Prolactin measurement is very common in standard clinical practice. It is indicated not only in the study of pituitary adenomas, but also when there are problems with fertility, decreased libido, or menstrual disorders, among other problems.
Inadequate interpretation of prolactin levels without contextualizing the laboratory results with the clinical, pharmacological, and gynecological/urological history of patients leads to erroneous diagnoses and, thus, to poorly based studies and treatments.
Macroprolactinemia, defined as hyperprolactinemia due to excess macroprolactin (an isoform of a greater molecular weight than prolactin but with less biological activity), is one of the main causes of such erroneous diagnoses, resulting in poor patient management when not recognized.
There is no unanimous agreement as to when macroprolactin screening is required in patients with hyperprolactinemia. At some institutions, macroprolactin testing by polyethylene glycol (PEG) precipitation is routinely performed in all patients with hyperprolactinemia, while others use a clinically based approach. There is also no consensus on how to express the results of prolactin/macroprolactin levels after PEG, which in some cases may lead to an erroneous interpretation of the results.
The objectives of this study were:
1. To establish the strategy for macroprolactin screening by serum precipitation with PEG in patients with hyperprolactinemia: universal screening versus a strategy guided by the alert generated by the clinician based on the absence or presence of clinical symptoms or by the laboratory when hyperprolactinemia is detected.
2. To create a consensus document that standardizes the reporting of prolactin results after precipitation with PEG to minimize errors in the interpretation of the results, in line with international standards.
La medición de la prolactina es muy frecuente en la práctica clínica habitual. Está indicada no solo en el estudio de los adenomas hipofisarios, sino también cuando hay problemas de fertilidad, disminución de la libido o trastornos menstruales, entre otros.
Una interpretación incorrecta de la concentración de la prolactina sin contextualizar los resultados analíticos con la historia clínica, farmacológica y gineco/urológica de los pacientes lleva a diagnósticos erróneos y, por lo tanto, a estudios y tratamientos mal fundamentados.
La macroprolactinemia, definida como la hiperprolactinemia debida al exceso de macroprolactina (isoforma de mayor peso molecular que la prolactina pero con menor actividad biológica), es una de las causas principales de estos diagnósticos erróneos, con el consecuente mal manejo del paciente, cuando no se reconoce.
No hay un acuerdo unánime en relación a cuando hay que realizar el cribado de macroprolactina en los pacientes con hiperprolactinemia. En algunos centros el estudio de macroprolactina mediante la precipitación con polietilenglicol (PEG) se realiza de forma rutinaria en todos los pacientes con hiperprolactinemia, mientras que en otros se realiza un enfoque basado en la clínica. Asimismo, no existe un consenso en la forma de expresar los resultados de la concentración de la prolactina/macroprolactina post PEG, lo que en algunos casos puede derivar en una interpretación errónea de los resultados.
Los objetivos de este documento son:
1. Establecer la estrategia de cribado de macroprolactina mediante precipitación del suero con PEG en pacientes con hiperprolactinemia: cribado universal vs estrategia guiada en función de la alerta originada por el clínico en base a la ausencia o presencia de sintomatología clínica o por el laboratorio ante la presencia de hiperprolactinemia.
2. Crear un documento de consenso que estandarice el informe de los resultados de la prolactina tras su precipitación con PEG para reducir errores en la interpretación de los resultados, siguiendo los estándares internacionales.
