by Pearson's chi-squared test or Fisher's exact test, and a McNemar test was applied in cases of paired proportions. To assess which variables of iodine intake could be protective factors against iodine deficiency, a multivariate binary logistic regression model was developed to predict UIC levels below 150μg/l. The independent variables used were habitual consumption of iodised salt and dairy products (daily servings of milk, yoghurt and cheese), and use of iodine supplements.
The data were registered in ACCESS-SQL 2010 and the statistical analysis was conducted using the program R (R Development Core Team), version 3.6.0, applying a level of significance of 0.05.
ResultsOf the 332 pregnant women recruited to the study, 14 were rejected on the basis of one or more of the exclusion criteria. Loss of subjects during the study was the result of miscarriage (n=47), diagnosis of gestational hypothyroidism detected in the first trimester of pregnancy (n=17), or because the patient did not comply with the follow-up regimen (n=13). A total of 241 pregnant women finished the study (Fig. 1). The average age was 34.1±5.1 years and the average gestational age was 25.5±2.6 weeks.
A total of 56.7% of the women habitually used iodised salt. In terms of the consumption of dairy products, 46.7% of the women consumed two or more servings a day, with an average consumption of 1.82±0.95 servings a day. Finally, 88.1% of the pregnant women studied took iodine supplements.
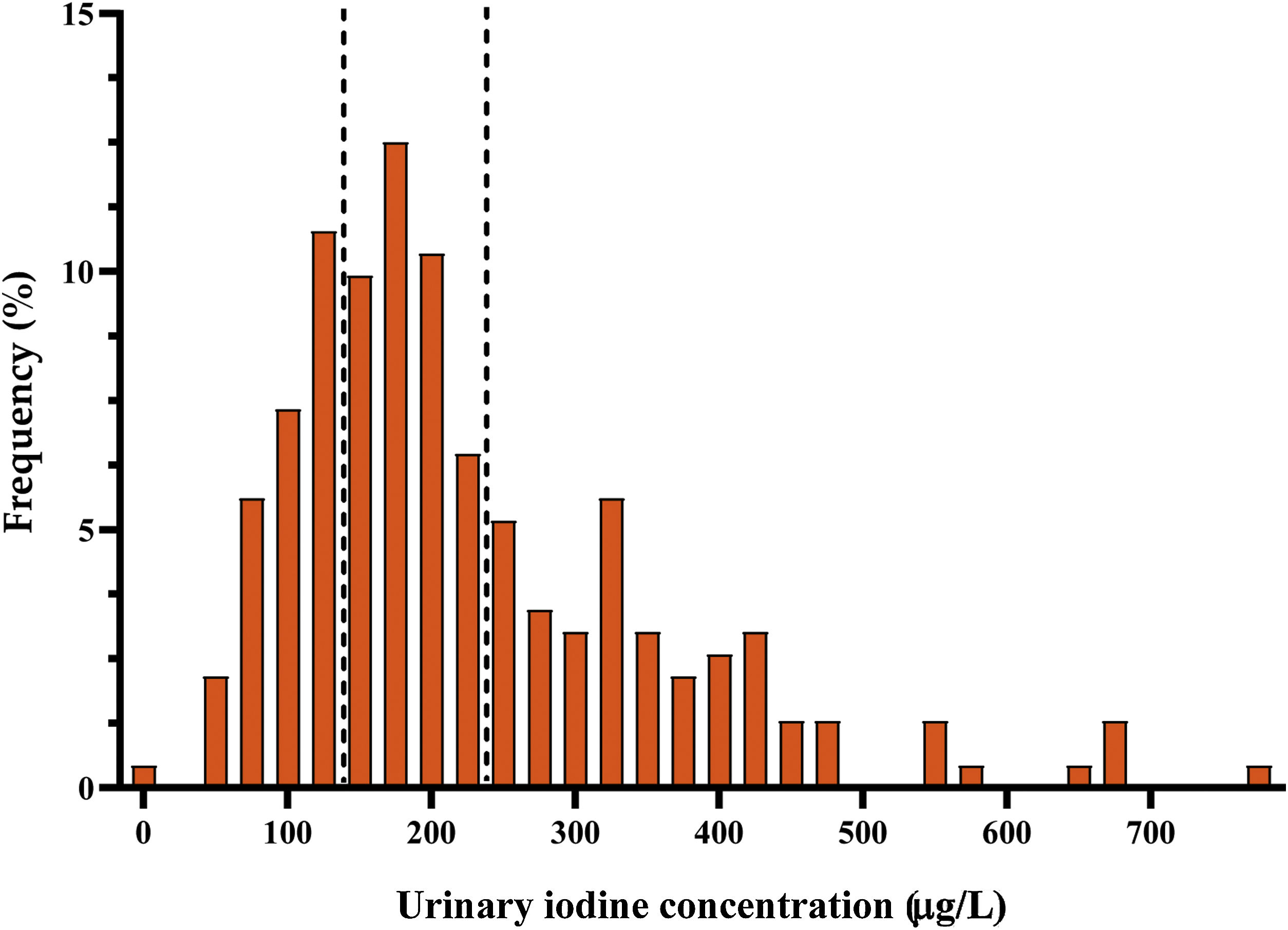
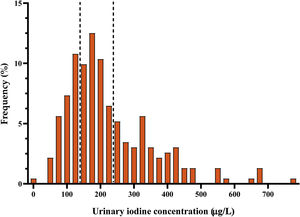
Urinary iodine concentrationMedian UIC in the second trimester of pregnancy was 191μg/l (interquartile range [IR]: 135.3–294μg/l). The distribution of ioduria is shown in Fig. 2. A comparison of these results with those obtained in the first trimester of gestation, showed a significant increase in UIC in the second trimester (p=0.02).
