Bariatric surgery (BS) is effective in improving chronic joint pain (CJP). However, the long-term effects on this comorbidity are poorly understood.
ObjectivesTo determine the prevalence of CJP in a sample of patients who had undergone BS with a minimum follow-up of 18 months. To determine whether or not there was any relationship between CJP and clinical or psychological outcomes after BS.
Material and methodsCross-sectional study. The Lattinen index (LI) was used to evaluate CJP, using the cut-off point of 10 to define significant CJP (SCJP).
ResultsOf the 110 subjects assessed, 31.2% (35/110) had SCJP. The patients with SCJP were older (57.4±13 vs 47.8±11.6 years; p<0.0001) and more time had elapsed since their BS (105.6±54.3 vs 78.5±39 months; p=0.023). The last BMI was higher in subjects with SCJP (35±5 vs 33.3±6.9kg/m2; p=0.05) and the percentage of patients who took significant regular exercise was lower (2.9% vs 68%; p<0.0001). Trauma problems after BS were more common in subjects with SCJP (61.8% vs 22.7%; p<0.0001). More patients with SCJP met depression criteria (47.1% vs 5.3%; p<0.0001) and/or were treated with antidepressants (38.2% vs 17.3%; p=0.003). Patients with SCJP reported fewer hours of sleep (6±1.4 vs 6.8±1.2h; p=0.003).
ConclusionsSCJP is highly prevalent in patients who have had BS once they reach the weight plateau phase. There is an association between having SCJP and worse psychological and functional status, with potential detrimental metabolic effects.
La cirugía bariátrica (CB) resulta eficaz en la mejoría del dolor crónico articular (DC); sin embargo, sus efectos a largo plazo sobre esta comorbilidad son poco conocidos.
ObjetivosDeterminar la prevalencia de DC en una muestra de pacientes intervenidos de CB con un seguimiento mínimo de 18 meses. Analizar si existe relación alguna entre el DC y los resultados clínicos o psicológicos tras la CB.
Material y métodosEstudio transversal. Se utilizó el índice de Lattinen (IL) para evaluar el DC, utilizando el punto de corte de 10 para definir DC significativo (DCS).
ResultadosDe los 110 sujetos evaluados un 31,2% (35/110) presentaban DCS. Los pacientes con DCS eran mayores (57,4±13 vs. 47,8±11,6 años; p<0,0001) y con un mayor tiempo desde la CB (105,6±54,3 vs. 78,5±39 meses; p=0,023). El IMC último era superior si existía DCS (35±5 vs. 33,3±6,9kg/m2; p=0,05) y el porcentaje de pacientes con ejercicio habitual significativo era inferior (2,9% vs. 68%; p<0,0001). La presencia de problemas traumatológicos tras CB era mayor en los casos de DCS (61,8% vs. 22,7%; p<0,0001). Existía un mayor porcentaje de pacientes con DCS con criterios de depresión (47,1% vs. 5,3%; p<0,0001) y/o tratados con antidepresivos (38,2% vs. 17,3%; p=0,003). Las horas de sueño referidas eran inferiores con DCS (6±1,4 vs. 6,8±1,2 horas; p=0,003).
ConclusionesLa prevalencia de DCS en pacientes sometidos a CB una vez alcanzada la fase meseta del peso es altamente prevalente. Existe una asociación entre la presencia de DCS y un peor estado psicológico y funcional, con un potencial detrimento metabólico.