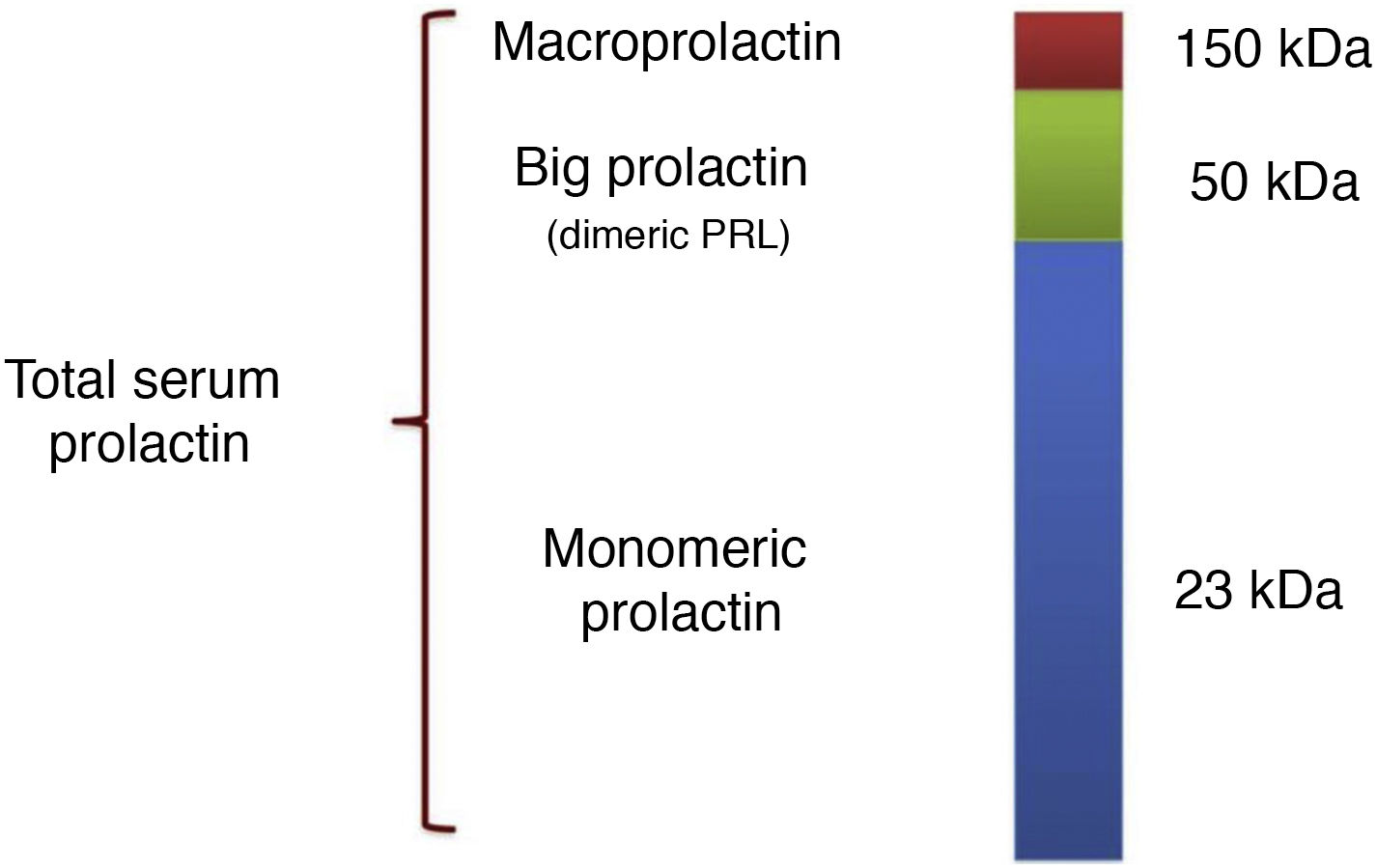
Prolactin is a polypeptide hormone synthesised mainly by the lactotroph cells of the adenohypophysis. The prolactin gene encodes a 227 amino acid polypeptide that, after translation and cleavage of the signal peptide, gives rise to the 23 kDa prolactin monomer. This is what is known as bioactive monomeric prolactin and represents 65–85% of total circulating prolactin.1 In addition, there are post-translational modifications (produced by dimerisation, polymerisation, phosphorylation, glycosylation, sulfation, deamidation, and covalent bonding)2 that generate prolactin isoforms that can interfere with the measurement of monomeric prolactin concentration. We highlight three isoforms as being the most relevant in this regard: glycosylated prolactin (25 kDa), whose boding facilitates aggregation into dimers; the 50−60 kDa prolactin isoform (20–25% of total serum prolactin),3 which exhibits reduced bioactivity and variable reactivity in different immunoassays; and lastly, the higher molecular weight complexes (150 kDa), formed by glycosylation, aggregation and covalent or non-covalent bonding to each other and to immunoglobulins (generally IgG and less frequently to IgA) that make up the so-called macroprolactin, which in most individuals represents less than 10% of total prolactin.1,2,4
Thus, the usual pattern in serum samples consists of three main components of prolactin (Fig. 1): (1) monomeric (23 kDa); (2) dimers or big prolactin (50−60 kDa), formed by the aggregation of glycosylated monomers, and (3) macroprolactin (150 kDa), also known as big-big prolactin. This distribution of isoforms is observed in most normoprolactinaemic subjects and also in those with hyperprolactinaemia due to physiological, pharmacological or pathological causes. In subjects with prolactin concentrations within the reference values, the prevalence of macroprolactinaemia is around 3.7%, but this proportion increases in patients with hyperprolactinaemia.