In 68.1% of cases, the women had a UIC of 150μg/l or above. In 31.9%, deficient ioduria was recorded (67.6% with mild deficiency), while levels were adequate in 36.6%. In a further 27.6%, ioduria was evaluated as ‘more than adequate’, and in 3.9% as ‘excessive’.
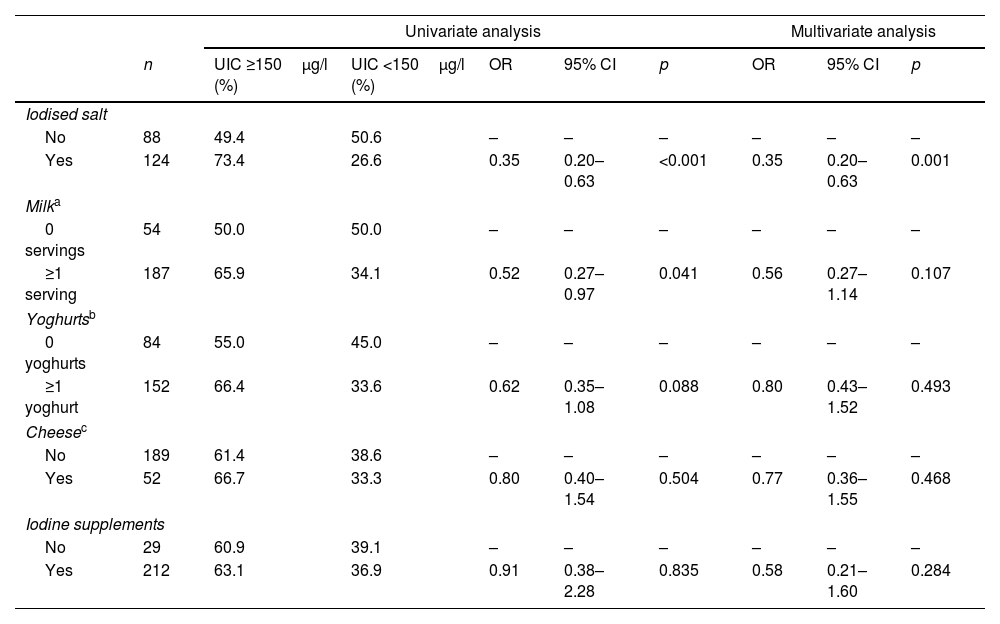
The relationship between ingestion of iodine and UIC was examined through both univariate and multivariate analysis, summarised in Table 1. In the univariate analysis, the consumption of iodised salt was associated with UIC ≥150μg/l (p<0.001). The consumption of at least one glass of milk a day was associated with UIC ≥150μg/l (p=0.041), although other dairy products and iodine supplements were not. In the multivariate binary logistic regression analysis, UIC <150μg/l was used as a dependent variable. The items on the iodine consumption questionnaire were considered as independent variables. In this analysis, the consumption of iodised salt was linked to a protection against iodine insufficiency (odds ratio [OR] 0.35 [0.20–0.63], p=0.001). This protective effect was not seen for either the consumption of milk and dairy products or the use of iodine supplements.
