Graves’ disease is an autoimmune disorder characterised by excessive production of thyroid hormones, which induces increased cellular metabolism in most tissues and increased production of reactive oxygen species (ROS). The aim of this work was to analyse the effect of ROS on cell viability and the expression of catalase (CAT), glutathione peroxidase-1 (GPx-1), superoxide dismutase (SOD-1) and DNA methyltransferase-1 (DNMT-1) in peripheral blood mononuclear cells (PBMC) from patients with newly diagnosed Graves’ disease or treated with methimazole.
Patients and methodsFor this study, women patients with newly diagnosed Graves’ disease (n=18), treated with methimazole (n=6) and healthy subjects (n=15) were recruited. ROS were evaluated by flow cytometry, and the viability/apoptosis of PBMC was analysed by flow cytometry and fluorescence microscopy. Genomic expression of CAT, GPx-1, SOD-1 and DNMT-1 was quantified by real-time PCR.
ResultsWe found high levels of ROS and increased expression of CAT, GPx-1, SOD-1 and DNMT-1 in PBMC from patients with newly diagnosed Graves’ disease. Methimazole treatment reversed these parameters. Cell viability was similar in all study groups.
ConclusionsROS induces the expression of CAT, GPx-1, and SOD-1. The activity of these enzymes may contribute to the protection of PBMC from the harmful effect of free radicals on cell viability. Increased expression of DNMT-1 may be associated with aberrant methylation patterns in immunoregulatory genes contributing to autoimmunity in Graves’ disease.
La enfermedad de Graves es un trastorno autoinmune caracterizado por una producción excesiva de hormonas tiroideas, que induce un aumento del metabolismo celular en la mayoría de los tejidos y una mayor producción de especies reactivas de oxígeno (ROS). El objetivo de este trabajo fue analizar el efecto de las ROS sobre la viabilidad celular y la expresión de catalasa (CAT), glutatión peroxidasa-1 (GPx-1), superóxido dismutasa (SOD-1) y ADN metiltransferasa-1 (DNMT-1) en células mononucleares de sangre periférica (PBMC) de pacientes con enfermedad de Graves recién diagnosticada o tratados con metimazol.
Pacientes y métodosSe seleccionó a mujeres con enfermedad de Graves recién diagnosticada (n=18), tratadas con metimazol (n=6) y a sujetos sanos (n=15). La producción de ROS fue evaluada por citometría de flujo. La viabilidad y apoptosis de las PBMC fue analizada por citometría de flujo y microscopía de fluorescencia. La expresión genómica de CAT, GPx-1, SOD-1 y DNMT-1 fue cuantificada por PCR en tiempo real.
ResultadosEncontramos altos niveles de ROS y una mayor expresión de CAT, GPx-1, SOD-1 y DNMT-1 en PBMC de pacientes con enfermedad de Graves recién diagnosticada. El tratamiento con metimazol revirtió estos parámetros. La viabilidad celular fue similar en todos los grupos de estudio.
ConclusionesLas ROS inducen la expresión de CAT, GPx-1 y SOD-1. La actividad de estas enzimas podría contribuir a la protección de las PBMC del efecto nocivo de los radicales libres sobre la viabilidad celular. El aumento de la expresión de DNMT-1 podría estar asociado con patrones de metilación aberrantes en genes inmunorreguladores que contribuyen a la autoinmunidad en la enfermedad de Graves.
Graves’ disease is an autoimmune pathology characterised by high serum levels of l-3,3′,5-triiodothyronine (T3) and l-thyroxine (T4) and low levels of thyrotropin (TSH), and the presence of autoantibodies against the receptor of the thyroid stimulating hormone (TRAb), thyroglobulin (ATG), and thyroid peroxidase (ATPO). Clinical manifestations of Graves’ disease include diffuse goitre, pretibial myxedema, and orbitopathy, and, rarely, abnormalities of the fingertips and nails. Graves’ disease is the most common cause of hyperthyroidism, with an annual incidence of 20–50 cases per 100,000 persons. Approximately 3% of women and 0.5% of men will develop the disease during their lifetime.1
Thyroid hormones regulate many physiological functions, increase cellular metabolism and mitochondrial activity in most tissues. Mitochondria produce large amounts of ROS through the oxidation-reduction reactions carried out in the respiratory chain.2 Evidence indicates that under physiological conditions, ROS may participate in redox signalling, but excessive ROS production may cause oxidative damage to proteins, lipids and DNA, and affect cellular functionality.3 Several studies indicate the role of oxidative stress in the progression of autoimmune thyroid diseases, such as Graves’ disease.4
ROS are neutralised by the antioxidant enzymes SOD, CAT, and GPx. Three SOD isozymes are expressed in human cells, a cytosolic (SOD-1), a mitochondrial (SOD-2), and an extracellular (SOD-3) isoform. These enzymes catalyse the conversion of the superoxide anion radical (O2−) to hydrogen peroxide (H2O2) and molecular oxygen (O2). The SOD-1 and SOD-3 isoforms also exhibit peroxidase activity, inactivating H2O2. Catalase converts H2O2 into O2 and H2O. The GPx enzyme catalyses the oxidation reaction of glutathione to glutathione disulfide using H2O2. GPx-1 is the cytosolic isoform that is expressed in most tissues, although there are other isoforms with tissue-specific distribution.5
There are numerous studies on the role of oxidative stress and antioxidant defenses in the thyroid gland, adipocytes, and orbital muscle cells,6 plasma, erythrocytes,5,7,8 or urine9 of patients with Graves’ disease, but the expression of antioxidant enzymes or the redox status in PBMC has not been evaluated.