The incidence of obesity has increased dramatically in recent decades and it has now become a major global health challenge.1 Alongside metabolic and psychological comorbidities, severe obesity is also associated with significant joint pain as well as with impaired physical function.2 Excess weight can lead to joint damage and pain, resulting in reduced activity.3 Obesity can also contribute to pain and physical impairment through factors such as deficient cardiorespiratory function,4 low grade systemic inflammation,5 low strength per body mass,6 and mood disorders.7 The lumbar spine and knee are the main two load-bearing sites for pain location.8 In addition to the overload on musculoskeletal structures caused by the excess weight, obesity can cause and maintain chronic joint pain due to an upregulation of adipokines, which leads to a low-grade inflammation.9
Obesity is responsible not only for metabolic complications and chronic pain but also for mood disorders. The incidence of depression is much higher among individuals with obesity, with epidemiological studies showing prevalence rates ranging from 5% to 23% and up to 31.5% among patients with obesity seeking bariatric surgery (BS).10 Moreover, chronic pain increases the risk for depression between 2.5 and 4.1 times.11 Additionally, patients with a major depression are three times more likely to suffer from non-neuropathic pain and six times more likely to suffer from neuropathic pain.12 These data support the hypothesis of a chronic low-grade inflammation as a common pathogenic factor for the three conditions.9
BS is effective at achieving weight loss and reducing many of the metabolic and psychologic comorbidities.13 However, although the evidence of improvements in pain and physical function has increased in recent years, the durability and degree of this improvement is still controversial.14 Furthermore, there is even less evidence of pain improvement once weight loss has reached a plateau, in the long term after BS.9,14
On the other hand, the tools used to measure pain and impaired physical function have not been validated pain-specific instruments. Instead, many studies have assessed pain and physical dysfunction using the bodily pain domain of the SF-36 (Medical Outcomes Study 36-Item Short Form Health Survey).8,14–16
The aim of our study was to analyse the frequency of clinically significant joint pain among subjects who underwent BS, once weight loss had reached a plateau. Also, we aimed to assess the influence of significant joint pain on clinical and biochemical outcomes in this BS sample. Finally, we aimed to specifically explore the relationship between chronic joint pain and depressive symptoms among this BS sample.
MethodsPatientsPatients with a minimum follow-up of 18 months after undergoing a sleeve gastrectomy (performed between 2013 and 2017) were consecutively invited to participate in this study and a total of 110 subjects were included. Sleeve gastrectomy involves the resection of a large portion of the stomach along the greater curvature without interfering with innervation or with the passage of food. It is a widely accepted bariatric procedure due to relatively better outcomes in terms of weight loss in the short and medium term, its relative operational simplicity, and its low operation risk. All operations were performed by two trained surgeons using the same surgical procedure. Exclusion criteria were the same as for individuals included in our bariatric surgery protocol: AIDS, an active neoplasm, or any medical or psychiatric disease that could interfere with the outcomes of the surgical procedure. The study was approved by the CEI-IB, the ethics committee of the hospital. Written informed consent was obtained from all patients prior to study participation.
Assessment of chronic joint painJoint pain intensity and interference with everyday activities were assessed using the Lattinen index (LI). The LI is an instrument widely used for the measurement of subjects who experience some kind of chronic pain which has been approved for use in Spain. This is a self-report 5-item scale designed to measure pain features over the past month using a Likert scale. Individuals answer five questions based on a 0–4 scale, indicating the frequency, intensity, regular use of drugs for pain relief, and degree of impairment in everyday activities.17,18 Also, an additional question about the mean number of hours of sleep per day is also reflected in the questionnaire. This test is shown in Table 1.
Lattinen index.
| Pain intensity | Null | 0 |
| Light | 1 | |
| Annoying | 2 | |
| Intense | 3 | |
| Unbearable | 4 | |
| Pain frequency | Do not | 0 |
| Rarely | 1 | |
| Frequent | 2 | |
| Very frequent | 3 | |
| Continuous | 4 | |
| Analgesic consumption | Do not | 0 |
| Occasionally | 1 | |
| Regular and few | 2 | |
| Regular and many | 3 | |
| Very many | 4 | |
| Disability | Do not | 0 |
| Light | 1 | |
| Moderate | 2 | |
| Help needed | 3 | |
| Total | 4 | |
| Sleeping quality | As usual | 0 |
| Something worse than usual | 1 | |
| He wakes up frequently | 2 | |
| Less than 4h | 3 | |
| Accurate hypnotics | 4 |
To rule out depressive syndrome, all participating patients rated the presence and severity of depressive symptoms by using the Spanish version of the Beck Depression Inventory (BDI), a 21-item questionnaire that assesses mood over the previous month.19,20 Total scores range from 0 to 63, with higher scores indicating greater symptoms of depression. The BDI has been widely used as a screening tool for major depression in the general population. In this setting, a cutoff score equal to or greater than 13 is indicative of significant depression. For obesity, as for diabetes, a BDI score of ≥16 was considered positive for significant depressive symptoms because this cut-off exhibited the best balance between sensitivity and positive predictive value.20
Height and weightHeight and weight were measured while each participant was wearing indoor clothing without shoes. Body mass index (BMI) was calculated as weight divided by height squared. Significant weight regain was defined as an increase of more than 10% of the minimum weight loss achieved.