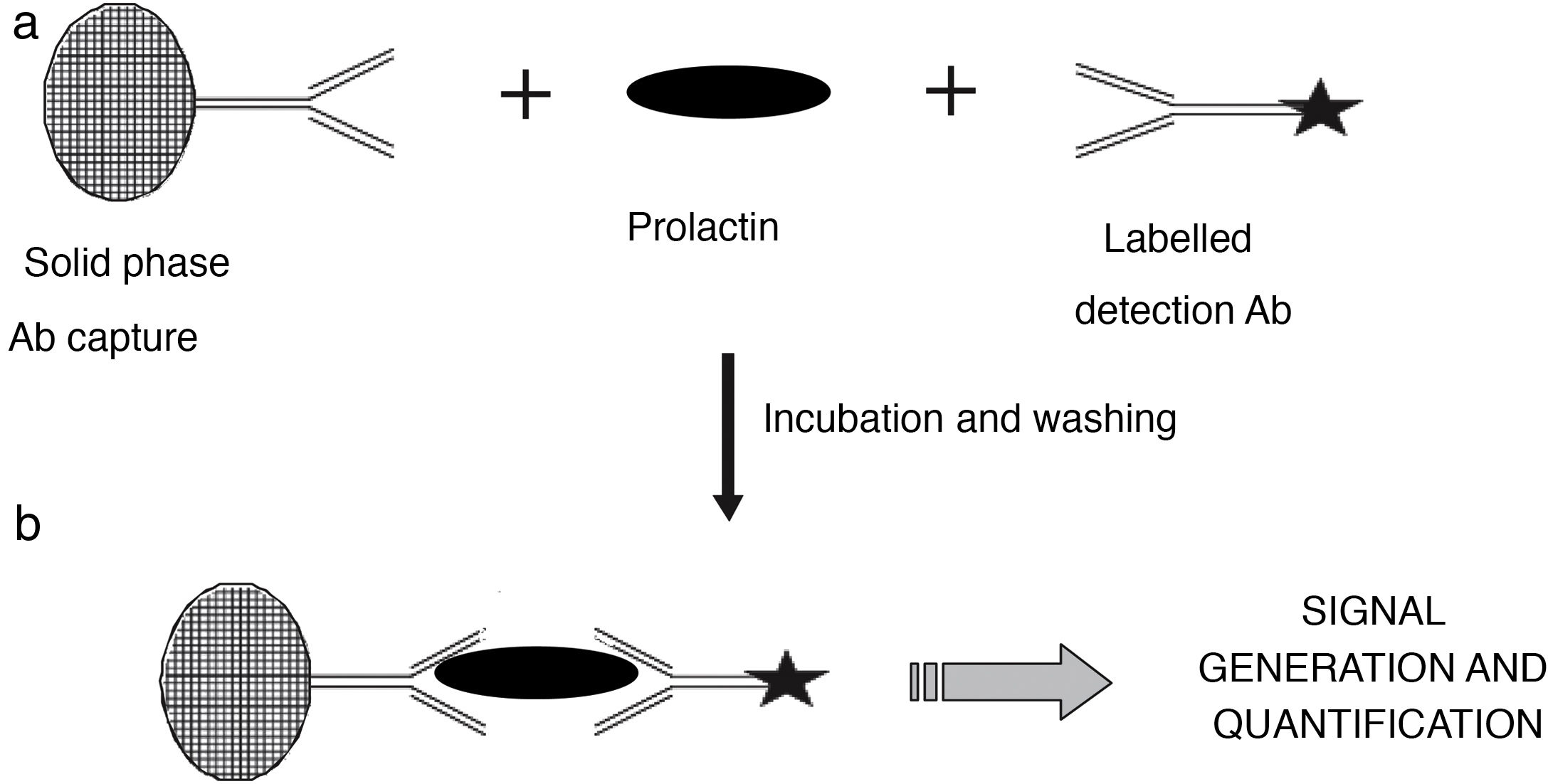
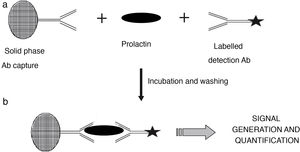
Measurement of serum prolactin concentrationSerum prolactin concentration is currently measured with automated immunometric immunoassays in which the prolactin molecule reacts with a capture antibody that is immobilised in a solid phase and with a labelled antibody that is used for detection. After elimination of the excess of labelled antibody that has not formed immune complexes, the signal generated is proportional to the concentration of prolactin present in the sample (Fig. 2). These methods provide fast and accurate results over a wide concentration range and have good reproducibility.
Diagram illustrating the principle of the immunometric immunoassay. Two antibodies specific for different epitopes of prolactin are used: a capture antibody, in this case bound to a solid phase matrix, together with a labelled detection antibody. During the incubation stage (a), reactive antibodies react with serum prolactin to form a sandwich. After a wash step to remove unbound material, the now bound detection antibody (b) generates a signal whose magnitude is directly related to the concentration of serum prolactin.
Most of the current methods use as a reference the WHO’s third international standard (3°IS 84/500), a preparation composed exclusively of human monomeric prolactin. However, despite standardisation there is a lack of commutability between the values produced by the different methods, so each must have its own reference ranges.
Currently, immunoassays have greatly improved in terms of their specificity, mainly thanks to the use of monoclonal antibodies and the addition of blocking agents that reduce the possibility of interference due to the presence of heterophile antibodies and other molecules. All immunoassays detect macroprolactin, although in a variable manner depending on the degree of reaction with the epitopes targeted by the capture or detection antibodies of the method.1
Measurement of macroprolactinIn some individuals, the high molecular weight forms account for a very high proportion of total circulating prolactin, a condition known as macroprolactinaemia or macroprolactin. According to the reference population studied, macroprolactin is present in 4–40% of patients with hyperprolactinaemia.4–6 In Spain, the percentage according to the different studies ranges between 9% and 31%.6–9 These disparities may be due to differences in the criteria used in the different studies in relation to the prolactin recovery percentage cut-off established for diagnosis, the immunoassay used (antibodies from different manufacturers have different cross-reactivities for macroprolactin) or the reference interval established for the concentration of prolactin after its precipitation with polyethylene glycol (PEG).