Reports in peripheral blood lymphocytes indicate an association between certain polymorphisms, mutations in immunoregulatory genes or an altered expression of regulatory T cells, and the development of pathology.10 In recent years, the study of epigenetic alterations in immunoregulatory genes has become more important. DNA methylation is one of the main epigenetic mechanisms involved in the regulation of gene expression. DNMT-1 catalyses the transfer of methyl groups at the 5-position of the cytosine residue in regions rich in cytosine-guanine dinucleotides called CpG islands, and is responsible for maintaining methylation patterns during DNA replication.11 Several studies indicate an association between redox imbalance and aberrant methylation patterns in various pathologies, including autoimmune diseases.12–14 However, the role of epigenetic mechanisms in the development of Graves’ disease has not been fully elucidated.
The aim of this work was to analyse the effect of oxidative stress induced by the hyperthyroid state on the cellular viability and the regulation of the enzymatic antioxidant system and analyse the expression of the epigenetic enzyme DNMT-1 in PBMC from patients with newly diagnosed Graves’ disease or treated with methimazole.
Materials and methodsSubjects of studyA prospective cross-sectional study was conducted recruiting women patients with newly diagnosed Graves’ disease (n=18), patients with Graves’ disease treated with methimazole for more than two months (n=6) and healthy euthyroid individuals (n=15). Patients with a history of other autoimmune diseases, pregnancy, treated with immunosuppressive drugs or smokers were excluded from this study. The research was conducted in accordance with the Helsinki Declaration and was approved by the Bioethics Committee of the British Hospital of Buenos Aires (approval number #585). All patients agreed to participate in the study by signing a written informed consent.
Biochemical analysesPlasma concentration of T3, total o free T4 and TSH were quantified by a chemiluminescent immunometric assay (QLIA, Architect ci8200, Abbott). Plasma concentrations of ATPO, ATG and TRAb were determined by electrochemiluminescence immunoassay (EQLIA, Cobas e411, Roche Diagnostics).
ROS quantificationPBMC were isolated by Ficoll-Hypaque gradient centrifugation and resuspended in phosphate-buffered saline (PBS). Cells (1×106/ml) were incubated with 10μM 2′-7′dichlorofluorescin diacetate (DCFH-DA) for 30min at 37°C. The fluorescence intensity of the oxidised probe (DCF) was measured by flow cytometer (BD Accuri™, Becton Dickinson Biosciences) at 488nm.
Genomic expression of antioxidant enzymes and DNMT-1 by quantitative PCRPBMC were homogenised in Tri-Reagent (Genbiotech SRL) and total RNA was isolated following conventional protocols. cDNA was synthesised by retrotranscription using the Omniscript kit (Qiagen NV). Quantitative PCR was carried out using a commercial master mix for real-time PCR containing SYBR Green fluorescent dye (Biodynamics SRL). The amplification reaction was performed in the Applied Biosystems 7500 equipment (ThermoFisher Scientific). Primers (Biodynamics SRL) used were: CAT 5′-GCCTTTGGC TACTTTGAGGTC-3′ and 5′-GAGAACCGAACTGCGATGG-3′; GPx-1 5′-TATCGAGAATGTGGCGTCCC-3′ and 5′-TCTTGGCGTTCTCCTGATGC-3′; SOD-1 5′-GGGCAAT GTGACTGCTGAC-3′ and 5′-ACAAGCCAAACGACTTCCAG-3′; DNMT-1 5′-CGACTACATCAAAGGCAGCAACCTG-3′ and 5′-TGGAGTGGACTTGTGGGTGTTCTC-3′; β2-microglobulin 5′-AGATGAGTATGCCTGCCGTGTGAA-3′ and 5′-TGCTGCTTACA TGTCTCGATCCCA-3′. Quantification of gene expression was performed using the comparative cycle threshold (Ct) method.
Cell viability/apoptosis assaysPBMC (1×106cells) were resuspended in 1ml staining buffer (10mM HEPES/NaOH pH 7.5, 0.14M NaCl and 2.5mM CaCl2) and then incubated with 5μl of annexin V-FITC (1mg/ml; Sigma Chemical Co.) and 10μl of propidium iodide (PI; 1mg/ml; Sigma–Aldrich) for 15min at 37°C. The percentage of viable and apoptotic cells was evaluated by flow cytometry (Accuri™ C6, BD Biosciences). Aditionally, PBMC (1×106cells/ml) were plated on slides and fixed with methanol-glacial acetic acid (3:1). Then, cells were stained with Hoechst 33258 (10μg/ml; Molecular Probes) for 10min. Nuclear morphology was examined by fluorescence microscope (Nikon Inc.). To assess changes in mitochondrial membrane potential, PBMC (1×106cells/ml) were incubated with 1μM rhodamine-123 for 20min at 37°C and then the fluorescence was analysed by flow cytometry.
