Enteral nutrition (EN) constitutes a way to provide nutritional support to patients who are not able to meet their nutritional requirements. However, it is not exempt of complications such gastrointestinal symptoms (GI) or diarrhea. Although is widely accepted EN can cause diarrhea, there is little evidence to support it.1 Formula composition has been postulated as a possible triggering factor, blaming FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) due to their osmotic effect. Maltitol, as polyol molecule, given in a high-enough dose would induce laxative effect. Following, we describe a case of possible diarrhea secondary to use of an EN formula maltitol-enriched.
In January of 2018, an 83-year-old woman with the relevant medical history of type 2 diabetes mellitus, hypertension, dyslipidemia, penicillin allergy and dependent for the activities of daily living after suffering a stroke in the past, was brought by her daughter to the Accident and Emergency Department. On admission, patient had swallowing difficulties, disability to eat and drink, right face drooping and speech difficulty. Her regular medicines were: Captopril 50mg one tablet once a day (OD), Dipotassium Chlorazepate 10mg one capsule OD, Furosemide 40mg one tablet OD, Metformin 850mg one tablet OD, Nimodipine 30mg one tablet twice a day.
Patient was diagnosed of ischemic stroke (21st January), which was confirmed by brain scan. She was admitted in Internal Medicine Department. Aspirin 100mg OD, Atorvastatin 80mg OD, Candesartan 16mg OD, Enoxaparin 40mg OD and Omeprazole 40mg OD were added to her regular medicines. Next day (22nd January) patient had temperature (38°C) starting empirical treatment with Levofloxacin 500mg OD 7 days course for possible respiratory infection. Levofloxacin and Omeprazole were stopped after 7 and 8 days of treatment respectively (28th January). There were not changes on the rest of her regular medicines during this admission, and after 13 days patient was discharged (2nd February).
Despite patient had a positive evolution on this episode, she had severe dysphagia from admission date, which did not improve, requiring texturized food and thickened fluids in the beginning. After 2 days (23rd January), EN via nasogastric tube was started due to patient did not tolerate oral diet. Diabetic EN (Diason Energy HP®, Nutricia Advanced Medical Nutrition®) 500mL divided in 5 shots per day was prescribed, increasing 250mL daily up to a maximum of 1000mL daily divided into five doses. EN was well tolerated. After five days (28th January), this diet was changed to Glucerna Select® (Abbot Nutrition®) 1000mL in 24h, divided in 5 shots because Diason Energy or similar EN was not available due to manufacturing problems. At 28th January, patient presented gastrointestinal disorders (abdominal pain, flatulence and watery diarrhea). Nausea or vomiting was not reported.
After two days (30th January) having those gastrointestinal problems the physician contacted pharmacy to monitor her EN. Patient was assessed and reviewed by pharmacy team (Nutrition Support Specialist Pharmacists). Also an interview with the patient's relatives and nurses was conducted to check the correct administration technique (no changes on administration technique were reported and it was correct). In addition, patient's relatives denied any food allergy, intolerance, digestive disorders or gastrointestinal disease.
Nutritional requirements were calculated for the patient, estimating 1591kcal per day2 (80kg, 160cm, Body Mass Index: 31.25kg/m2), however due to her obesity, pharmacy team agreed a low-calorie and high-protein nutrition (1000kcal per day and 80–96g protein) would be appropriated. Thereby, EN was changed to Diben Estandar®, Fresenius Kabi 1000mL over 24h divided in 5 doses initially. Administration technique change to continuous feeding was discarded due to high suspicion that the EN formula was the cause of GI disorders and patient's family preferences. Next day (31st January), diarrheic stools ceased and rest of gastrointestinal symptoms. Patient continued with same EN for three more days until discharge from the hospital with good tolerance and follow up with Nutrition Support Unit to optimize patient's EN.
Diarrhea is one of the most common complications associated with EN. There are multiple contribute factors involved on its pathogenesis, such medication, infection, underlying disease and enteral nutrition.1
Drugs have been reported as major cause of loose stools in hospitalized patient with enteral nutrition.3 In our patient, medication was reviewed as a possible cause. Omeprazole and Levofloxacin have been reported as cause of diarrhea, however both medicines were stopped (28th January) and GI episode continues. Thereby, it seems related with the enteral formula change, in regards of the start and stop diarrhea timeline.