Gel filtration chromatography (GFC) is the reference method to evaluate the presence of macroprolactin in the sample, since it enables identification and confirmation of the different molecular forms of prolactin. However, this technique is laborious and expensive when compared to other automated methods, so it is not suitable as a routine technique in clinical laboratories. As an alternative, there are several procedures, some of which are also complex: (1) HPLC10; (2) immunoassay after elimination of macroprolactin by ultrafiltration; (3) immunoadsorption of IgG species with protein A, protein G, or anti-human IgG, and (4) precipitation with PEG.1,11
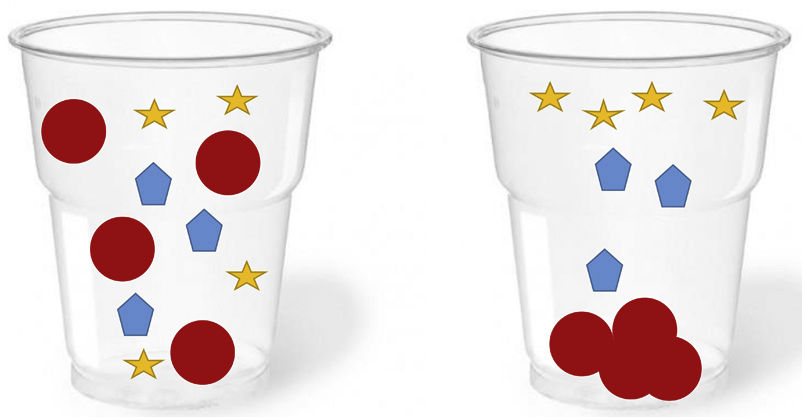
PEG precipitation is the technique that best correlates with GFC.11 It is also simple, fast, cheap, accessible and reproducible, and for this reason it is the most widely used and recommended in clinical laboratories for screening for the presence of macroprolactin in patients with hyperprolactinaemia. PEG acts as an inert molecular “sponge” that absorbs and dehydrates proteins, reducing their solubility and leading to their precipitation. Applied to serum, PEG is relatively specific for the precipitation of immunoglobulins and immunoglobulin complexes, hence its effectiveness in the precipitation of the most common form of macroprolactin containing IgG (Fig. 3). However, this procedure has some limitations:
- 1
PEG can also produce a partial precipitation of monomeric prolactin (20–25%) and, therefore, reduce its specificity and produce an underestimation or misinterpretation of prolactin concentration in cases with the simultaneous presence of macroprolactin and increased monomeric prolactin.1
- 2
It has been shown that the presence of PEG in the sample can interfere with some immunoassays used to measure prolactin, so it is recommended that each laboratory establish specific reference values for its method, derived from the study of prolactin precipitation with PEG of sera from healthy individuals.
- 3
In rare cases where the sera contain very high concentrations of gamma globulin, false positives may occur for the presence of macroprolactin.12
- 4
False negatives can occur in macroprolactin sera with IgA, since PEG precipitation of IgA is partial.
However, despite all these limitations, PEG prolactin precipitation is still the most widely used technique for macroprolactin screening, reserving GFC as a confirmation method when the results obtained with PEG precipitation are discrepant with the clinical data.
Macroprolactin bioactivityThe classic symptoms of hyperprolactinaemia, such as galactorrhoea, infertility, decreased libido and menstrual irregularity, are the consequence of supraphysiological concentrations of monomeric prolactin that suppress the gonadotropic axis. The biological activity of macroprolactin in vivo is low, probably due to its inability to bind to prolactin receptors, since the high molecular weight of the complex formed between the monomeric form and IgG prevents it from crossing the capillary membrane.13 Therefore, the presence of asymptomatic hyperprolactinaemia and clinical-biochemical discrepancies are what should alert the clinician to the possible coexistence of macroprolactin. On the other hand, the existence of macroprolactin does not rule out the presence of pituitary disease, so this biochemical finding does not exclude radiological examination when the clinical data are relevant.
Strategy for the evaluation of hyperprolactinaemia/macroprolactinaemiaIn the evaluation of hyperprolactinaemia, it is extremely important to take into account the conditions for extracting the sample, which must be done with the patient on an empty stomach, 2−3 h after waking up and without excessive stress in the venipuncture.