Statistical analysisData are expressed as mean±standard error or median and interquartile range. Statistical analysis was carried out using ANOVA followed by the Newman–Keuls Multiple Comparison test. For data that did not follow a normal distribution, the Kruskal–Wallis non-parametric test followed by Dunn's Multiple Comparison posthoc was used (SPSS 21.0 software).
ResultsEvaluation of biochemical parametersThe plasma concentration of TSH, thyroid hormones, and autoantibodies in patients with Graves’ disease and healthy subjects is shown in Table 1. Patients with Graves’ disease showed an overproduction of T3 and T4 and low levels of TSH. They also had high levels of antibodies directed against TPO, TG and the TSH receptor. The treatment of patients with methimazole restored all hormonal parameters to values similar to those of euthyroid subjects, although the concentration of anti-thyroid antibodies remained high as a result of the autoimmune process.
Evaluation of biochemical parameters in women patients with Graves’ disease and healthy subjects.
| Biochemical parameters | Euthyroid subjectsn=15Age:36 [30–46] | Patients with Graves’ disease | |
|---|---|---|---|
| Newly diagnosedn=18Age:40 [26–48] | Treated with methimazolen=6Age:33 [25–40] | ||
| T3(ng/dl) | 125[109–130] | 231(a)[156–414]p<0.0001 | 122(b)[80–184]p<0.0001 |
| T4(μg/dl) | 7.90[6.90–8.90] | 18.65(a)[11.95–23.55]p<0.0001 | 9.90(b)[5.37–15.83]p<0.0006 |
| fT4(pmol/l) | 13.0[13.0–14.0] | 26.5(a)[19.6–25.0]p<0.0001 | 12.7(b)[10.1–13.0]p<0.0001 |
| TSH(mIU/l) | 2.16[1.54–3.07] | 0.02(a)[0.02–0.02]p<0.0001 | 0.48(b)[0.14–1.96]p<0.0001 |
| ATPO(IU/ml) | 11.0[6.0–17.0] | 181.5(a)[16.3–435.3]p<0.001 | 600.0(c)[39.0–647.0]p<0.0011 |
| ATG(IU/ml) | 23[18–37] | 50(a)[20–196]p<0.0001 | 105(c)[16–172]p<0.0011 |
| TRAb(UI/l) | 5.00[1.45–5.00] | 18.64(a)[9.24–43.25]p<0.0001 | 11.10(c)[8.02–34.31]p<0.0003 |