Metabolic and inflammatory profileBlood samples were drawn for the following analyses: blood count, coagulation, fasting glucose, glycated haemoglobin (HbA1c), total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, apolipoproteins, ionogram, plasma creatinine, hepatic profile, albumin, prealbumin, 25OH vitamin D, Vitamins A, E, B1, B12, C and folic acid, zinc and selenium, serum cortisol, leptin, insulin, and thyroid profile. Inflammatory markers used for assessing changes in the low-grade inflammatory state were: C-reactive protein, homocysteine, ferritin, erythrocyte sedimentation rate, fibrinogen, and platelet count. First void urine samples were collected to determine random albumin-to-creatinine ratio. All measurements were made at 8:00 a.m. after an overnight fast of at least 8h.
Other assessmentsWe obtained initial weight and BMI from computerised medical history. Comorbidities related to obesity prior to surgery, such as diabetes, hypertension, dyslipidemia, sleep apnea syndrome, hyperuricemia, orthopedic comorbidities (which include osteoarthritis and musculoskeletal and/or bone problems associated with mobility impairment or impaired daily activity), as well as presurgical psychiatric conditions were also recorded.
Statistical analysesInitial analyses were descriptive and included calculation of mean and standard deviation (SD) for continuous variables and as frequencies for categorical variables. The distribution of the sample was analysed by the Kolmogorov–Smirnov test and normal probability plots. Patients were divided in two groups according LI. Comparison between the two groups was analysed by an unpaired Student's test for variables with a normal distribution and the Mann–Whitney U test for the variables without a normal distribution. Categorical data were compared by a Chi-square test or Fisher exact test. ROC curves of quantitative risk factors associated with positive LI were performed. The optimal cut-off values were determined by the maximum Youden index (J), defined as sensitivity+specificity−1. Binary logistic regression models were used to identify risk factors associated to positive LI, with negative LI as the reference (odds ratio [OR]=1). Analysis was performed using the Backward stepwise regression method.
A p value <0.05 on the two-tailed test was considered to indicate statistical significance. Data were analysed using SPSS version 24 statistical software (SPSS Inc., Chicago, IL, USA).
ResultsAfter taking the LI, out of 110 subjects, 35 (31.8%) had a score equal to or greater than 10, whereas the remaining 75 patients did not report significant joint pain.
When we compared sociodemographic variables, subjects with a pathological LI were older compared with patients with a negative LI (57.4±13 vs. 47.8±11.6 years; p<0.00001). On the other hand, both groups were comparable in terms of gender, employment situation, or emotional status.
Moreover, subjects with a positive LI had a longer duration since BS compared with patients without significant pain (105.6±54.3 vs. 78.5±39 months; p=0.023). Although both groups were comparable in terms of baseline BMI, minimum weight loss achieved, %EWL, and in the proportion of patients with significant weight regain, subjects with significant joint pain had a greater BMI at the time of the evaluation (35±5.1 vs. 33.3±6.9kg/m2; p=0.05).
When we took comorbidities related to obesity into account, we found that, despite the two groups having no significant differences regarding presurgical orthopedic problems, long-term postsurgical orthopedic comorbidities were more frequent among patients with a positive LI (61.8% vs. 22.7%; p<0.00001). We did not find any other differences with other either presurgical or postsurgical complications associated with weight (type 2 diabetes, dyslipidemia, hypertension, and obstructive sleep apnea syndrome). Moreover, the frequency of admissions after BS was also comparable between the two groups.
The proportion of subjects who smoked or had a consumption of more than 20g of alcohol per day was also comparable between both groups. Furthermore, no differences were seen between the two groups regarding both the standardised follow-up visits according to our hospital's protocol and treatment adherence.
