Neuronal populations involved in the regulation of food intake, particularly the orexigenic (appetite-stimulating) hormone ghrelin, are found in the hypothalamus. Changes in plasma ghrelin levels have been observed following different bariatric surgery procedures, but the results from different studies are contradictory. Much remains unknown regarding the role of ghrelin in the weight loss process following bariatric surgery.
Our objective was to describe the behavior of fasting ghrelin levels, comparing the changes occurring between 2 different surgical techniques (gastric bypass versus vertical sleeve gastrectomy) and their correlation with weight loss.
Patients and methodA prospective, observational, analytic cohort study of 54 patients (27 for each surgical technique) with a 12-month follow-up period. We analyzed demographic data, anthropometric data, comorbidities, weight loss and evolution of fasting ghrelin.
ResultsThe behavior of acylated ghrelin was similar with the 2 surgical techniques, with no significant differences between gastric bypass and vertical sleeve gastrectomy. With both procedures, there was an increase in acylated ghrelin on day 5 and a subsequent decrease, and levels then gradually increased to reach values at 12 months that were higher than those reported preoperatively. This increase in ghrelin levels did not affect weight loss, since one year post-surgery, 30% weight loss was achieved with both types of surgery.
ConclusionsWe observed an increase in fasting acylated ghrelin levels at one year of follow-up with both surgical techniques, with 30% weight loss.
En el hipotálamo existen poblaciones neuronales involucradas en la regulación de la ingesta, destacando la ghrelina como hormona orexígena (estimula el apetito). Después de los diferentes procedimientos de cirugía bariátrica se han observado cambios en los niveles plasmáticos de ghrelina, siendo los resultados de los estudios contradictorios. Existen muchas lagunas en cuanto al papel que desempeña la ghrelina en el proceso de pérdida de peso después de cirugía bariátrica.
Nuestro objetivo es describir el comportamiento de ghrelina en ayunas, comparando los cambios acontecidos en 2 técnicas quirúrgicas (bypass gástrico versus gastrectomía vertical) y su correlación con la pérdida ponderal.
Pacientes y métodoEstudio observacional de cohortes analíticas prospectivo, donde se incluyen 54 pacientes (27 por cada técnica quirúrgica) y un período de seguimiento de 12 meses. Se analizaron datos demográficos, datos antropométricos, comorbilidades, pérdida ponderal y evolución del comportamiento de ghrelina en ayunas.
ResultadosCon ambas técnicas quirúrgicas el comportamiento de ghrelina acilada fue similar, sin diferencias significativas entre bypass gástrico y gastrectomía vertical. Con ambos procedimientos se produce un ascenso de ghrelina acilada al 5.o día y caída posterior, para luego ir ascendiendo hasta alcanzar valores superiores a los preoperatorios a los 12 meses. Este aumento en los niveles de ghrelina no afecta a la pérdida ponderal, ya que al año de la cirugía con las 2 técnicas quirúrgicas se alcanza un 30% de pérdida de peso.
ConclusionesObservamos un incremento de los niveles de ghrelina acilada en ayunas al año de seguimiento con ambas técnicas quirúrgicas, cuando existe una pérdida ponderal del 30%.
There are neurohormonal signals involved in homeostatic regulation that are essential for understanding the pathogenesis of obesity disorders. The aim of these signals is to achieve/maintain an energy balance, with stable weight and optimum nutrient intake.1
The hypothalamus contains two neuronal populations that are involved in the control of food intake.2 The first population consists of neurons that express neuropeptide Y and agouti-related protein, which stimulate the orexigenic pathway (i.e., they increase appetite). Of particular relevance in this regard is ghrelin (produced mainly in the gastric fundus and, to a lesser extent, in other organs such as the intestine, pancreas, the hypothalamus itself, etc.). The second population consists of neurons that express proopiomelanocortin (POMC) and the cocaine- and amphetamine-regulated transcript (CART), which stimulate the anorexigenic pathway (i.e., they decrease appetite). Examples of hormones intervening in this pathway are peptide YY (PYY) and glucagon-like peptide-1 (GLP-1).3
The ghrelin gene4 is a complex gene, the processing of which gives rise to a broad variety of proteins with multiple biological activities: acylated ghrelin, non-acylated ghrelin, and obestatin. Acylated ghrelin is the molecule conventionally referred to as “ghrelin”, and possesses a unique feature that differentiates it from other peptides and which proves crucial for its biological activity: the addition of an octanoyl group in serine 3, mediated by the enzyme ghrelin O-acyltransferase. This confers on it the ability to cross the blood-brain barrier, and its actions are mediated through subtype 1a of the growth hormone secretagogue receptor (GHS-R). The molecule has a broad range of biological effects5: it stimulates growth hormone (GH) secretion, regulates food intake and energy balance, reduces insulin secretion, possesses cardiovascular effects, and modulates cell proliferation. Non-acylated ghrelin presents higher plasma levels (70–90%), does not bind to subtype 1a of GHS-R or affect GH secretion, and its receptor has not yet been identified.