T3, triiodothyronine; T4, thyroxine; TSH, thyroid stimulating hormone; TRAb, anti-TSH receptor antibodies; ATG, anti-thyroglobulin antibodies; ATPO, anti-thyroid peroxidase antibodies. Data are median and interquartile range. (a,c)Differ from the healthy subjects; (b)Differ from newly diagnosed Graves’ disease patients.
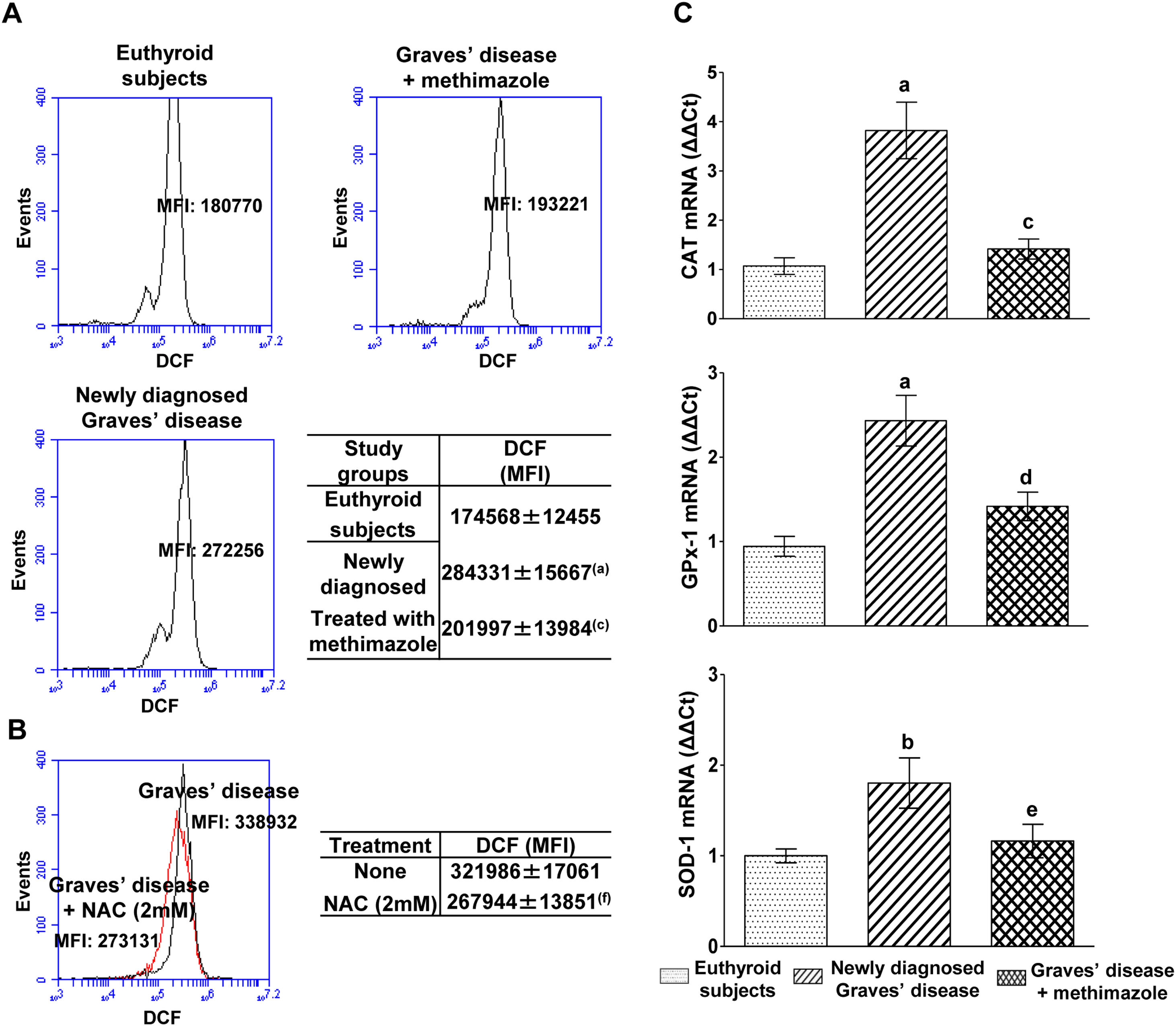
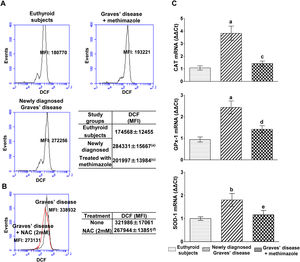
PBMC from patients with untreated Graves’ disease showed higher levels of ROS compared to euthyroid subjects (Fig. 1A). In vitro treatment of these cells with a glutathione synthesis precursor, N-acetyl-cysteine (NAC, 2mM) for 24h reduced intracellular ROS levels (Fig. 1B). Patients with Graves’ disease treated with methimazole for more than two months and who restored their hormonal values to euthyroidism, showed ROS levels in PBMC similar to those of healthy subjects (Fig. 1A).
Effects of hyperthyroidism on the induction of ROS and the genomic expression of antioxidant enzymes. Quantification of ROS in PBMC from healthy subjects, patients with newly diagnosed Graves’ disease or treated with methimazole (A), or in PBMC from patients with newly diagnosed Graves’ disease incubated with NAC (2mM) for 24h (B). The mean fluorescence intensity (MFI) of DCF±ES is indicated in the tables. The mRNA expression of CAT, GPX-1 and SOD-1 was quantified by real-time PCR (C). Newly diagnosed Graves’ disease vs euthyroids p<0.001(a) or p<0.05(b); Treated with methimazole vs newly diagnosed Graves disease p<0.001(c), p<0.01(d) or p<0.05(e); Treated with NAC vs no treatment p<0.01(f).
We analysed the genomic expression of CAT, GPx-1 and SOD-1 to evaluate the enzymatic antioxidant capacity of cells in counteracting the increase in ROS induced by hyperthyroidism. Patients with newly diagnosed Graves’ disease showed increased levels of mRNA expression of CAT (3.82±1.28-fold increase), GPx-1 (2.43±0.67-fold increase) and SOD-1 (1.80±0.62-fold increase) compared to euthyroid subjects. Patients with Graves’ disease treated with methimazole showed mRNA expression levels of antioxidant enzymes similar to those of healthy subjects (Fig. 1C).