Conversely, when we assessed lifestyle habits, the proportion of subjects who reported more than 150min of aerobic exercise per week was lower among patients with a positive LI (2.9% vs. 68%; p<0.00001). However, we could not find differences between the two groups regarding the proportion of macronutrients in the diet.
These data are summarised in Table 2.
Comparison of sociodemographic features, months since BS, anthropometric variables and comorbidities related to obesity among subjects with criteria for FA and individuals without it.
| All subjects (n=110) | Positive LI (n=35) | Negative LI (n=75) | p | |
|---|---|---|---|---|
| Gender (male/female) (%) | 27.5/72.5 | 17.6/82.4 | 32/68 | NS |
| Age (years) | 50.8±12.8 | 57.4±13 | 47.8±11.6 | 0.00001 |
| Months since BS | 87±45.9 | 105.6±54.3 | 78.5±39 | 0.023 |
| BMI (baseline, kg/m2) | 46.9±6.8 | 47.6±7.6 | 46.7±6.4 | NS |
| BMI (current, kg/m2) | 33.8±6.4 | 35±5.1 | 33.3±6.9 | 0.05 |
| Minimum weight achieved (kg) | 85.1±16.6 | 83.1±14.3 | 86±17.6 | NS |
| %EBL | 29.3±10.9 | 27±9.4 | 30.4±11.4 | NS |
| Tobacco use (%) | 15.6 | 17.6 | 14.7 | NS |
| Alcohol (%) | 6.4 | 5.9 | 6.7 | NS |
| T2DM (preBS, %) | 19.3 | 26.5 | 16 | NS |
| T2DM (current, %) | 14.7 | 14.7 | 14.7 | NS |
| Insulin use (preBS, %) | 5.5 | 5.9 | 5.3 | NS |
| Hypertension (preBS, %) | 45.9 | 50 | 44 | NS |
| Hypertension (current, %) | 11.5 | 11.8 | 10.7 | NS |
| Dyslipidemia (preBS, %) | 33 | 35.3 | 32 | NS |
| Dyslipidemia (current, %) | 28.4 | 32.4 | 26.7 | NS |
| Orthopedic problems (preBS, %) | 40.4 | 52.9 | 36.1 | NS |
| Orthopedic problems (current, %) | 34.9 | 61.8 | 22.7 | 0.00001 |
| Sleep apnea (preBS, %) | 63.3 | 67.6 | 61.3 | NS |
| Sleep apnea (current, %) | 31.2 | 41.2 | 27 | NS |
| Admissions after BS (%) | 52.3 | 64.7 | 46.7 | NS |
Data are mean±SD or %. BMI, body mass index. LI, Lattinen index. %EBL, percent excess BMI loss. T2DM, type 2 diabetes.
As shown in Table 3, when we looked at the biochemical parameters, we did not find any differences in metabolic or nutritional values between the two groups.
Comparison of biochemical parameters among subjects with a positive LI and individuals without it.