The mechanism regulating ghrelin secretion is not exactly known, and the interpretation of ghrelin levels is difficult. Contrarily to what might be expected, the levels are decreased in obese individuals and elevated in subjects with a low body mass index (BMI), cachexia or anorexia nervosa, despite which hunger is not incremented. In addition to a short-term regulatory role, ghrelin is involved in long-term weight regulation. Plasma ghrelin levels are inversely related to the BMI, and fluctuate in a compensatory manner according to variations in body weight.6
Anatomical changes secondary to bariatric surgery have been related to modifications in the production of different hormones. In vertical sleeve gastrectomy (VSG), the gastric fundus is removed, causing a drastic decrease in ghrelin secretion7 and an acceleration of gastric emptying. This consequently results in the elevation of plasma GLP-1 and PYY,8 both of which are related to satiety sensation. But the satiety mechanism is much more complex; there is an intermediate step between the digestive stimulus and the central nervous system response in the form of satiety sensation. Over 100 hormones and neuropeptides are involved in this neurohormonal pathway, and are currently the focus of many scientific studies.
In 2002, Cummings et al.7 published a study that has since become a reference in the literature on ghrelin modifications related to surgery for obesity. The decrease in plasma ghrelin concentration in response to oral carbohydrate intake is well known, though studies that have measured the acylated ghrelin fraction response are not so numerous.9 A cross-sectional study7 found gastric bypass (BP) to be associated with the inhibition of fasting ghrelin levels and to an absence of fluctuations in plasma concentration in relation to meals. Subsequent studies have not confirmed this observation, however, and all kinds of changes in ghrelin have in fact been reported after BP.10
The results of the different studies are difficult to compare, because most of them do not specify which ghrelin fraction is being measured11,12 (total, non-acylated or acylated), and data moreover are not provided regarding the analytical procedure or the collection method used. Studies measuring ghrelin after intake predominate, and fewer studies have described the course of fasting ghrelin9,13 and its relationship to body weight loss.
The main objective of the present study is to describe the behavior of acylated ghrelin under fasting conditions, without food stimulation, establishing comparisons of the changes observed with two surgical techniques (BP versus VSG). Body weight loss and its correlation to ghrelin is analyzed as a secondary objective.
Material and methodsPatientsThe patients included in the study protocol met the inclusion and exclusion criteria for bariatric surgery as established by the Spanish Society of Surgery for Obesity (Sociedad Española de Cirugía de la Obesidad [SECO]).14,15 A total of 60 patients were consecutively recruited as they were entered on the surgical waiting list and met the mentioned inclusion criteria. Finally, only 54 patients were included for data analysis, 27 corresponding to each surgical procedure (BP versus VSG); the rest were excluded because of sample losses and sample reading error.
All patients were simultaneously monitored by the Departments of Endocrinology and General Surgery, and were informed about the risks and benefits of each procedure. Written informed consent was obtained according to the guidelines of the Ethics Committee of our hospital for blood sampling and freezing, and the subsequent storage of blood products.
The operations were performed via laparoscopic surgery and by the same surgical team of four bariatric surgeons. The BP technique involved the construction of a gastric reservoir (20–30ml), with a 150-cm feed loop and a 100-cm biliopancreatic loop. Gastrojejunal anastomosis was performed using a circular, mechanical end-to-side 21mm endostapler. For VSG, a gastroplasty was performed with a 32Fr tutor, with gastric sectioning starting 4–5cm from the pylorus and reaching the angle of His with a roticulator linear endostapler.
Study designThe study was carried out at the Department of General Surgery of Complejo Hospitalario de Cartagena (Cartagena, Murcia, Spain) between 2011 and 2013, and involved a follow-up period of 12 months. A prospective, analytical observational cohort design was used.
All the patients included in the study underwent a thorough evaluation before surgery. Abdominal ultrasound, esophagogastric transit, and upper gastrointestinal endoscopy were performed. In addition to the demographic parameters, we recorded comorbidities (arterial hypertension, type 2 diabetes, obstructive sleep apnea syndrome [OSAS] and dyslipidemia) and follow-up data (percentage overweight lost [POL] and variations in laboratory parameters over time).
Blood sampling was performed at the outpatient clinic, with fasting for 12h in all cases. Each patient underwent venous puncture with the collection of a tube of blood. The blood tube used (BDTM P800 [2ml]) contained specific additives for stabilization of the different plasma metabolic markers. It contained a dry EDTA-K2 spray as an anticoagulant and a cocktail of specific dipeptidyl peptidase-IV inhibitors. Due to these protease inhibitory properties, the use of the P800 tube is especially recommended in ghrelin plasma detection and quantification assays, as it allows for the protection and stabilization of these hormones. The tube was kept refrigerated at −2 to 4°C, and centrifugation was performed for 10min at 3500rpm within 72h after extraction of the sample. Two Eppendorf tubes were labeled for each blood sample, stating the patient's case history number and sampling time (preoperative and 5 days and 1, 3, 6 and 12 months after surgery). The Eppendorf tubes were stored at −30°C.