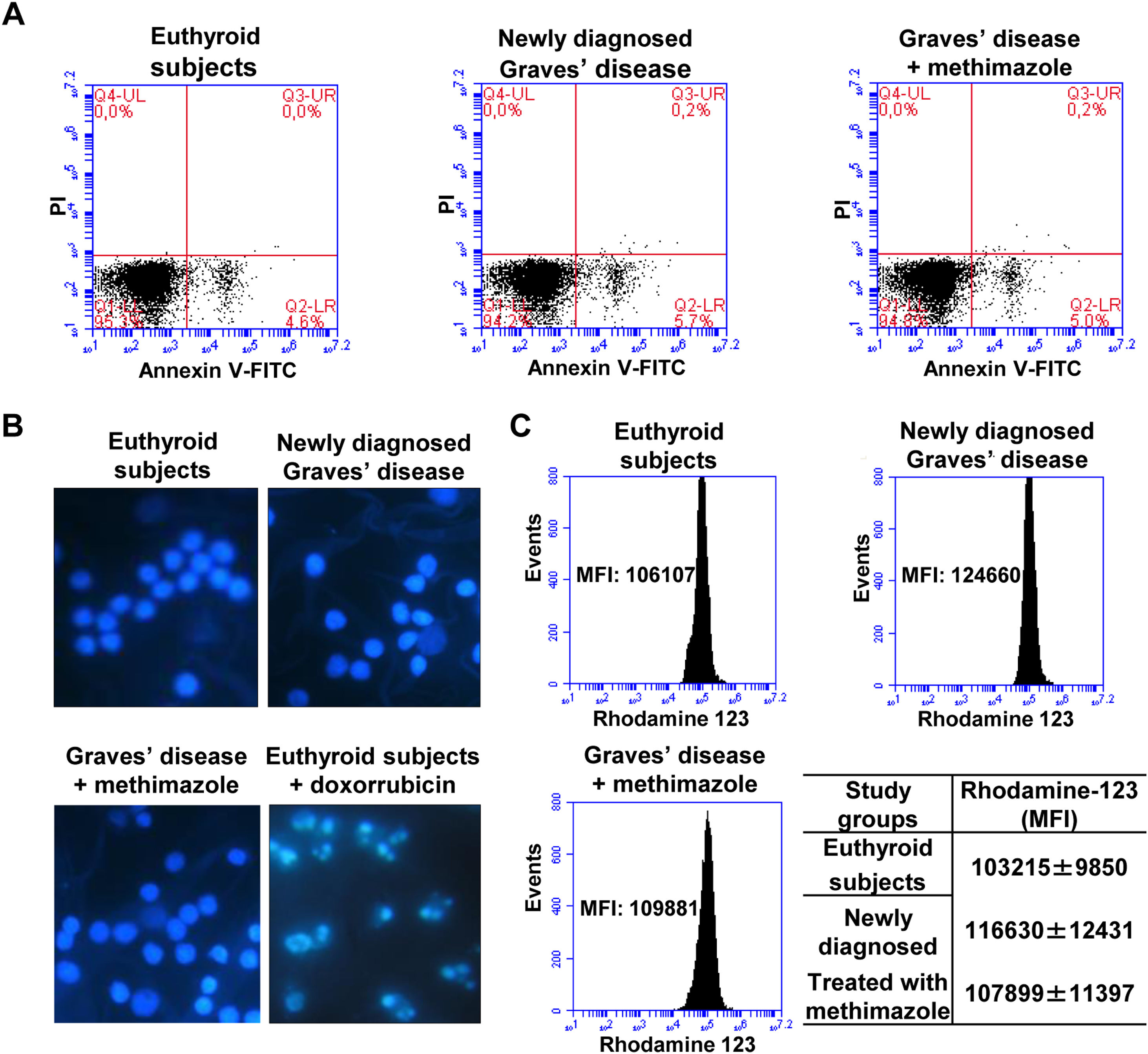
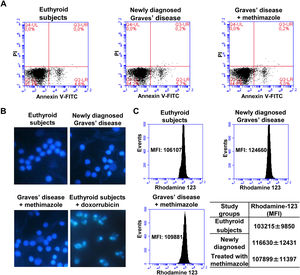
Effects of oxidative stress on the viability of PBMCWe analysed the percentage of viable cells, in early apoptosis, late apoptosis, or necrosis in PBMC from patients with Graves’ disease (newly diagnosed or treated with methimazole) and euthyroid subjects (Fig. 2A). The percentage of viable cells (greater than 94%) and in early apoptosis stage (approximately 5%) was similar in all study groups and there was no presence of cells in late apoptosis or necrosis. To corroborate the absence of apoptosis, we stained the PBMC with Hoeschst 33258 and analysed the nuclear morphology by fluorescence microscopy (Fig. 2B). PBMC from patients with newly diagnosed Graves’ disease or treated with methimazole exhibited intact nuclei without chromatin condensation or nuclear fragmentation and a similar appearance to cells from healthy individuals. PBMC from healthy individuals were treated with doxorubicin (4μM) for 8h and used as a positive control of apoptosis. Furthermore, rhodamine-123 staining showed that the mitochondrial membrane potential was not altered in PBMC from patients with Graves’ disease (Fig. 2C).
Effects of oxidative stress on the viability of PBMC. PBMC were labelled with annexin V-FITC and PI and the percentage of viable cells in apoptosis or necrosis was analysed by flow cytometry (A). PBMC were stained with Hoeschst 33258 and the nuclear morphology was analysed by fluorescence microscopy. PBMC treated with doxorubicin (4μM) for 8h were used as a positive control of apoptosis (B). PBMC were incubated with rhodamine 123 and the mitochondrial membrane potential was quantified by flow cytometry (C). The mean fluorescence intensity (MFI) of rhodamine 123±ES is indicated in the table.