| All subjects (n=110) | Positive LI (n=35) | Negative LI (n=75) | p | |
|---|---|---|---|---|
| HbA1c (%) | 5.5±0.9 | 5.8±1.3 | 5.3±0.6 | NS |
| Fasting plasma glucose (mg/dl) | 94.5±25 | 100.3±39.7 | 91.7±13 | NS |
| Plasma creatinine (mg/dl) | 0.7±0.2 | 0.7±0.1 | 0.8±0. 3 | NS |
| Total cholesterol (mg/dl) | 182.7±35.8 | 183±34.2 | 182.6±36.8 | NS |
| LDLc (mg/dl) | 111.6±29.6 | 109.6±28.5 | 112.6±30.2 | NS |
| HDLc (mg/dl) | 51.8±13.1 | 52.1±12.5 | 51.6±13.4 | NS |
| Triglycerides (mg/dl) | 97.4±44.1 | 104.4±47.2 | 95±42.6 | NS |
| AST (U/L) | 20.5±33.2 | 17.8±4.7 | 21.8±40.2 | NS |
| ALT (U/L) | 18.5±13 | 17.9±8 | 18.8±14.8 | NS |
| GGT (U/L) | 18.7±13.1 | 19.7±17 | 18.2±10.9 | NS |
| TSH (μUI/mL) | 1.8±0.9 | 1.9±1.1 | 1.7±0.8 | NS |
| Calcium (mg/dl) | 9.3±0.4 | 9.3±0.4 | 9.3±0.4 | NS |
| Magnesium (mg/dl) | 2.2±1 | 1.9±0.1 | 2.3±1.2 | NS |
| 25OHVitD (ng/ml) | 24.2±13.1 | 24.1±12.6 | 24.2±13.5 | NS |
| Iron (μg/dl) | 83.2±35.4 | 78±27.5 | 85.8±38.7 | NS |
| Ferritin (ng/ml) | 45.4±38 | 32±25.1 | 51.8±44.8 | NS |
| Transferrin (mg/dl) | 275.2±48.5 | 274.1±42.9 | 275.8±51.5 | NS |
| Transferin Saturation (%) | 22.8±10.3 | 21.2±7.7 | 23.6±4.2 | NS |
| Folate (ng/ml) | 8.6±4.2 | 9.4±4.6 | 7.6±3.7 | NS |
| Cianocobalamin (pg/ml) | 384.4±361.2 | 459±310 | 350.5±230.8 | NS |
| Prealbumin (mg/dl) | 24.5±8.3 | 21.6±4.2 | 26.7±10.5 | NS |
| Uric acid (mg/dl) | 5.1±1.5 | 4.9±1.8 | 5.1±1.4 | NS |
Data are mean±SD or %. BMI, body mass index. LI, Lattinen index. GFR, glomerular filtration rate.
Regarding psychological outcomes, we found relevant differences between subjects with significant joint pain compared with subjects without this condition. Subjects with a positive LI had higher scores in the BDI test compared with patients with a LI below 10 (15.1±9.9 vs. 5.6±6.4; p<0.00001). Therefore, the proportion of patients either having a diagnosis of depressive disorder or taking antidepressants at the time of the evaluation was greater among subjects with a pathological LI (41.7% vs. 5.3% and 38.2% vs. 17.3%; p<0.00001 and p=0.003, respectively).