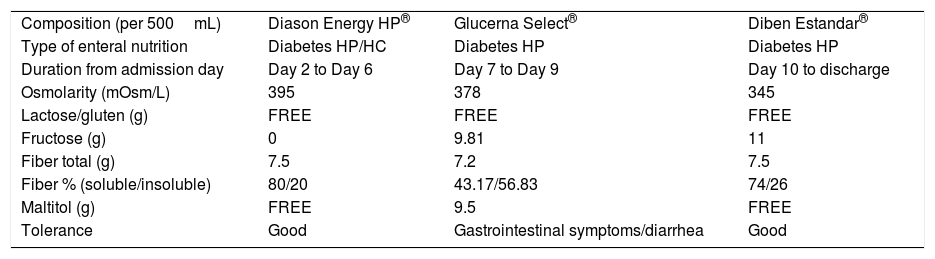
Enteral nutrition also has been blamed as a cause of diarrhea. Enteral formula has been suggested as a contributing factor to (GI) symptoms. Three different EN formulas with different composition were prescribed during patient's admission (Table 1).
Composition of different enteral nutrition formulas given during patient's admission.
| Composition (per 500mL) | Diason Energy HP® | Glucerna Select® | Diben Estandar® |
| Type of enteral nutrition | Diabetes HP/HC | Diabetes HP | Diabetes HP |
| Duration from admission day | Day 2 to Day 6 | Day 7 to Day 9 | Day 10 to discharge |
| Osmolarity (mOsm/L) | 395 | 378 | 345 |
| Lactose/gluten (g) | FREE | FREE | FREE |
| Fructose (g) | 0 | 9.81 | 11 |
| Fiber total (g) | 7.5 | 7.2 | 7.5 |
| Fiber % (soluble/insoluble) | 80/20 | 43.17/56.83 | 74/26 |
| Maltitol (g) | FREE | 9.5 | FREE |
| Tolerance | Good | Gastrointestinal symptoms/diarrhea | Good |
HC: high calorie nutrition; HP: high protein nutrition.
Patient developed diarrhea after starts Glucerna Select® EN and it stops after changing to Diben Estandar®. As stated in Table 1, the main difference between Glucerna Select® formula and the rest of EN formulas was its Maltitol content, which was 19g per day (1000mL) its intake. Maltitol is a slow absorption disaccharide polyol with a good glycaemic profile, being hypocaloric (2.1kcal/g) and non-cariogenic molecule. In the healthy population cases of mild laxative effect and flatulence have been reported with daily amounts of 30–40g.4,5 In addition, there are evidence that suggest the link between EN-associated diarrhea and polyols, such maltitol.6,7 In our case, GI symptoms appeared and gone suddenly, so it seems that a boosted mechanism could explain it. As known, Maltitol is absorbed around 40%, previous hydrolysis by the intestinal brush-border disaccharidases, to glucose and sorbitol.8 The unabsorbed amount (60%) is fermented through the intestinal flora. The Sorbitol that is a product of the hydrolysis of Maltitol, is absorbed only 25% and the rest, like the Maltitol, is fermented through the intestinal flora.7 Both Maltitol and Sorbitol are osmotic molecules that can lead in GI symptoms.6,7 As previously mentioned, patient was treated before start with Glucerna Select® EN with Levofloxacin without any GI disturbances. A marked reduction in commensal microbiota has been reported after a course of broad-spectrum antibiotic.2 In our case, treatment with Levofloxacin could have altered gastrointestinal microbiota, decreasing Maltitol fermentation. A prolonged stay of this polyol at gastrointestinal level could have increased the osmotic charge. This fact would explain patient's abdominal discomfort and diarrhea. In addition, less soluble fiber proportion of Glucerna Select® could contribute with the mechanism suggested.
The Naranjo algorithm indicated that Maltitol content in Glucerna Select® was the possible cause of the patient's diarrhea.9
Low dose of Maltitol could be beneficial as additive for the EN, due to its glycemic profile and its low caloric content. However, in some circumstances, in which the gastrointestinal function is compromised, could be harmful leading on side effects (GI symptoms and diarrhea). Thus a deep patient's assessment and EN formula choice could be a key factor to avoid EN related problems.