The worldwide prevalence of type 2 diabetes mellitus increases in parallel to that of obesity. Liraglutide (LRG), a glucagon-like peptide-1 receptor agonist, can reduce body weight. This study assessed the metabolic efficacy of LRG in real-world clinical practice.
MethodsAn observational, retrospective cohort study including patients treated with LRG for at least one year (187 patients). Anthropometric and metabolic variables, a composite endpoint, factors predicting response to LRG, and cardiovascular risk over time were assessed. A linear mixed-effects model with a bivariate structure was constructed to investigate the time-dependent relationship between weight and HbA1c values.
ResultsHbA1c levels and weight significantly decreased in the first 12 weeks, and the decrease persisted at 12 and 24 months in all subgroups studied. Mean weight and HbA1c decreases after 24 months were 8.5kg and 1.7% respectively. HbA1c values <7% were achieved by 42% of patients at 12 months and by 40% at 24 months. Treatment with LRG allowed for reduction in insulin dose. No serious adverse events were noted. Cardiovascular risk decreased from high to moderate-low.
ConclusionsUnder standard clinical practice conditions, LRG achieved a better metabolic response than seen in clinical trials. Efficacy at 12 weeks of treatment is a good predictor of response. LRG allows for delaying or reducing insulin dose by improving both weight and glucose control. Cardiovascular risk improved.
La prevalencia mundial de diabetes mellitus tipo 2 aumenta junto a la de la obesidad. Liraglutida (LRG), un agonista del receptor del péptido similar al GLP1, es un fármaco antidiabético capaz de reducir peso. Evaluamos en práctica clínica de vida real su eficacia metabólica.
MétodoEstudio de cohorte observacional retrospectivo. Se incluyeron los pacientes tratados al menos durante un año con LRG (187 pacientes). Evaluamos variables antropométricas, metabólicas, objetivos combinados, factores predictivos de respuesta y evolución del riesgo cardiovascular. Se construyó un modelo de efectos mixtos lineales de estructura bivariante para investigar la relación tiempo-dependiente entre el peso y los valores de HbA1c.
ResultadosDescenso significativo de los valores de HbA1c y peso en las primeras 12 semanas de tratamiento, mantenido a los 12 y 24 meses, en todos los subgrupos estudiados. Reducción media de peso y HbA1c tras 24 meses de tratamiento de 8,5 kg y 1,7%. El valor de HbA1c fue <7% en 42% de pacientes a los 12 meses, 40% a los 24 meses. El tratamiento con LRG permitió reducir la dosis de insulina. No registramos eventos adversos graves. El riesgo cardiovascular mejoró.
ConclusionesBajo condiciones de práctica clínica habitual la respuesta metabólica a LRG resultó mejor que la observada en ensayos clínicos. La eficacia a las 12 semanas de tratamiento es un buen predictor de respuesta. LRG permite retrasar o reducir la insulinoterapia. Los pacientes mejoraron su riesgo cardiovascular.
Type 2 diabetes mellitus (T2DM) affects people throughout the world. Its prevalence has been increasing dramatically as a result of aging populations and the rising prevalence of obesity.1,2
Insulin resistance is an underlying cause of T2DM and is associated with obesity. Consequently, weight reduction is a key goal of treatment. Whenever possible, medications should be chosen to promote weight loss.
Weight gain can offset the beneficial effects of good glycemic control and discourage patients from adhering to treatment. Even moderate weight loss has been shown to improve glycemic control.3,4 Guidelines3,5,6 recommend a patient-centered approach.
The safety and efficacy profile of the GLP1-RA liraglutide (LRG) has been evaluated in clinical trials,7,8 which have shown that, in addition to controlling glycemia, LRG can reduce body weight. A meta-analysis9 evaluating the results of head-to-head trials showed LRG to be one of the most effective drugs for control of glycemia and obesity.
Conditions affecting people with T2DM (hypertension, dyslipidemia, obesity, physical inactivity) increase the risk of heart disease. LRG10 and empagliflozin11 reduce cardiovascular and all-cause mortality when added to standard care in clinical trials. Ongoing studies are investigating the cardiovascular benefits of other agents in these drug classes. Real-world and post-marketing studies are being promoted to provide data that will help clinicians to choose appropriate options and corroborate safety.
The primary objective of the present study was to evaluate the metabolic effectiveness and safety of LRG in T2DM patients in real-world clinical practice over a longer period than during clinical trials. The secondary objectives were to evaluate potential clinical predictors of response, effectiveness of LRG in various patient subgroups, and changes in cardiovascular risk. A special analysis of patients receiving insulin treatment was performed.
MethodsStudy designWe performed a 1-year observational, retrospective cohort study (October 2015 to October 2016). We identified patients treated with LRG according to the indications of usual clinical practice.12
The study was approved by the Ethics Committee of the Galician Health Service (SERGAS) and conducted according to the requirements of the Declaration of Helsinki and the principles of Good Clinical Practice.
PatientsThe study population comprised outpatients followed under conditions of routine clinical practice. The inclusion criteria were T2DM, age ≥18 years, and body mass index (BMI) ≥30kg/m2.
Study variablesThe variables we recorded at baseline were sex, age, diabetes duration, cardiovascular risk factors (hypertension, dyslipidemia, smoking, general vascular disease, sleep apnea syndrome), and previous treatment (metformin, combined oral antidiabetic drugs, insulin).