Moreover, there were significant differences between the two groups regarding the scores obtained in all different compounds of the BDI, either psychological-cognitive or negative or somatic-biological symptoms (6.4±5.7 vs. 1.4±3.6, 1.4±0.8 vs. 0.3±0.5, 7.7±4.1 vs. 3.5±3.2, p<0.0001, p<0.0001 and p<0.0001, respectively).
Finally, when we looked at the sleeping patterns, subjects with a LI equal to or greater than 10 reported fewer sleeping hours in comparison with patients with a non-pathological LI (6±1.4 vs. 6.8±1.2h; p=0.003). All these variables are summarised in Table 4.
Comparison of adherence to healthy lifestyle habits, pharmacologic treatment and follow-up and psychological variables among subjects with a positive LI and patients without significant chronic joint pain.
| All subjects (n=110) | Positive LI (n=35) | Negative LI (n=75) | p | |
|---|---|---|---|---|
| Subjects with non-adherence to protocol (%) | 29.4 | 29.4 | 29.3 | NS |
| Subjects with non-adherence to pharmacologic treatment (%) | 18.3 | 20.6 | 17.3 | NS |
| Subjects with adherence to prescribed diet (%) | 65.1 | 68.8 | 65.3 | NS |
| More than 150min/week exercise (%) | 47.7 | 2.9 | 68 | 0.00001 |
| Depressive disorder (%) | 18.3 | 34.4 | 11.8 | 0.00001 |
| Antidepresant use (%) | 23.9 | 38.2 | 17.3 | 0.003 |
| BDI global score | 8.6±8.8 | 15.1±9.9 | 5.6±5.4 | 0.0001 |
| Psychological cognitive subscore | 3.3±4.8 | 6.4±5.7 | 1.9±3.6 | 0.0001 |
| Negative emotions subscore | 0.7±1.1 | 1.4±0.8 | 0.3±0.5 | 0.0001 |
| Somatic/biological subscore | 4.8±4 | 7.7±4.1 | 3.5±3.2 | 0.0001 |
| Sleep hours | 6.5±1.3 | 6±1.4 | 6.8±1.2 | 0.003 |
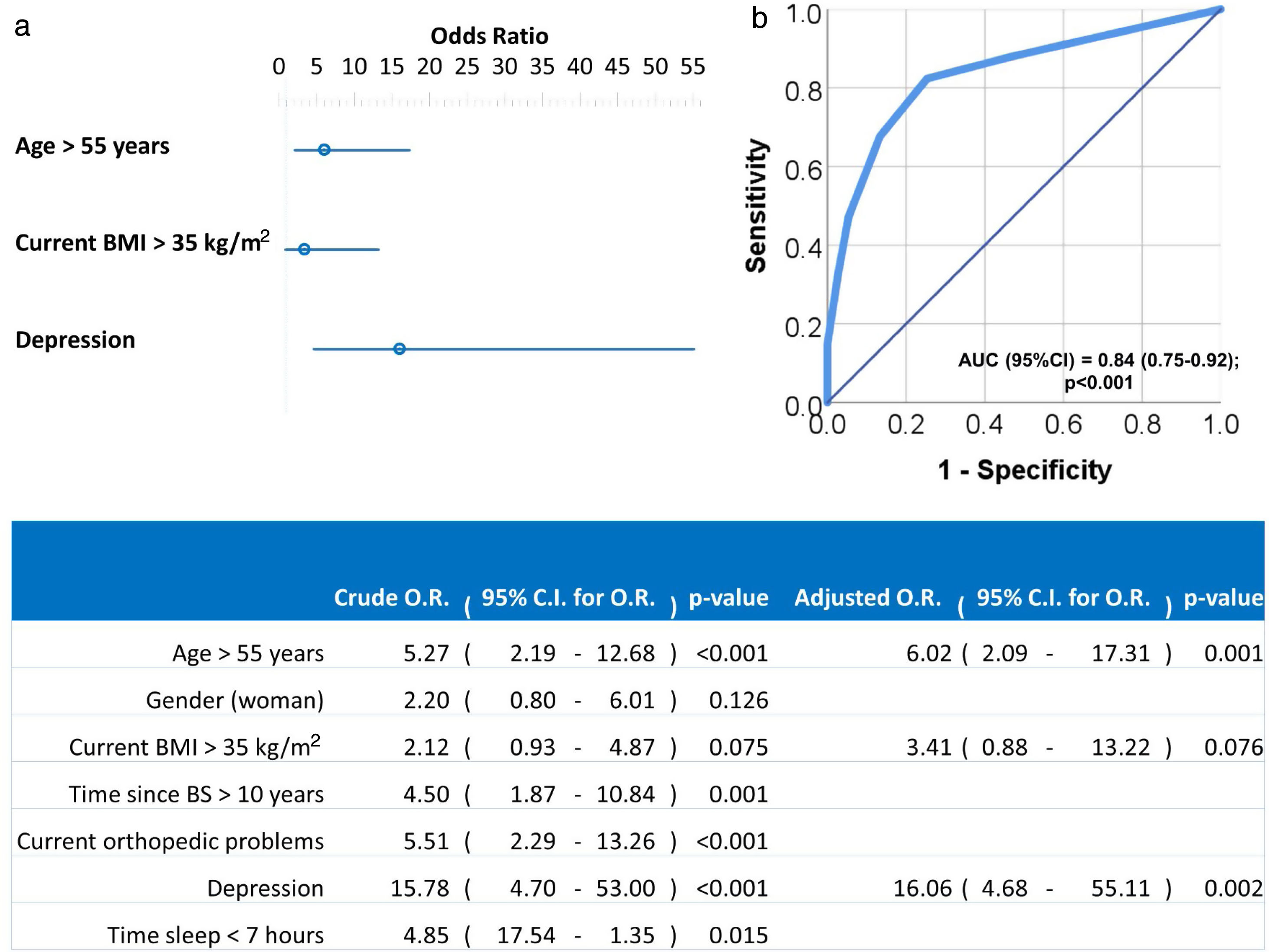
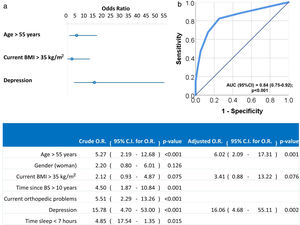
Univariate and multivariate logistic regression analysis were used to investigate independent factors associated to positive LI. All previously listed factors (p<0.05 in Tables 2 and 3) were included initially in the model before stepwise and backward elimination. The final model included age above 55 years old, current BMI greater than 35kg/m2 and diagnosis of depression as the independent risk factors associated with positive LI (Fig. 1).