Relationship between UIC and nutritional intake of iodine. Univariate analysis and multivariate binary logistic regression were carried out using UIC <150μg/l as a dependent variable.
| Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | UIC ≥150μg/l (%) | UIC <150μg/l (%) | OR | 95% CI | p | OR | 95% CI | p | |
| Iodised salt | |||||||||
| No | 88 | 49.4 | 50.6 | – | – | – | – | – | – |
| Yes | 124 | 73.4 | 26.6 | 0.35 | 0.20–0.63 | <0.001 | 0.35 | 0.20–0.63 | 0.001 |
| Milka | |||||||||
| 0 servings | 54 | 50.0 | 50.0 | – | – | – | – | – | – |
| ≥1 serving | 187 | 65.9 | 34.1 | 0.52 | 0.27–0.97 | 0.041 | 0.56 | 0.27–1.14 | 0.107 |
| Yoghurtsb | |||||||||
| 0 yoghurts | 84 | 55.0 | 45.0 | – | – | – | – | – | – |
| ≥1 yoghurt | 152 | 66.4 | 33.6 | 0.62 | 0.35–1.08 | 0.088 | 0.80 | 0.43–1.52 | 0.493 |
| Cheesec | |||||||||
| No | 189 | 61.4 | 38.6 | – | – | – | – | – | – |
| Yes | 52 | 66.7 | 33.3 | 0.80 | 0.40–1.54 | 0.504 | 0.77 | 0.36–1.55 | 0.468 |
| Iodine supplements | |||||||||
| No | 29 | 60.9 | 39.1 | – | – | – | – | – | – |
| Yes | 212 | 63.1 | 36.9 | 0.91 | 0.38–2.28 | 0.835 | 0.58 | 0.21–1.60 | 0.284 |
UIC: urinary iodine concentration; OR: odds ratio; CI: confidence interval.
Data relating to thyroid function and thyroid autoimmunity was collected from 118 pregnant women.
There were 88 women with no autoimmune thyroid disease, their average TSH being 2.29±0.96mIU/l and average FT4 0.97±0.12ng/dl. A comparison of these results with those obtained in the first trimester, showed no difference in levels of TSH (p=0.66) and a decrease in levels of FT4 in the second trimester (p<0.01). For 64.8% of these women, TSH levels were <2.5mIU/l. In the group of 30 women with autoimmune thyroid disease, average TSH was 2.76±1.30mIU/l, and average FT4 was 0.99±0.17ng/dl (no statistically significant difference between the first and second trimesters). Of these women, 40.0% had TSH levels <2.5mIU/l.
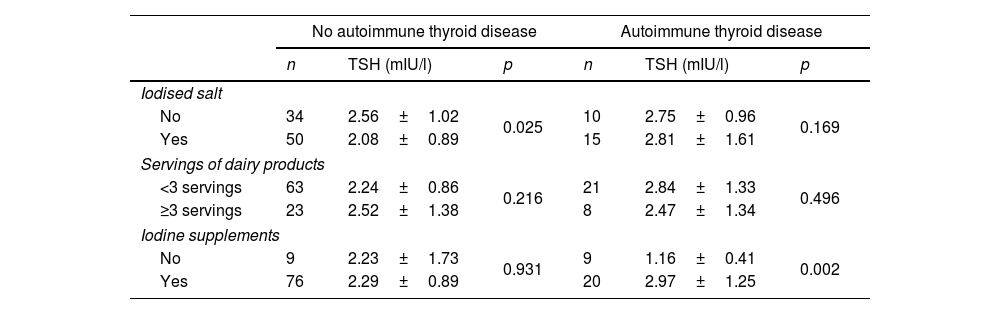
The relationship between maternal thyroid function and nutritional iodine intake was analysed with no statistically significant differences. In a second approach, the analysis was carried out separately for those women with and without autoimmune thyroid disease (Table 2). Those pregnant women without autoimmune thyroid disease that consumed iodised salt had levels of TSH below those that did not (2.08±0.89mIU/l vs. 2.56±1.02mIU/l, p=0.025), and there was no difference depending on servings of dairy products or the use of iodine supplements. With respect to women with autoimmune thyroid disease, there was no difference in terms of consumption of iodised salt or dairy products, but those who were taking iodine supplements had higher levels of TSH than those who did not (2.97±1.25mIU/l vs. 1.16±0.41mIU/l, p=0.002). Of the women with autoimmune thyroid disease taking iodine supplements, 77.8% had levels of TSH ≥2.5mIU/l. No association was observed between FT4 and iodine intake.