The parameters evaluated during follow-up (at 3, 6, 12, 18, 24, 30, and 36 months) were body weight, BMI, percentage of excess BMI lost (% EBMIL), HbA1c, fasting blood glucose, systolic and diastolic blood pressure, and lipid profile.
We also evaluated the proportion of patients achieving an HbA1C target of <7% and a composite endpoint (CEP), which was defined as follows:
- •
CEP I: HbA1c reduction ≥1 and weight loss ≥5%
- •
CEP II: HbA1c reduction ≥1 and weight loss ≥3%
- •
CEP III: HbA1c reduction ≥0.4 and weight loss ≥5%
- •
CEP IV: HbA1c reduction ≥0.4 and weight loss ≥3%
- •
CEP V: HbA1c reduction ≥0.4 and no weight increase or weight loss >3% with no increase in HbA1c
We evaluated baseline characteristics that could be predictive of response to LRG. We analyzed response (HbA1C, weight) by subgroups: age, sex, diabetes duration, treatment at baseline. Patients who were receiving insulin were evaluated to study the effect of adding LRG.
We evaluated the risk of cardiovascular disease using Regicor Calculator, which is adapted to the Spanish population.13
Statistical analysisA linear mixed-effects model14 was used to study the overall reduction in weight and HbA1c during treatment. The model enables us to study the effect of time and duration of diabetes flexibly using a quadratic function to determine whether the responses are linear or not over time.
The linear mixed effects model is constructed with a bivariate structure to investigate the time-dependent relationship between weight and HbA1c values. We used the bivariate longitudinal analysis introduced by Thiébaut et al.,15 which enables testing of the difference between the longitudinal outcomes and joint testing of a treatment effect on a set of outcomes.
The statistical analysis was performed using R, Version 2.12.0 (R Development Core Team, Vienna, Austria) and SAS, Version 9.2 (SAS Institute Inc., Cary, North Carolina, USA). Statistical significance was set at p<0.05.
ResultsBaseline sampleThe study population comprised 209 patients. Twenty-two were withdrawn. Metabolic response was evaluated in the remaining 187 cases. Of these, 171 patients completed 12 months on treatment (Group A), 85 completed 24 months (Group B), and 20 completed 36 months (Group C).
The reasons for withdrawal were LRG-related adverse events (13 cases), death (5 cases), acute complications (2 cases), and lost cases (2 cases).
LRG was discontinued in 34 cases (18%) owing to lack of efficacy, which the physician considered as inadequate glycemic and/or weight response according to his individualized objectives. Most of them discontinued treatment between 3 and 6 months from the start. These patients were considered for the effectiveness evaluation.
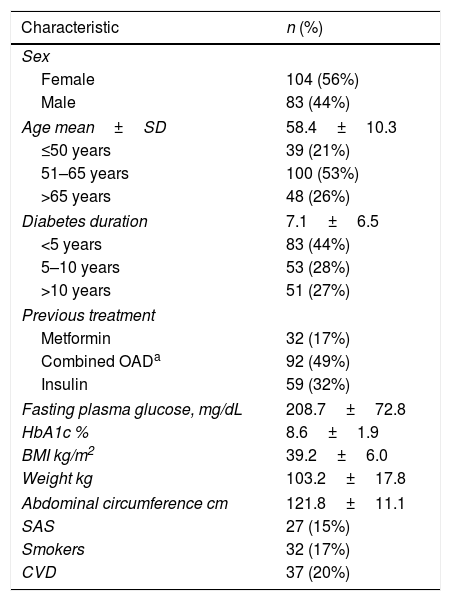
We evaluated the descriptive baseline characteristics of the basal 187 patients (Table 1).
Baseline characteristics (n=187).
| Characteristic | n (%) |
|---|---|
| Sex | |
| Female | 104 (56%) |
| Male | 83 (44%) |
| Age mean±SD | 58.4±10.3 |
| ≤50 years | 39 (21%) |
| 51–65 years | 100 (53%) |
| >65 years | 48 (26%) |
| Diabetes duration | 7.1±6.5 |
| <5 years | 83 (44%) |
| 5–10 years | 53 (28%) |
| >10 years | 51 (27%) |
| Previous treatment | |
| Metformin | 32 (17%) |
| Combined OADa | 92 (49%) |
| Insulin | 59 (32%) |
| Fasting plasma glucose, mg/dL | 208.7±72.8 |
| HbA1c % | 8.6±1.9 |
| BMI kg/m2 | 39.2±6.0 |
| Weight kg | 103.2±17.8 |
| Abdominal circumference cm | 121.8±11.1 |
| SAS | 27 (15%) |
| Smokers | 32 (17%) |
| CVD | 37 (20%) |
CVD: cardiovascular disease; HbA1c: glycated hemoglobin A1c; ND: no data; SAS: sleep apnea syndrome; SD: standard deviation.