Forest plot (a) and ROC curve (b) of the multivariate logistic regression of risk factors associated to LI. Multivariate analysis was performed using stepwise backward method. Crude and adjusted Odds Ratios (O.R.) are indicated in the table. Comparison of the expected and observed frequencies by the Hosmer–Lemeshow goodness-of-fit test (p-value=0.949) and by ROC curve (AUC=0.84; p<0.001) indicated a good fit for the model.
Our study aimed to find out the frequency of significant joint pain among a BS sample in the long-term. In addition, we wanted to assess whether the presence of pain among these subjects had some influence on clinical or psychological parameters.
We found that significant joint pain was present in 31.8% of the sample. The prevalence of musculoskeletal pain in at least one region of the body among patients evaluated before BS is almost 100%21,22 compared with rates of moderately intense pain reported by European people of 20%.23 However, obesity surgery has been shown to reduce the presence of musculoskeletal problems, at least at short-term, up to three years postoperatively. One year after BS, most participants have clinically meaningful presurgery to postsurgery improvements for bodily pain and physical function, which has been assessed using the SF-36 health-related quality of life survey. Not only have the majority of patients’ subjective amelioration in pain but also in disability among individuals with a mobility deficit at baseline.16 Rates of improvement in joint function and mobility deficit remission did not differ between the first and third years. But, by the third year, subjects reported more pain and less physical function than in the first year. Despite these medium-term postsurgery deteriorations, in the third year, overall status was significantly better than the baseline situation.16 Moreover, 6,328 subjects from the SOS Study were asked about musculoskeletal pain two and six years after BS, and they were compared with 1,135 individuals from the general population. Self-reported work-restricting pain was more common in the subjects with obesity than in the general population. Patients who underwent BS had a lower incidence of work-restricting pain after two years. However, although there was still amelioration in pain after six years among subjects with BS, the difference was reduced.9
Thus, weight loss after BS often results in decreased joint pain. The largest effect is seen in joints in the lower extremities, hips, knees, and ankles, as well as in the wrists or in the lumbar spine.24 It is still not clear whether pain relief after weight loss is only related to a decreased mechanical loading, which would only affect knee or ankle pain, or rather if it is also related to improvements in peripheral and/or central sensitisation, which may have effects beyond these joints. Therefore, a significant weight loss, a secondary decrease in obesity-related inflammation, and/or mechanical loading may be sufficient to lead to improvements in sensitisation.25 For all this, BS would result not only in reduced pain, but also in opioid use. However, studies have shown that there was an increase in postsurgical chronic opioid use, irrespective of presurgical chronic pain and/or depression diagnoses.26 Plausible explanations for this could be a higher sensitivity to pain, a lower pain detection threshold, and altered pain processing present among subjects with obesity that may persist after BS.27
We found that, after a minimum follow-up of 18 months since BS, the patients who reported more joint pain were older, had a longer duration since their surgical procedure, and had a greater BMI at the time of the evaluation. Few studies have assessed whether there is any relationship between the presence of significant joint pain and clinical outcomes. Older age, lower income, and pre-existing medical conditions, such as cardiovascular diseases and diabetes before BS, were among the factors independently associated with a lower likelihood of improvement in pain postsurgery. Additionally, a greater weight loss was associated with a higher likelihood of amelioration,16 while the type of surgical procedure, independent of weight loss, was not.9,22
Furthermore, exercise was less frequent among subjects with joint pain compared with patients who underwent BS without significant pain at the time of the evaluation. The relationship between pain and physical activity is bidirectional, reinforced through a positive feedback loop.8 The relationship between obesity and pain could be seen as a vicious circle that challenges rehabilitation efforts: pain-inactivity – obesity–pain.28 Also, pain can be both caused and relieved by exercise. The mechanisms involved likely have to do with inflammation. Obesity itself is also associated with a chronic pro-inflammatory state. In addition, the onset of pain could be attributed in part to these inflammatory pathways. Whereas acute bouts of physical activity result in an exaggerated inflammatory response, regular aerobic exercise in previously sedentary subjects decreases this systemic inflammation, the largest effects of which are observed in subjects with obesity. Therefore, this group of patients has much to gain from the attenuated inflammatory response of both regular aerobic and resistance activity. But, when these periods of exercise are followed by prolonged sedentary behaviour among these subjects with obesity, it could increase not only the inflammatory response but also the experience of pain, which could be a barrier for further engagement. Indeed, it has been shown that patients with obesity report more severe pain than non-obese chronic pain patients.29