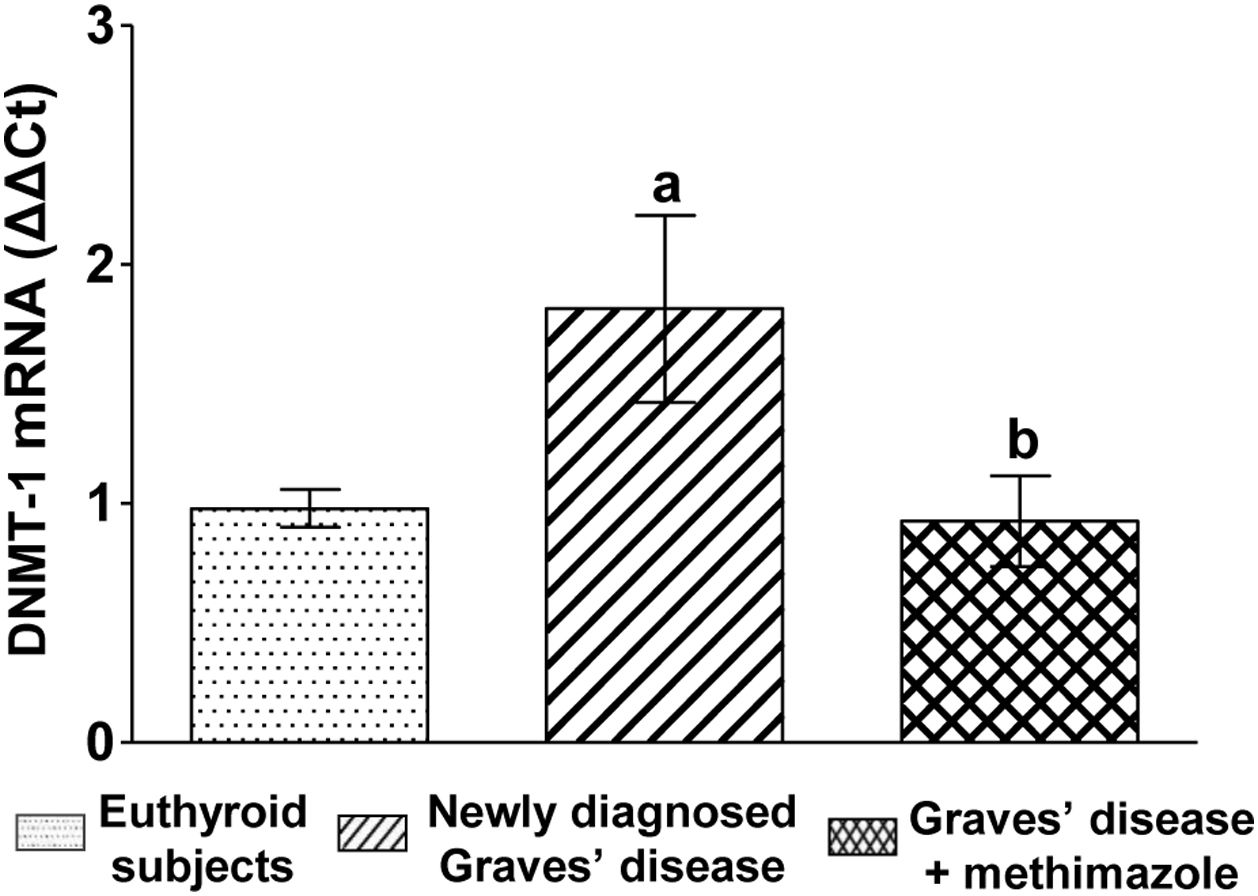
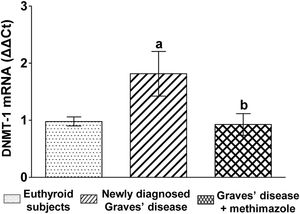
We analysed the genomic expression of DNMT-1 to evaluate the modulation of autoimmune hyperthyroidism on enzymes that participate in epigenetic processes. Patients with newly diagnosed Graves’ disease showed increased levels of DNMT-1 mRNA expression compared to euthyroid subjects (1.81±0.87-fold increase). Patients with Graves’ disease who restored their hormonal values to euthyroidism by treatment with methimazole and also presented lower levels of ROS, showed a lower genomic expression of DNMT-1 than untreated patients (Fig. 3).
DiscussionWe first investigated the role of the hyperthyroid state on the induction of oxidative stress in PBMC from patients with Graves’ disease. The results show a significant increase in ROS in PBMC of patients with newly diagnosed Graves’ disease, while the patients treated with methimazole showed ROS levels similar to those of euthyroid individuals. This may be the result of decreased plasma levels of thyroid hormones and recovery from euthyroidism, but the antioxidant effect of thiamazole should also be considered.15 We found that in vitro treatment of PBMC from patients with Graves’ disease with NAC (2mM) significantly reduced intracellular ROS levels after 24hours of culture due to its action as precursor to reduced glutathione synthesis or as direct scavenger of ROS.16 In Graves’ disease, an increase in ROS has been reported in the thyroid, blood tissue, and periorbital tissue of patients with orbitopathology,5–8 but the increase in ROS in PBMC has not been previously described.