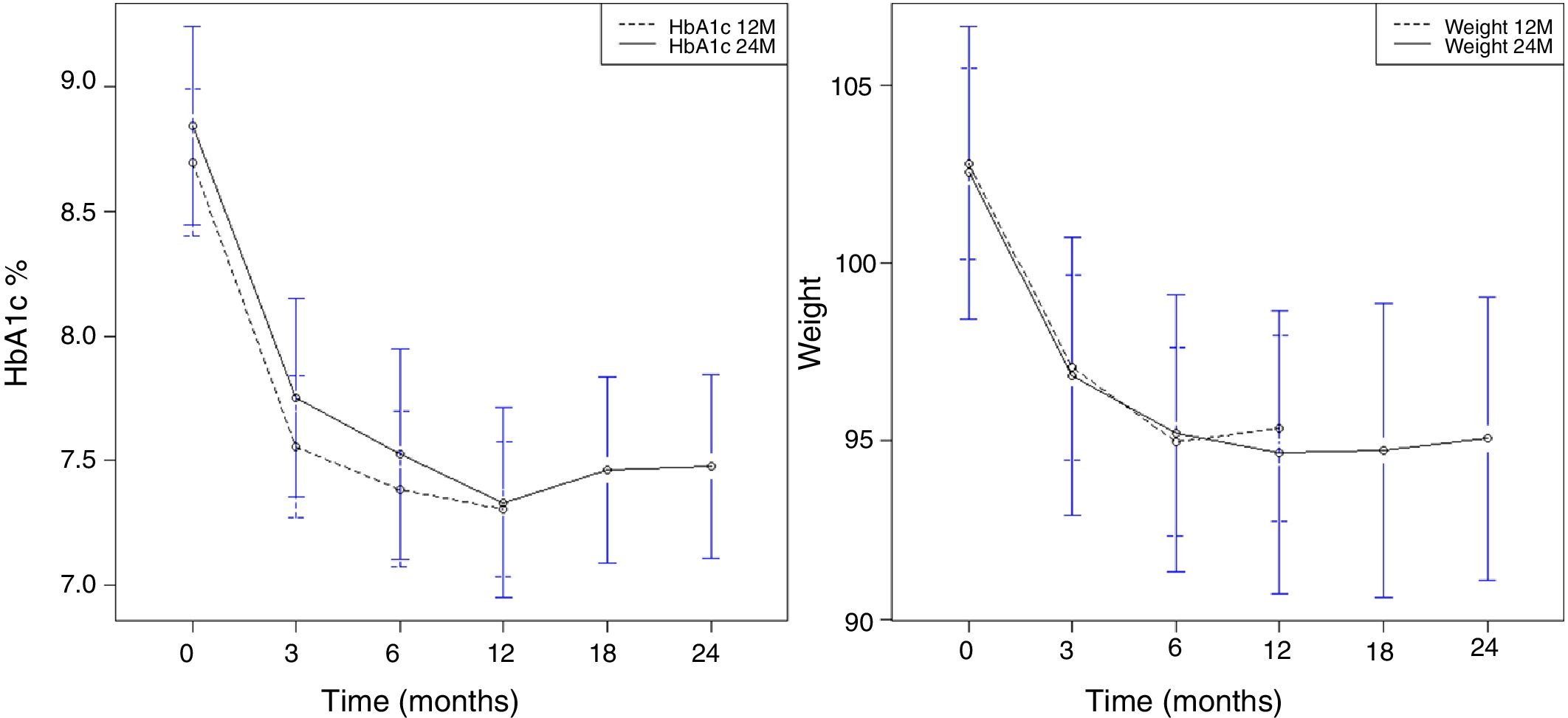
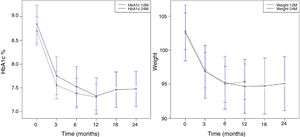
Overall, the mean reduction in weight was 8.6kg at 12 months, 8.5kg at 24 months, and 9kg at 36 months. The mean reduction in HbA1c was 1.7% at 12 months, 24 months, and 36 months. A significant reduction in HbA1c (Fig. 1A) and weight (Fig. 1B) was observed as early as week 12, and this was maintained at 12 and 24 months.
