Pituitary adenoma (PA) constitutes 15% of intracranial tumors. Non-functioning PA (NFPA) is the second most common type of PA after prolactinoma, with a prevalence of 22.2 cases/100,000 inhabitants.1 In most cases, NFPA is a macroadenoma (≥1cm) whose clinical presentation is variable; from an incidental oligosymptomatic finding to a true giant PA with great local involvement of neighboring structures and different degrees of hypopituitarism.2 Its clinical management varies depending on the size of the tumor, its growth over time and neuro-ophthalmic involvement. Surgical treatment followed by radiotherapy of the remnant or tumor recurrence is the usual treatment. The clinical experience with medical treatment with somatostatin analogs (SSA) and dopamine agonists (DA) as a primary treatment in these tumors is poorly defined.
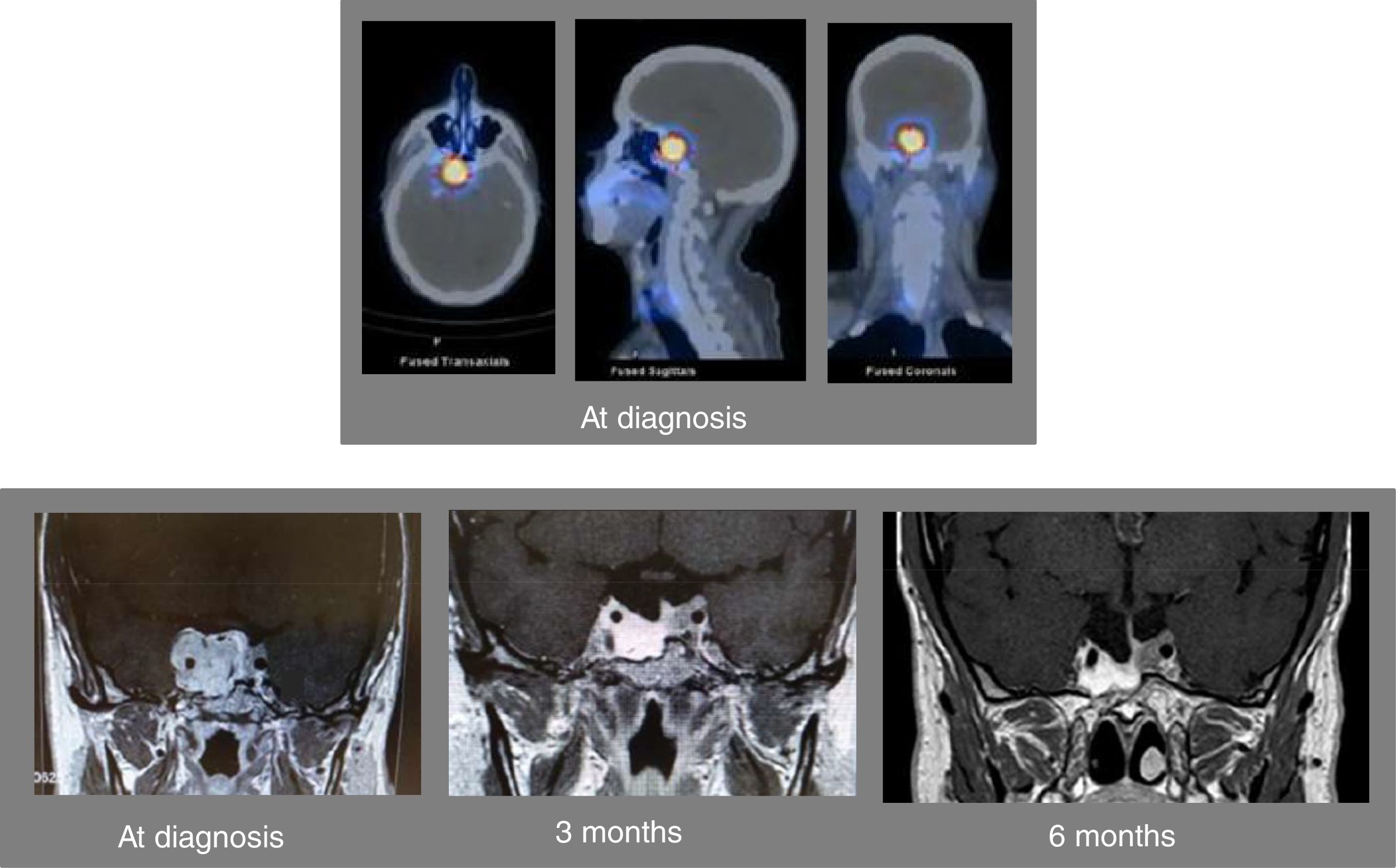
A 46-year-old man was referred to us for assessment of pituitary tumor discovered in a cranial CT during the study of headache in the last 5–6 months. Clinically, the patient did not show symptoms of adenohypophyseal hypo-hyperfunction or diabetes insipidus. No galactorrhea or erectile dysfunction, and libido was preserved. Hormonal evaluation showed central hypogonadism [FSH 2.3mIU/ml (NR, 1.4–18), LH 1.5mUI/ml (1.5–9.3), testosterone 93.9ng/dl (241 – 827)], and mild hyperprolactinemia [PRL 49.1ng/ml (2.2–17.7), PRL after dilution 47.8ng/ml]. The rest of the pituitary function was normal [TSH 2.05μIU/ml (0.35–5.0), free T4 1.09ng/dl (0.7–1.98), ACTH 56.3pg/ml (9.0–55.0), cortisol 13.6μg/dl (4.3–22.4), urinary free cortisol 16μg/24h (11.0–71.0), Nugent test: cortisol 0.8μg/dl, GH 0.8ng/ml (4.3–6.3), and IGF-I 134ng/ml (55.0–420.0)]. Pituitary MRI confirmed the presence of an invasive pituitary tumor of 2.0×1.7×3.0cm with suprasellar extension with greater involvement of the right side, extending to both cavernous sinuses and encompassing the right and partially left carotid (Fig. 1). The campimetric study of the visual fields was normal. A 99mTc-EDDA/HYNIC-Tyr3-Octreotide (tektrotyd) scintigraphy revealed a hypercaptant lesion at pituitary level (Fig. 1). With the clinical diagnosis of invasive non-functioning pituitary macroadenoma associated with mild hyperprolactinemia, due to probable compression of the pituitary stalk, and central hypogonadism, he started therapy with lanreotide autogel 120mg/month sc and oral cabergoline 0.5mg/week, and was referred to the Neurosurgery Department for surgical treatment of the PA. Shortly after starting medical treatment, the patient showed a marked clinical improvement of headaches, without visual disturbances. A new pituitary MRI performed 3 months after starting medical treatment showed a marked reduction in pituitary adenoma size (1.2×1.5×2.5cm) (Fig. 1). Due to the excellent clinical and radiological evolution, it was decided to postpone surgery. At 6 months after starting medical treatment, the patient referred notable clinical improvement of headaches without visual disturbances, the adenohypophyseal function was controlled (testosterone 496.10ng/dl and PRL 0.5ng/ml) and pituitary MRI showed marked reduction of the macroadenoma size with respect to the previous MRI (Fig. 1). From that moment the patient was treated with lanreotide autogel 120mg/month sc and oral cabergoline 0.25mg/week. A year and a half after starting treatment, pituitary function remained controlled (testosterone 418ng/dl and PRL 0.5ng/ml) and tumor size remained stable with respect to the last pituitary MRI performed at sixth month.
Axial (left above), sagittal (center above), and sagittal (right above) images of the SPECT-TC with 99mTc-EDDA/HYNIC-Tyr3-Octreotide (tektrotyd) scintigraphy at diagnosis showing a hypercaptant lesion at the level of the pituitary tumor. Pituitary MRI at diagnosis showing an invasive pituitary tumor of 2.0×1.7×3.0cm with suprasellar extension with greater involvement of the right side, extending to both cavernous sinuses and encompassing the right and partially left carotid (left down). Pituitary MRI 3 months (center down) and 6 months (right down) after starting combined therapy with lanreotide and cabergoline.