The clinical practice guideline for the diagnosis and treatment of hyperprolactinaemia14 establishes that a single measurement of elevated serum prolactin (without excessive venipuncture stress) establishes the diagnosis of hyperprolactinaemia. In case of doubtful increases in prolactin or those discordant with clinical symptoms, the extraction can be repeated by placing an intravenous catheter and extracting a baseline sample and one at 20 min to minimise the effect of the venipuncture.14,15
After ensuring that the blood extraction has been carried out under the appropriate conditions, we continue with the differential diagnosis based on first ruling out known causes of hyperprolactinaemia, such as physiological causes (pregnancy, lactation, stress, exercise…), those induced by drugs (mainly dopamine receptor antagonists, including some typical antipsychotics and antiemetics, among others), and secondary causes, such as hepatorenal insufficiency, hypothyroidism or polycystic ovaries, after which we would focus on hypothalamus-pituitary disorders (Table 1).
Causes of hyperprolactinaemia.
| Analytical |
| Macroprolactin |
| Physiological |
| Pregnancy |
| Breast stimulation/lactation |
| Stress |
| Medications |
| Antipsychotics |
| Antiemetics |
| Antihypertensives |
| Dopamine antagonists |
| Oestrogens |
| Secondary |
| Kidney or liver failure |
| Primary hypothyroidism |
| Polycystic ovary syndrome |
| Pituitary gland |
| Prolactinoma |
| Non-functioning adenoma |
| Hypophysitis |
| Pituitary stalk transection |
| Hypothalamus |
| Tumours |
| Infiltrative disease |
However, as mentioned above, macroprolactinaemia is a common cause of hyperprolactinaemia and should be taken into account in the study of the latter.
In this sense, the following approaches would exist in practice:
- 1
Clinician-guided study of macroprolactinaemia. This means that it is the clinician who requests the study of macroprolactinaemia in cases of patients with hyperprolactinaemia but without clinical translation. This approach is recommended by some international guidelines and is widely used in Spain.14,15 It has the problem that it depends on the skill of the clinician and, therefore, can lead to underdiagnosis of macroprolactinaemia and erroneous diagnosis that in many cases results in additional unnecessary studies and inappropriate treatment of patients.5,16
- 2
Laboratory-guided macroprolactin study. In this case, the laboratory takes on an active role, and together with the results of hyperprolactinaemia it issues an alert “recommending that a macroprolactin study be requested in cases of clinical disparity with the results issued”. This alert is intended to remind clinicians who are less familiar with the pathology resulting from prolactin disorders of the existence of this possible interference.
- 3
Universal study of macroprolactinaemia. In this case, macroprolactin would be determined in all patients in whom hyperprolactinaemia is detected. This strategy is followed routinely in some countries, such as England and Ireland, and in some laboratories in our setting. It has the advantage that it would avoid misdiagnosis and unnecessary tests and treatments, but the disadvantage is that it increases work and costs for the laboratory.
For the study of macroprolactin precipitation with PEG, the following protocol is used: an equal volume of serum and PEG 6000 solution at 25% (weight/volume, for example, dissolving 25 g of PEG 6000 in 100 ml of NaCl 0.9% or distilled water, stored at 4 °C) is mixed for 30 s with a vortex mixer and centrifuged at 3500 rpm for 30 min. The prolactin concentration in the supernatant is measured and corrected for the dilution factor (1:2). The recovery percentage is calculated using the initial prolactin (PRL) concentration and the post-PEG PRL concentration according to the following formula:
The post-PEG PRL concentration (corrected by the dilution factor) is the patient’s monomeric prolactin.
Clinical interpretation of the presence of macroprolactinaemia. Results report and follow-up.
Recovery (%) = (post-PEG PRL (multiplied by the dilution factor)/initial PRL) × 100
There are disparities even in the very definition of macroprolactinaemia. Some authors use the term to indicate that macroprolactin is the predominant form of immunoreactive prolactin in hyperprolactinaemic patients, as originally described by Jackson et al.17 Other authors use the term to indicate that macroprolactin is the predominant molecule, regardless of the existence of hyperprolactinaemia, even in sera with prolactins in the normal range.18 It is important to point out that the presence of a substantial proportion of macroprolactin does not exclude a simultaneous high concentration of bioactive monomeric prolactin, which would explain why in some cases in which macroprolactin is detected we find active symptoms.1,4 For this reason, the way the results are presented acquires special relevance.