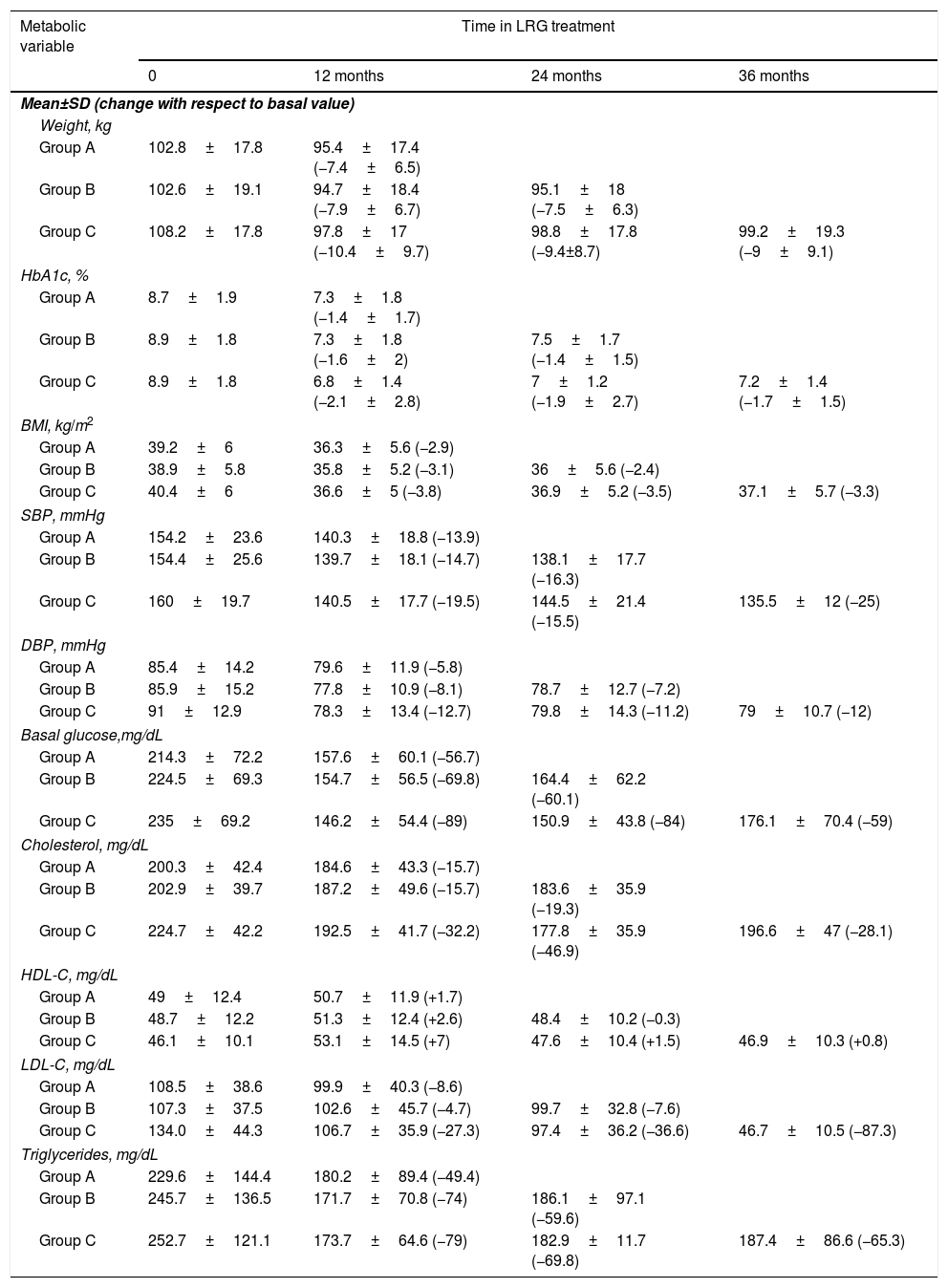
Table 2 shows the progress of metabolic variables for patients who had received treatment for 12 months, 24 months, and 36 months. We found a marked and stable improvement over time in most of the metabolic parameters analyzed.
Evolution of metabolic variables according to the group studied: Group A (n 171) patients treated for 12 months, Group B (n 85) patients treated for 24 months and Group C (n 20) patients treated for 36 months.
| Metabolic variable | Time in LRG treatment | |||
|---|---|---|---|---|
| 0 | 12 months | 24 months | 36 months | |
| Mean±SD (change with respect to basal value) | ||||
| Weight, kg | ||||
| Group A | 102.8±17.8 | 95.4±17.4 (−7.4±6.5) | ||
| Group B | 102.6±19.1 | 94.7±18.4 (−7.9±6.7) | 95.1±18 (−7.5±6.3) | |
| Group C | 108.2±17.8 | 97.8±17 (−10.4±9.7) | 98.8±17.8 (−9.4±8.7) | 99.2±19.3 (−9±9.1) |
| HbA1c, % | ||||
| Group A | 8.7±1.9 | 7.3±1.8 (−1.4±1.7) | ||
| Group B | 8.9±1.8 | 7.3±1.8 (−1.6±2) | 7.5±1.7 (−1.4±1.5) | |
| Group C | 8.9±1.8 | 6.8±1.4 (−2.1±2.8) | 7±1.2 (−1.9±2.7) | 7.2±1.4 (−1.7±1.5) |
| BMI, kg/m2 | ||||
| Group A | 39.2±6 | 36.3±5.6 (−2.9) | ||
| Group B | 38.9±5.8 | 35.8±5.2 (−3.1) | 36±5.6 (−2.4) | |
| Group C | 40.4±6 | 36.6±5 (−3.8) | 36.9±5.2 (−3.5) | 37.1±5.7 (−3.3) |
| SBP, mmHg | ||||
| Group A | 154.2±23.6 | 140.3±18.8 (−13.9) | ||
| Group B | 154.4±25.6 | 139.7±18.1 (−14.7) | 138.1±17.7 (−16.3) | |
| Group C | 160±19.7 | 140.5±17.7 (−19.5) | 144.5±21.4 (−15.5) | 135.5±12 (−25) |
| DBP, mmHg | ||||
| Group A | 85.4±14.2 | 79.6±11.9 (−5.8) | ||
| Group B | 85.9±15.2 | 77.8±10.9 (−8.1) | 78.7±12.7 (−7.2) | |
| Group C | 91±12.9 | 78.3±13.4 (−12.7) | 79.8±14.3 (−11.2) | 79±10.7 (−12) |
| Basal glucose,mg/dL | ||||
| Group A | 214.3±72.2 | 157.6±60.1 (−56.7) | ||
| Group B | 224.5±69.3 | 154.7±56.5 (−69.8) | 164.4±62.2 (−60.1) | |
| Group C | 235±69.2 | 146.2±54.4 (−89) | 150.9±43.8 (−84) | 176.1±70.4 (−59) |
| Cholesterol, mg/dL | ||||
| Group A | 200.3±42.4 | 184.6±43.3 (−15.7) | ||