Since we found that hyperthyroidism increases ROS levels in PBMC, we decided to analyse the genomic expression of the antioxidant enzymes CAT, GPx-1, and SOD-1. The mRNA expression of the three enzymes was increased in PBMC from patients with newly diagnosed Graves’ disease and restoration to euthyroidism by treatment with methinazole showed genomic expression levels similar to those of healthy subjects. Some authors have found a significant increase in the activity of SOD, CAT or GPx in red blood cells17 or plasma from patients with untreated Graves’ disease.5,17–19 Conversely, Bednarek et al. and Lassoued et al. found lower GPx activity in plasma from patients with newly diagnosed Graves’ disease compared to healthy subjects.18,19 Some studies have analysed the activity of antioxidant enzymes in patients with different duration of hyperthyroid state. Thus, Abalovich et al. found a decrease in SOD and CAT activities in red blood cells of patients with Graves’ disease with more than 6 months of hyperthyroidism.20 Aslan et al. also reported a decrease in total antioxidant capacity in serum of patients with Graves’ disease with more than 2.3 months of hyperthyroidism, and a positive correlation between the duration of hyperthyroidism and the degree of oxidative stress.21 In contrast, Bednarek et al. found an increase in SOD and CAT activity in plasma from patients with Graves’ disease with less than 2 months of hyperthyroidism.22 The discrepancies found between the authors may be due to the duration of the hyperthyroid status at the time of diagnosis of the disease. Despite these discrepancies, restoration to euthyroid status of patients by methimazole treatment is associated with a reversal of the abnormalities of the antioxidant defense system.17,20,22
Numerous studies have reported oxidative damage to lipids5,8,17,18 and plasma proteins,4 as well as DNA oxidation8 in PBMC from patients with newly diagnosed Graves’ disease. We analysed the effect of oxidative stress on the viability of PBMC from patients with newly diagnosed Graves’ disease or treated with methimazole. Flow cytometry analysis showed no evidence of apoptosis or necrosis in PBMC of all study groups and showed a high percentage of cell viability that correlates with an unaltered mitochondrial membrane potential. In addition, analysis by microscopy confirmed the absence of morphological changes compatible with apoptosis. In agreement with our results, Mihaljevic et al. found a lower percentage of apoptosis in lymphocytes from patients with Graves’ disease compared to healthy subjects, despite finding greater genomic instability in these cells.23 Similarly, Klatka et al. found a lower percentage of apoptosis in lymphocytes from patients with Graves’ hyperthyroidism, which increased after restoration to euthyroidism by treatment with methimazole. These authors also found that the number and subgroups of white blood cells do not differ between Graves’ disease patients and healthy individuals.24 These results, together with ours, demonstrate that excess circulating thyroid hormones do not induce apoptosis of PBMC. The absence of apoptosis in the PBMC from patients with Graves’ hyperthyroidism may be attributed to alterations in apoptotic signalling pathways or a lower intensity of Fas expression in these cells.25 Conversely, increased apoptosis has been found in lymphoid cell lines or in peripheral blood T lymphocytes treated in vitro with thyroid hormones for different times.26,27 Furthermore, increased apoptosis was found in PBMC from patients with untreated Graves’ disease after 24h of in vitro culture.27 Hara et al. found similar results, but they did not find significant differences in PBMC survival between untreated Graves patients and euthyroid controls at the beginning of the culture.28 These findings suggest that thyroid hormones have the potential to induce cell death of human PBMC in vitro. However, our results seem to reveal that thyroid hormones also exert a negative or even no influence on cellular apoptosis in vivo. More studies are required to elucidate the mechanisms of action of thyroid hormones that may explain the observed differences.
Epigenetic alterations in immunoregulatory genes have been found in several autoimmune diseases, including Graves’ disease29,30. However, the role of epigenetic mechanisms in the pathogenesis of Graves’ disease has not yet been elucidated. We found a higher expression of the DNMT-1 enzyme in the PBMC from patients with newly diagnosed Graves’ disease. The expression of the DNMT-1 in PBMC from patients who achieved euthyroidism by treatment with methimazole was similar to that of healthy subjects and was accompanied by a decrease both in oxidative stress in PBMC and in serum thyroid hormones, while the autoantibodies ATPO, ATG, and TRAb in the blood of these patients remained elevated.
Several authors found a strong association between high ROS levels and epigenetic alterations in some autoimmune diseases.31,32 Thyroid hormones have also been shown to regulate gene expression and immune response in lymphocyte cells,33 as well as being able to induce epigenetic alterations.34,35 Considering these previous reports and our results, the participation of oxidative stress or thyroid hormones, or both together, may be responsible for the regulation of DNMT-1 enzyme expression.
In agreement with our data, Limbach et al. found, in CD4+ and CD8+ cells from patients with Graves’ disease, hypermethylated CpG sites in genes involved in T cell signalling.36 Other authors have found both hypermethylation30,37 and hypomethylation30,37,38 in genes involved in the immune and inflammatory response in peripheral blood leukocytes from patients with Graves’ disease.
In contrast to our results, Guo et al. found global DNA hypomethylation and lower DNMT-1 expression in T and B lymphocytes purified from peripheral blood from Caucasian male and female patients with newly diagnosed Graves’ disease.39 Similar results were obtained by Cai et al. using PBMC from Asian female and male patients, and also demonstrated that the degree of global DNA methylation was influenced by the gender of the patients studied.30 In the current study, we found an increase in DNMT-1 expression in PBMC from Caucasian female patients with newly diagnosed Graves’ disease. The discrepancies found may be attributed to differences in the inclusion criteria of the patients, the heterogeneity of the cell types analysed, the ethnic variability of the study populations and the duration of the hyperthyroidism at the time of diagnosis.