When reporting results using the macroprolactin PEG precipitation method, there are two approaches to identifying patients with macroprolactinaemia.
- 1
Based on recovery percentage after PEG precipitation. The percentage of prolactin recovered after treatment of the serum with PEG is measured with respect to the concentration of prolactin obtained in the untreated serum. As it is method-dependent, varying based on the method used, the cut-off point is usually between 40% and 60%. A low recovery percentage indicates a predominance of macroprolactin molecules and will be reported as positive for macroprolactin. A high recovery percentage will indicate a predominance of monomeric prolactin molecules that will be reported as negative for macroprolactin.16
- 2
Concentration of monomeric prolactin. Prolactin concentration after PEG precipitation corrected with the dilution factor is measured along with corresponding reference values for each method. It is also method-dependent. There are monomeric prolactin reference values in the literature for the most widely used commercial immunoassays.19–21 This type of report allows the clinician to know if the patient’s biologically active prolactin (the monomeric prolactin) is elevated or normal, thereby decreasing the possibility of misclassifying patients with macroprolactin who have concomitant monomeric hyperprolactinaemia.
Regarding the follow-up of patients with macroprolactin, although there is no established and agreed guideline, some studies have been published that support the stability of this condition over time. The Wallace et al.22 group followed 51 patients with macroprolactinaemia for 10 years without finding clinical or analytical changes during the follow-up. In the study by Hattori et al.,18 macroprolactin screening was performed on 654 workers at their hospital. They detected the presence of macroprolactin in 27 subjects, and during the four years of follow-up, macroprolactin values remained stable. Moreover, none of the remaining 627 subjects developed macroprolactinaemia in this period. Therefore, serial monitoring of macroprolactin does not seem to be justified.
Working group recommendationsA survey was sent to the working group that was answered by all the co-authors, establishing the following recommendations:
- •
Investigate the presence of macroprolactin only in cases of hyperprolactinaemia.
- •
Regarding when the macroprolactin study should be performed: the working group is in favour of universal screening, although it recognises the limitations that may hinder this being established.
- •
PEG precipitation is the recommended screening method to rule out the presence of macroprolactin in clinical laboratories, reserving the GFC method for discrepant cases in which confirmation is required.
- •
Results report: indicate in the report of the PEG precipitation study the presence/absence of macroprolactin and the concentration of monomeric prolactin post-PEG together with the reference interval (specific for method and sex) to avoid interpretation errors (Appendix A).
- •
The percentage of macroprolactin with respect to total prolactin is a value that remains stable over time and does not require serial monitoring. Therefore, reassessment of macroprolactin in a given patient (that is, re-quantification of post-PEG monomeric prolactin) would only be indicated in the case of symptoms compatible with hyperprolactinaemia and an increase in total prolactin concentrations compared to previous values.
The SEEN [Sociedad Española de Endocrinología y Nutrición (Spanish Society of Endocrinology and Nutrition)] and SEQCML [Sociedad Española de Medicina de Laboratorio (Spanish Society of Laboratory Medicine)] Laboratory working group values multidisciplinary debate, and aspires to ensure that the laboratory can respond to the needs of the clinician. This channel of communication is a solid basis for improving diagnostic and follow-up strategies for endocrinological disorders. The recommendations agreed upon in this document are a true reflection of this joint work.
FundingNo financial support has been received.
AuthorshipAll authors have participated and approved the final version of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Biagetti B, Ferrer Costa R, Alfayate Guerra R, Álvarez García E, Berlanga Escalera E, Casals G, et al. Macroprolactina: del laboratorio a la práctica clínica. Recomendaciones del grupo de trabajo de laboratorio de la SEEN y de la comisión de hormonas de la SEQCML sobre la medición e informe del resultado de la macroprolactina. Endocrinol Diabetes Nutr. 2022;69:63–69.