| Group B | 202.9±39.7 | 187.2±49.6 (−15.7) | 183.6±35.9 (−19.3) | |
| Group C | 224.7±42.2 | 192.5±41.7 (−32.2) | 177.8±35.9 (−46.9) | 196.6±47 (−28.1) |
| HDL-C, mg/dL | ||||
| Group A | 49±12.4 | 50.7±11.9 (+1.7) | ||
| Group B | 48.7±12.2 | 51.3±12.4 (+2.6) | 48.4±10.2 (−0.3) | |
| Group C | 46.1±10.1 | 53.1±14.5 (+7) | 47.6±10.4 (+1.5) | 46.9±10.3 (+0.8) |
| LDL-C, mg/dL | ||||
| Group A | 108.5±38.6 | 99.9±40.3 (−8.6) | ||
| Group B | 107.3±37.5 | 102.6±45.7 (−4.7) | 99.7±32.8 (−7.6) | |
| Group C | 134.0±44.3 | 106.7±35.9 (−27.3) | 97.4±36.2 (−36.6) | 46.7±10.5 (−87.3) |
| Triglycerides, mg/dL | ||||
| Group A | 229.6±144.4 | 180.2±89.4 (−49.4) | ||
| Group B | 245.7±136.5 | 171.7±70.8 (−74) | 186.1±97.1 (−59.6) | |
| Group C | 252.7±121.1 | 173.7±64.6 (−79) | 182.9±11.7 (−69.8) | 187.4±86.6 (−65.3) |
BMI: body mass index; DBP: diastolic blood pressure; HbA1c: glycated hemoglobin A1c; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; SBP: systolic blood pressure.
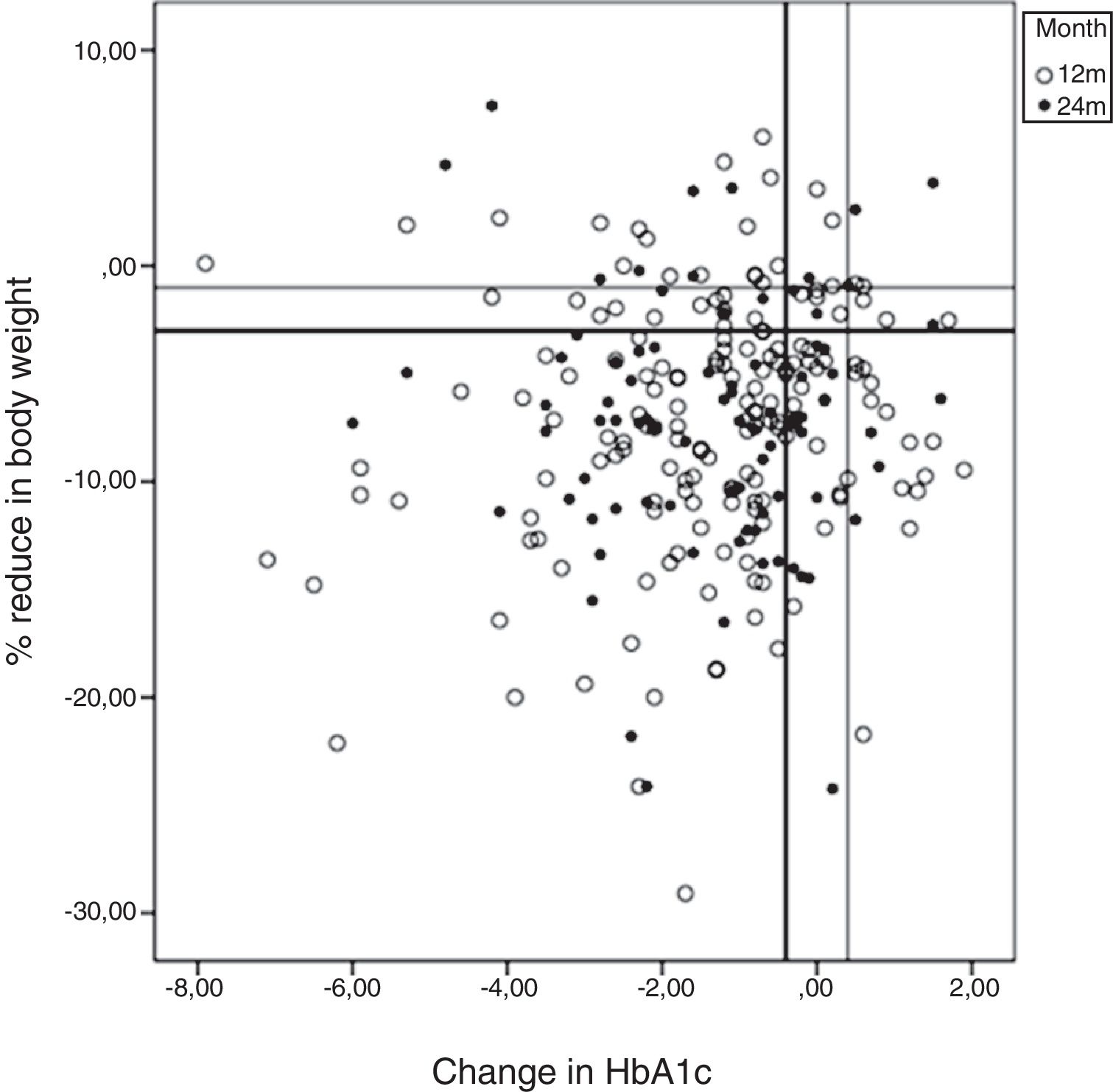
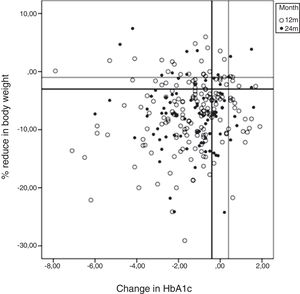
HbA1c <7% was achieved by 42% of patients at 12 months and by 40% at 24 months. The results at 12–24 months for each of the CEP defined (see Material and Methods, Study variables) were as follows: CEP I, 34%–36%; CEP II, 39%–44%; CEP III, 49%–48%; CEP IV, 59%–57%; and CEP V, 74%–79%. There were no differences between sexes. The results for HbA1c compared with changes in body weight from baseline to 12 and 24 months are shown in Fig. 2 (scatterplot). Most patients achieved a reduction in both parameters.
Correlation between metabolic response and study variablesWe evaluated HbA1c over time (at 12/24 months) according to age, diabetes duration, baseline antidiabetic medication and sex. With respect to age, the variation was as follows: <50 years, −2.3%/−1.68%; 51–65 years, −1.3%/−1.2%; >65 years, −1.3%/−1.5%. According to duration of diabetes: <5 years, −1.7%/−1.5%; 5–10 years, −1.5%/−1.2%; >10 years, −1.2%/−1.1% and baseline antidiabetic medication: metformin, −1.7%/−1.5%; oral agents, −1.6%/−1.2%; insulin, −1.4%/−1.5%. According to sex: female, −1.5%/−1.4%; male −1.56%/−1.3%.