On the other hand, we found that both significant depressive symptomatology and the proportion of patients taking antidepressants were higher among the group with chronic joint pain. Depressive disorder or depressive symptoms are more frequent among subjects with obesity than in the general population. There is a wide spectrum of mechanisms that might cause this association, such as a low-grade chronic inflammation or lifestyle habits. It is well known that, in the short term after BS, depressive symptoms decrease significantly.30 Therefore, this amelioration in mood may contribute to the perception of pain after surgery.16
Moreover, some epidemiological studies have shown that chronic pain increases the risk for depression up to 4-fold. Similarly, patients with major depression are three times more likely to suffer from non-neuropathic pain and up to six times more likely to suffer from neuropathic pain. Therefore, a potential explanation for this relationship could be a common pathogenic factor between chronic pain and depression. Accumulating evidence suggests that chronic subclinical neuro-inflammation plays a critical role in the pathogenesis of both depression and chronic pain.
The number of sleeping hours was reduced among subjects with chronic joint pain. In fact, chronic pain is a common cause of poor quality sleep. Furthermore, abnormal eating patterns are frequent among subjects with depression. A lack of sleeping hours may cause disturbances in the circadian rhythm of cortisol. Consequently, it may worsen and/or maintain this low grade chronic inflammatory state present in the physiopathology of obesity, depression, and chronic pain.14
The present study evidenced new and interesting findings, but some limitations should be considered. As a cross-sectional study, we cannot make any definitive conclusions regarding a causal relationship between the presence of chronic joint pain and the relationship with BS and its outcomes. Also, the lack of a non-surgical control group precludes us from establishing that BS caused observed changes in chronic joint pain. We used self-reported assessments that were not specific to subjects with obesity or to BS samples and that may have questionable reliability and validity, thus limiting the determination of a clinical diagnosis. Finally, we could not extrapolate our results to other populations because all our patients were Caucasian. On the other hand, the strengths of our study are the use of a specific questionnaire for pain rather than the reporting of pain being limited to the bodily pain domain of the SF-36, which had been used in the majority of previously performed studies. Also, our sample size was big enough to get statistical power. Besides, the majority of studies previously published have assessed chronic joint pain at short-term, not after weight loss has reached a plateau.
We consider that these findings stimulate and justify the routine screening of chronic joint pain among patients with obesity with a validated tool, before and after BS, as part of the standardised protocol. There is an association between chronic pain and negative psychological, functional and metabolic outcomes. For all these reasons, it is extremely important to diagnose the presence of chronic pain among these individuals in order to treat them promptly. Future research should also continue to explore both the relationship between obesity and chronic joint pain and potential targets for treatment.
Author contributionsJN was responsible for designing the protocol, conducting the search, interpreting the results and writing the manuscript.
IR was responsible for collecting the data, and approving the final version of the manuscript.
KD was responsible for collecting the data, and approving the final version of the manuscript.
LA was responsible for collecting the data, and approving the final version of the manuscript.
PS was responsible for the statistical analysis and the interpretation of the results.
MIT was responsible collecting the data and approving the final version of the manuscript.
SP was responsible for collecting the data and approving the final version of the manuscript.
AC was responsible for collecting the data and approving the final version of the manuscript.
LM was responsible for designing the protocol and approving the final version of the manuscript.
All authors read and approved the final version of the manuscript.
Conflict of interestThe authors declare they have no conflict of interest.
None.