Results of thyroid function tests by autoimmune thyroid disease status and according to nutritional intake of iodine.
| No autoimmune thyroid disease | Autoimmune thyroid disease | |||||
|---|---|---|---|---|---|---|
| n | TSH (mIU/l) | p | n | TSH (mIU/l) | p | |
| Iodised salt | ||||||
| No | 34 | 2.56±1.02 | 0.025 | 10 | 2.75±0.96 | 0.169 |
| Yes | 50 | 2.08±0.89 | 15 | 2.81±1.61 | ||
| Servings of dairy products | ||||||
| <3 servings | 63 | 2.24±0.86 | 0.216 | 21 | 2.84±1.33 | 0.496 |
| ≥3 servings | 23 | 2.52±1.38 | 8 | 2.47±1.34 | ||
| Iodine supplements | ||||||
| No | 9 | 2.23±1.73 | 0.931 | 9 | 1.16±0.41 | 0.002 |
| Yes | 76 | 2.29±0.89 | 20 | 2.97±1.25 | ||
TSH: thyroid stimulating hormone.
Finally, the relationship between thyroid function and UIC was examined after dividing the results according to thyroid autoimmunity status, with no statistical differences found.
DiscussionThe pregnant women in our health area were found to have adequate nutritional iodine status in the second trimester of gestation, even with a mild increase in ioduria compared to the first trimester results, with a median UIC of 171.5μg/l.9 In studies of populations not taking iodine supplements, a decrease in ioduria is observed as the pregnancy progresses, probably related to the increasing iodine requirements of the foetus over time, but also to increased maternal renal clearance, the de-iodinising action of the placenta and increased maternal thyroxine synthesis. In a cross-sectional study conducted in China among 2378 iodine-sufficient pregnant women who were not given iodine supplements,10 analysis of their UIC showed that, although their iodine sufficiency was maintained in each trimester, there was a progressive decrease in ioduria. This reduction in UIC has been described in both iodine-deficient11 and iodine-sufficient12 populations of pregnant women. Meanwhile, in a randomised controlled trial conducted in Sweden among 200 pregnant women assigned (1:1) to daily intake of a multivitamin tablet with or without 150μg of iodine,13 ioduria and estimated urinary iodine excretion (eUIE) were insufficient in both groups at the start of the trial. Ioduria increased in the intervention group in the second trimester, without reaching iodine sufficiency, and was maintained in the third trimester, while a progressive decrease in ioduria was observed in the control group. However, when analysing eUIE, the intervention group reached iodine sufficiency in the second and third trimester, while in the control group eUIE decreased. In our study, 87.1% of the pregnant women were taking iodine supplements,9 which could explain why the UIC levels increased in the second trimester of pregnancy. Furthermore, an increased consumption of iodised salt and dairy products may have occurred during pregnancy, with a resultant increase in UIC levels.
The univariate analysis of the relationship between the UIC and ingestion of iodine demonstrated that the consumption of iodised salt and milk was associated with UIC ≥150μg/l. However, in the multivariate analysis of the data, only the use of iodised salt provided a protective effect against iodine deficiency in our sample of pregnant women. These results are similar to those obtained in the first trimester,9 in line with previously published works,14,15 which demonstrate that consumption of iodised salt is sufficient to achieve adequate iodine intake in the pregnant population, despite the fact that its consumption in Spain is still low. According to the TIROGEST study, consumption of iodised salt in Spanish pregnant women did not reach 50% of households.16 On the other hand, our results highlight that the use of iodine supplements, especially if not used in the preconception period, may not be enough to guarantee an adequate nutritional iodine status, as has been shown in other studies.17
In our cohort of pregnant women, TSH remained stable in both the first and second trimester of gestation, with a decrease in FT4 levels in the second trimester only in pregnant women with no autoimmune thyroid disease. The results in the literature are mixed. Corcino et al.18 described an increase in TSH and a decrease in FT4 in the third trimester, which they related to the decrease in ioduria despite the participants in their study being iodine sufficient. These same results were also observed in a study in India, although in an iodine deficient population.11 However, in the Swedish study,12 there were no significant differences in thyroid function, both TSH and FT4, between trimesters, and not even between the treated and the control groups. Censi et al.19 found that women receiving iodine supplementation had a decrease in TSH in the third trimester of pregnancy compared to the control group, even though their ioduria decreased to iodine deficiency in the third trimester, but no difference in FT4 was found between the iodine supplemented group and the control group. Recent systematic reviews on the use of iodine supplementation in pregnant women found inconsistent effects on thyroid function.20,21