The decrease in HbA1c was significant in all the subgroups, although there were no significant differences between the subgroups.
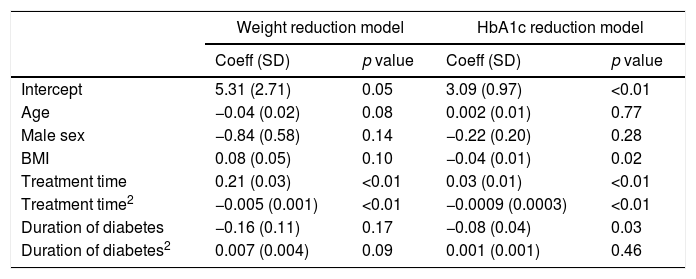
Statistical correlation studies results are shown at Table 3. The results of the bivariate linear mixed effects model (weight and HbA1c) are shown in Table 3A. No correlations were found between reduction in weight and HbA1c with respect to age or sex. According to the baseline BMI value, the reduction in HbA1c was inversely correlated with BMI, although weight reduction was not. Therefore, LRG reduced BMI equally well at all baseline BMI values. However, the decrease in HbA1c was lower with a higher baseline BMI.
Results of the bivariate linear mixed effects model.
| Weight reduction model | HbA1c reduction model | |||
|---|---|---|---|---|
| Coeff (SD) | p value | Coeff (SD) | p value | |
| Intercept | 5.31 (2.71) | 0.05 | 3.09 (0.97) | <0.01 |
| Age | −0.04 (0.02) | 0.08 | 0.002 (0.01) | 0.77 |
| Male sex | −0.84 (0.58) | 0.14 | −0.22 (0.20) | 0.28 |
| BMI | 0.08 (0.05) | 0.10 | −0.04 (0.01) | 0.02 |
| Treatment time | 0.21 (0.03) | <0.01 | 0.03 (0.01) | <0.01 |
| Treatment time2 | −0.005 (0.001) | <0.01 | −0.0009 (0.0003) | <0.01 |
| Duration of diabetes | −0.16 (0.11) | 0.17 | −0.08 (0.04) | 0.03 |
| Duration of diabetes2 | 0.007 (0.004) | 0.09 | 0.001 (0.001) | 0.46 |
SD: standard deviation. Treatment time2 and duration of diabetes2 are a quadratic effect of treatment time and duration of diabetes, respectively. These variables capture the non-linear effect of the corresponding outcomes.
The HbA1c reduction was inversely correlated with duration of diabetes, although weight reduction was not. The relationship was linear, that is, the lower the duration of diabetes, the greater the reduction in HbA1c.