NFPA is around 25–35% of pituitary tumors, being most of them gonadotropinomas, which account for as many as 40–50% of all pituitary macroadenomas.3 At diagnosis, most NFPAs are macroadenomas and, on many occasions, they are incidentally diagnosed.2 Surgery is the treatment of choice for symptomatic (headache, visual disturbances and neurological involvement) NFPAs, those associated with pituitary apoplexy and those that show growth during the surveillance. However, complete surgical resection, especially in invasive adenomas (Knosp grades 3–4) is exceptionally achieved. For this reason and, as a complementary treatment to a debulking surgery, radiotherapy is usually used to control the tumor remnant or its recurrence.4 Radiotherapy achieves an excellent long-term local tumor control but at the expense of a high rate of hypopituitarism.
As occurs with prolactinomas, where DAs constitute the first-line therapy, NFPAs also express dopamine D2 receptors (D2R). D2R expression has been reported in about 35–40% of NFPAs and DAs (bromocriptine and cabergoline) have been shown to decrease gonadotropin and alpha-subunit secretion in gonadotroph adenomas.5–7 Therefore, DAs could have a therapeutic role in the medical management of NFPAs. In fact, in these patients, pooled results have shown a reduction of tumor size and stabilization of disease in 30% and 58% of patients, respectively.8
On the other hand, NFPAs can also express somatostatin receptors (SSTRs),9 although its clinical relevance has not been fully defined. The finding of significant expression of SSTR type 2 (SSTR2) and type 5 (SSTR5) has suggested a possible therapeutic role regarding the use of SST analogs in preventing tumor recurrence in NFPAs.10 SSA as primary medical therapy for NFPAs has shown low tumor response rates (12–40%),11 however the combination therapy with DA and SSA can increase tumor response rate [mean reduction in tumor volume of 30±4% (18–46%) after 6 months of combination therapy with octreotide and cabergoline] in 60% of NFPA patients.12 SSTRs and D2R may have additive effects on cell proliferation in pituitary adenomas thought interaction by heterodimerization as shown for SSTR1-SSTR5, SSTR5-D2R, SSTR2-SSTR3, and SSTR2-D2R.8 In fact, SSA and cabergoline are usually used after surgery in invasive NFPA.
In our patient, the long-term clinical management with combined medical treatment with SSA and DA was encouraged for several facts: (1) the positive tumor uptake in scintigraphy with 99mTc-tektrotyd that was compatible with high SSTR expression by the tumor; (2) the spectacular and rapid clinical response of headache to medical treatment associated with good tolerance; (3) the adequate control of hyperprolactinemia and hypogonadism with cabergoline; (4) the low probability of complete surgical resection due to the high degree of invasiveness of the tumor (Knosp grades 3–4); and (5) the rapid (3 months) and striking response in terms of tumor size reduction (Fig. 1).
In summary, our clinical case suggests that those patients with large and invasive NFPAs without neuro-ophthalmological involvement and little likelihood of complete surgical resection, that are associated with mild hyperprolactinemia and positive tumor uptake in SSTR scintigraphy, combined treatment with DA and SSA could be considered as an initial therapeutic alternative to surgical treatment.
Informed consentThe patient signed the consent for the publication of the clinical case.
Conflicts of interestThe authors have no conflict of interest and financial support in relation to the present manuscript.