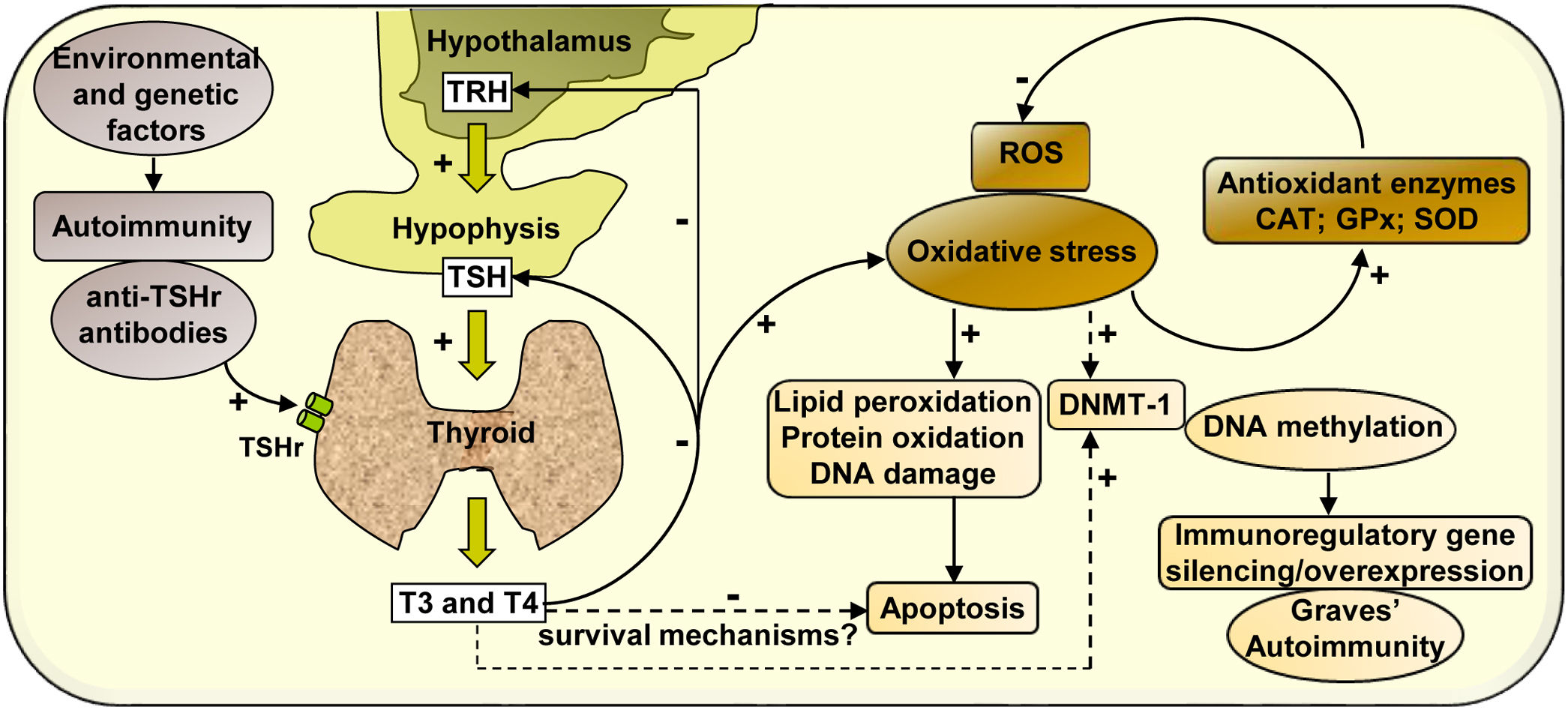
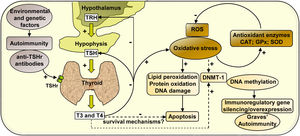
In summary, our study analyses the effect of hyperthyroidism on the regulation of the cellular redox state and the modulation of the enzyme DNMT-1, whose activity may activate or silence the expression of immunoregulatory genes, contributing to the development of autoimmunity in Graves’ disease (Scheme 1).
Mechanisms of action involved in the regulation of the cellular redox state and the expression of DNMT-1 mediated by hyperthyroidism in PBMC from patients with Graves’ disease. Graves’ disease is an autoimmune disorder characterised by the presence of antibodies against thyroid antigens. The interaction between stimulating autoantibodies and the TSHr in the thyroid induces an excessive activity of the gland that manifests with the overproduction of T3 and T4. High circulating levels of thyroid hormones produce a negative feedback on the pituitary, causing the inhibition of TSH secretion. High levels of circulating thyroid hormones affect the cellular metabolism in most tissues and induce an increase in ROS that leads to the oxidation of numerous macromolecules (lipids, proteins and DNA) and may affect cell viability. However, there may be hyperthyroidism-mediated survival mechanisms that may protect PBMC from apoptosis. The increased expression of antioxidant enzymes (CAT, GPx-1 and SOD-1) contributes to diminish the levels of oxidative stress generated by hyperthyroid conditions. In another way, thyroid hormones, ROS, or both, may modulate the expression of the enzyme DNMT-1, responsible for DNA methylation, causing the silencing or overexpression of immunoregulatory genes and contributing to the pathogenesis of Graves’ disease.
No potential conflict of interest relevant to this article was reported.
This work was supported by the National Agency for Scientific and Technological Promotion (PICT-2016-0696) and the Florencio Fiorini Foundation.