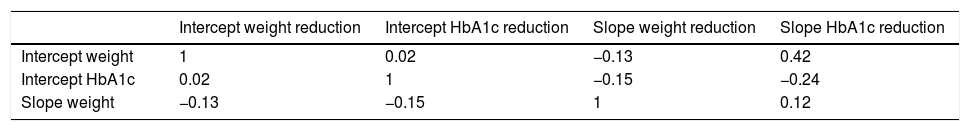
The correlation of the random intercept and slopes for the reduction in weight and HbA1c was obtained based on a longitudinal multivariate model (Table 3B). The intercept represents the correlation between the baseline measurements; the slope represents the time-dependent correlation between weight and HbA1c. The model therefore indicated that the greater the initial BMI, the lower the decrease in the HbA1c slope, and the higher the initial HbA1c, the greater the decrease in the slope of HbA1c.
The duration of therapy with LRG was directly correlated with the decrease in weight and HbA1c as a result of the initial and early response that was maintained over time. In this case, the relationship was quadratic, not linear (Fig. 1A and B).
Effect of adding LRG to previous insulin treatmentOf the 68 patients (36%) receiving insulin at baseline (0.55±0.4IU/kg), 59 patients were evaluated at 12 months and 23 (39%) had stopped insulin. Of the 30 patients evaluated at 24 months, 8 (26%) had stopped insulin. The patients who continued to take insulin reduced their dose to 0.41±0.2IU/kg at 12 months and to 0.43±0.2IU/kg at 24 months.
In patients receiving insulin, baseline HbA1c fell from 9.5±1.8 to 9.3±1.7% at 12 months (n=36) and to 8.9±1.3% at 24 months. In the subgroup of patients who stopped insulin, baseline HbA1c fell to 7.2±1.4% (n=23) at 12 months and to 7.7±1.5% (n=8) at 24 months. A significant reduction in weight was observed: from 98.1±15.6kg to 89.5±15.5kg at 12 months and 88.2±13.9kg at 24 months.
Evaluation of %EBMIL with LRG treatmentEBMIL is the criterion used to evaluate metabolic surgery, which was considered successful (EBMIL >50%) in 15 patients (9%) at 12 months and in 7 patients (8%) at 24 months. EBMIL was >10% in 125 patients (73%) at 12 months and in 65 patients (77%) at 24 months.
Adverse eventsNo serious adverse events were observed. Thirteen patients stopped treatment because of digestive intolerance (nausea and vomiting 7 cases, diarrhea 4 cases, and increased amylase 2 cases). No patients had pancreatitis or pancreatic cancer. Five patients died (cancer of the liver, lung, colon, bladder, and genitals). Acute complications (major intercurrent process or adverse event not related to LRG) were observed in 2 cases (ACVA and appendicitis).
Cardiovascular risk during treatment with LRGCardiovascular risk evolution was evaluated using REGICOR.13 Patients on LRG treatment for 12 months: at baseline at very high risk was 14 (10%) it decreased to 5 (3%) at 12months; 38 (26%) patients at high risk decreased to 17 (12%) at 12months; 58 (40%) patients at moderate risk increased to 71 (49%) and 34 (24%) patients at low risk increased to 51 (35%) at 12months.
Patients on LRG treatment for 24months: at baseline at very high risk was 7 (8%) it decreased to 3 (4%) at 24months; 24 (30%) patients at high risk decreased to 15 (19%) at 24months; 30 (38%) patients at moderate risk increased to 33 (41%) and 19 (24%) patients at low risk increased to 29 (36%) at 24months. Treatment with LRG for 12–24 months, combined with other treatments used in real-world practice, reduced cardiovascular risk from high to moderate-low.
DiscussionWe performed a retrospective observational cohort study to evaluate the metabolic effectiveness and safety of LRG in patients with T2DM in real-world clinical practice over a longer period than that usually assessed in clinical trials. We found that therapy with LRG reduced weight at 12, 24, and 36 months. Hb1Ac also decreased to target in almost half of the study population at 12 and 24 months. Most patients achieved the composite endpoint. Adding LRG to basal insulin treatment enabled nearly one-third of patients to stop insulin; the remainder were able to reduce their dose by one-third and weight reduction was significant.
In a recent review of 124 publications,16 HbA1c was significantly reduced after 6 months of treatment with LRG (mean change from baseline 0.9%–2.2%; HbA1c <7.0%, 29.5–65.0%). The NICE composite endpoint (HbA1c reduction ≥1% and weight loss ≥3%) was met in 16.9–47.0% of cases, and the absolute weight change was −1.3 to −8.65kg. Our results were consistent with the best of these, even though the time on treatment with LRG was longer than most of them.
When our results were compared with those of EVIDENCE,17 the most longer (24months) and big population sample (n 2019) in this review, the greater mean HbA1c and weight reduction in our series can be explained by the shorter duration of diabetes and higher initial weight.
This review includes three Spanish studies; Gomez Peralta et al.18 reported a 4.4kg reduction at 3months (n 158); Diaz-Soto G et al.,19 a 0.9% HbA1c reduction at 4months (n 59) and Mezquita-Raya et al.,20 a 1.1% HbA1c and 4.6kg reduction at 6 months of LRG treatment.
Later Lecube et al.21 reported a HbA1c decrease near 1.2% and weight loss 7.3kg during 6 months treatment.
A recent multicentric publication in Spain22 (24months treatment) reports a HbA1c reduction ranging from 1.0% to 1.5% and body weight from 5kg to 10kg.