In our study, the analysis of thyroid function with respect to ingestion of iodine in women without autoimmune thyroid disease revealed that pregnant women who consume iodised salt have levels of TSH below those of pregnant women who do not, an effect not observed in relation to iodine supplements. This can perhaps be explained by the fact that the majority of pregnant women in Spain who consume iodised salt do so for more than a year before getting pregnant.16 Pre-conception consumption of iodised salt guarantees that intrathyroidal iodine stores are full before pregnancy, thus ensuring better adaptation to the increase in iodine requirements during gestation.20 In contrast, when there is a tendency for women to start taking iodine supplements following conception,9 such women have higher levels of TSH than those who begin to consume iodised salt in the pre-conception period.22
On the other hand, the analysis of the thyroid function of pregnant women with autoimmune thyroid disease who take iodine supplements showed that they have higher levels of TSH in the second trimester of gestation than those who do not take supplements, results that were also observed in the first trimester.23 This may, once again, be related to the post-conception use of an iodine supplement and its greater risk of failing to ‘escape’ the Wolff-Chaikoff effect, especially in patients who are more susceptible, such as those with autoimmune thyroid disease.6
To achieve adequate iodine nutrition in the pregnant women population, the WHO recommends daily ingestion of 250μg of iodine per day24 and the US Institute of Medicine (IOM) recommends 220μg.25 That said, no safe maximum limit for the consumption of iodine has been established for pregnant women; the WHO recommends not taking more than 500μg of iodine a day, while the IOM raises the upper limit to 1100μg. A Chinese observational study of 7190 women in the first trimester of pregnancy,26 published in 2015, found the lowest levels of TSH to be associated with UIC of between 150 and 250μg/l, while the highest were found in the group with UIC of over 250μg/l, suggesting that “more than adequate” ioduria may have repercussions on thyroid function. A review by Farebrother et al.27 concluded that the incidence of thyroid dysfunction with respect to iodine ingestion followed a U-shaped pattern. It is necessary to know what the maximum limit for iodine ingestion in the general population is because an excess ingestion has not only been related to thyroid dysfunction, but also to increased blood pressure, greater risk of metabolic syndromes and alterations in lipid and glucose metabolism,28 Furthermore, UIC ≥400μg/l was found to be associated with an increase in mortality in the general population.29
On the basis of our results and previous studies,26 we suggest that the excess of iodine associated with iodine supplementation in pregnant women with autoimmune thyroid disease may be associated with an increase in maternal TSH, thereby increasing the risk of gestational hypothyroidism. Indeed, 77.8% of the pregnant women in our study who had autoimmune thyroid disease and were taking iodine supplements had levels of TSH >2.5mIU/l, so treatment with levothyroxine could be considered, according to the ATA recommendations.30
The principal limitation of our study is its sample size, which allowed us to examine our principal objectives but did not enable us to achieve statistically significant results with respect to the relationship between UIC and thyroid function. Another limitation of our study is that the iodine intake questionnaire was completed during the first visit to the midwife but was not repeated in conjunction with the second UIC and thyroid function follow-up. In addition, a high proportion of the pregnant women in our study were taking iodine supplements, which makes it difficult to draw solid conclusions about the influence of the ingestion of iodine on UIC and thyroid function, although this is partly corrected by the multivariate binary logistic regression analysis carried out. Finally, our research is an observational study, so there may be confounding factors that could compromise the obtained results.
ConclusionsThe pregnant women studied from Health Area IV in Asturias maintain adequate nutritional iodine status in the second trimester of gestation, with increasing ioduria and stable TSH with decreasing levels of FT4. In our sample of pregnant women, only the consumption of iodised salt was associated with adequate iodine nutrition, without affecting maternal thyroid function. Most women used iodine supplements, linked to higher levels of TSH in pregnant women with autoimmune thyroid disease.
FundingThis research received no external funding.
Authors’ contributionsSilvia González-Martínez conceptualisation, data curation, formal analysis, investigation, visualisation and writing of original draft, and its review and editing. Eduardo Martínez-Morillo data curation, resources, supervision and writing, review and editing. Noelia Avello-Llano data curation, resources, supervision and writing, review and editing. Ana Isabel Escudero-Gomis conceptualisation, methodology, supervision and writing, review and editing. Elías Delgado-Álvarez conceptualisation, methodology, supervision and writing, review and editing. Edelmiro Luis Menéndez-Torre conceptualisation, data curation, methodology, project administration, supervision and writing, review and editing.
Conflicts of interestThe authors declare no conflicts of interest.
We wish to thank the midwives who collaborated in the data collection and the pregnant women for participating as volunteers.