We found that the metabolic response was early (3–6 months) and maintained over time. This finding could be explained by the appropriate selection of responders in our real-world setting.
Evaluation of the reduction in HbA1c using the random intercept and slope model revealed a correlation with the baseline value (the higher the baseline value, the greater the reduction). In contrast, the reduction and intercept for the HbA1c slope were lower at higher BMI levels, probably because LRG was indicated for patients with relatively low baseline HbA1c and very high baseline BMI, with correction of high BMI being the main objective of therapy with LRG in this subgroup. Given the findings for baseline BMI and the subsequent reduction therefore, treatment with LRG reduced BMI equally well across all baseline BMI values. Consequently, the effect of LRG on weight could be independent of the effect on HbA1c. This result supports the indication of LRG for weight reduction.
The reduction in HbA1c was inversely correlated with duration of diabetes. The relationship was linear, that is, the lower the duration of diabetes, the greater the reduction in HbA1c. In contrast, no correlation was observed for the reduction in weight; the effect of therapy on weight was independent of duration of diabetes. These findings also support an independent effect of LRG on glycemia and on weight.
Treatment of diabetes and obesity should be intensified once the diagnosis has been confirmed, and every opportunity should be taken to create a legacy effect.23–25 The efficacy and maintained effect of LRG over time make it a promising option when attempting to create a legacy effect in obese patients with diabetes.
The decrease in weight and HbA1c was more pronounced as the time on LRG increased. However, this finding can be explained by the initial and early response, which was maintained over time. The statistical analysis showed that the initial glycemia and weight responses were based on a quadratic model and not on a linear model, thus confirming these early responses as major predictive markers of response to LRG.
Given that the effects of therapy with LRG were clear after 12 weeks, a 3-month trial period may be sufficient to demonstrate the potential of the drug to reduce both weight and HbA1c. Treatment could be discontinued in patients who do not respond within this period.
Addition of LRG to insulin has proven to be effective in reversing weight gain, decreasing the insulin dose, and improving glycemic control in obese patients.26 The results of the subgroup of patients who were taking insulin at baseline are particularly interesting. Some were able to stop or reduce insulin dose, and both weight and Hb1Ac improved considerably. Consequently, in obese patients with T2DM, treatment with GLP1-RA should be considered before starting with insulin3,5,6 or uptitrating its dose. We may be able to “rescue” patients who received intensive therapy with insulin before LRG was marketed. Our results of combined LRG and insulin therapy may help to understand the results of previous studies.22
Evaluation of the EBMIL enabled us to compare our results with those obtained with bariatric surgery.27,28 Our results suggest that LRG treatment in T2DM obese patients could facilitate or even eliminate the need for surgery in patients who could reach the target %EBMIL with LRG alone.
Most adverse effects involved the gastrointestinal tract and were mild. These were less frequent than in clinical trials.7 The favorable safety profile of LRG could facilitate adherence to dietary modifications and ensure continued health benefits over time.
Recent trials evaluating LRG,10 empagliflozin,11 semaglutide,29 canagliflozin30 showed improved cardiovascular outcomes in patients with T2DM. The mechanism underlying these results and the question of whether outcome was a class effect or not remain open to debate. Our results for cardiovascular risk are consistent with those of the LEADER trial.10 In our study, we cannot attribute the improvement in cardiovascular risk exclusively to LRG. The treatment schedule was multifactorial, as recommended in guidelines. The addition of LRG could enable high-risk patients to be reclassified as moderate-low risk.
This study has limitations mainly related to its observational retrospective design. The criterion to define lack of efficacy and discontinue LRG treatment was not predefined, it was individualized decided by the physician. We must consider the influence of confounding factors. The combined antidiabetic therapy prescribed did not include SGLT2i. Future results of metabolic effectiveness studies in real-world clinical practice including combined SGLT2i and GLP1-RA may be compared with our results just using GLP-RA.
Real-world data have the potential to improve the quality and delivery of medical care, reduce overall costs, and improve outcomes by accelerating our understanding of how best to incorporate new therapies into everyday clinical practice. Such data help to fill the knowledge gap between clinical trials and actual clinical practice.
ConclusionsIn conclusion, we found that therapy with LRG under conditions of routine clinical practice produced a better metabolic response (weight loss and reduction in Hb1Ac) than that observed in clinical trials. The early effect observed in responders at 3 months was maintained at 12 and 24 months and was the best predictor of response to LRG. The weight response was independent of baseline BMI, baseline HbA1c, and duration of diabetes. The HbA1c response was greater when baseline HbA1c was higher and duration of diabetes was shorter. LRG could delay or reduce the insulin dose in a significant subgroup of obese patients by improving weight simultaneously with glycemic control. We also observed an improvement in cardiovascular risk factors enabling patients to be transferred from the high-risk category to medium-low risk. Consequently, LRG would be a suitable candidate for inclusion in multifactorial interventions aimed at reducing the risk of cardiovascular events.
Founding sourcesMedical writing, editorial assistance (Cociente, S.L.), and the statistical analysis were funded by a grant from Novo Nordisk. The authors take full responsibility for the content and conclusions stated in this manuscript. Novo Nordisk neither influenced the content of this publication nor was involved in the study design or in the collection, analysis, or interpretation of the data reported.
Conflict of interestsThe authors declare no conflict of interests.