HormonesThe Human Metabolic Hormone Magnetic Bead, Panel 96 Well Plate Assay, MILLIPLEX® MAP Cat. # HMHMAG-34K kit was used to analyze the samples.
MILLIPLEX® MAP is a human metabolic panel designed for the study of biomarkers, analytically validated and integrated into a single panel. The kit allows us to investigate the expression of multiple analytes simultaneously, with the advantage of increased speed and sensitivity. The kit can be customized to design a personalized panel composition adapted to the needs of each case. In our case, the kit was designed to determine acylated ghrelin, each analyte being measured in duplicate. We also determined active GLP1 and total PYY, which are not addressed in this article but will be in a future study.
MILLIPLEX® MAP is based on Luminex® xMAP® technology, a rapid method that is capable of performing a variety of bioassays (immunoassays) on the surface of encoded fluorescent magnetic beads, known as MagPlex TM-C microspheres.
Analysis takes place when the microspheres pass quickly through a laser and are quantified, multiple results being obtained for each sample. Each kit comes with the control ranges for the analyte to be measured, quantified in pg/ml.
In our kit, the lower limit of detection for acylated ghrelin was 2pg/ml, with an intra- and inter-assay coefficient of variation (CV) of 2% and 8%, respectively.
Anthropometric parametersThe anthropometric parameters assessed before and after surgery were:
- -
Height (meters and centimeters)
- -
Weight (kilograms)
- -
Ideal weight (kilograms) according to the Metropolitan Life (MetLife) formula:
Ideal weight=(Height (cm)−150)×0.75+50
- -
The body mass index (BMI) or the Quetelet index, calculated from the formula:
BMI=Weight/Height2 (kg/m2)
We measured POL to assess weight loss over time, and surgery was considered to be successful when POL >65%, using the following formula:
POL=(Initial weight−Current weight/Initial weight−Ideal weight)×100
Statistical analysisThe data were analyzed using the SPSS version 20 statistical package for MS Windows (IBM, USA). The analyses were supervised by the Research Support Unit of Hospital Universitario de Santa Lucía (Cartagena, Murcia, Spain).
Quantitative variables were expressed as the mean and standard deviation (SD) or as the median and interquartile range (IQR), as appropriate. Categorical variables were expressed as frequencies and percentages. The normal distribution of variables was assessed using a graphical method (P-P and Q-Q curves) and a hypothesis test (Shapiro–Wilk).
Comparisons of quantitative variables between the comparator groups (BP versus VSG) were made using the Student t-test/analysis of variance (ANOVA) or Mann–Whitney U-test/Kruskal–Wallis test, as appropriate. Comparisons between categorical variables of independent groups (BP versus VSG) were based on the chi-squared test. The analyses were made at each of the study timepoints (preoperative and 5 days and 1, 3, 6 and 12 months after surgery). For quantitative variables, we plotted the mean and corresponding 95% confidence interval (95%CI) by calculating the standard error of the mean for each timepoint. The Friedman test was used to analyze paired data. Correlation analyses were performed with the Spearman test to assess the relationship between ghrelin and the BMI. Statistical significance was considered for p<0.05.
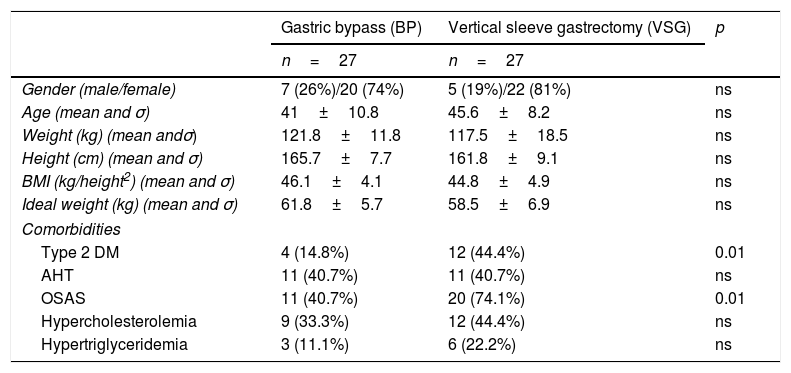
ResultsPreoperative characteristicsThe study groups showed no preoperative differences in terms of demographic data. By contrast, the VSG group had a significantly greater number of patients with type 2 diabetes mellitus and obstructive sleep apnea syndrome than the BP group (Table 1).
Demographic data and preoperative comorbidities.
| Gastric bypass (BP) | Vertical sleeve gastrectomy (VSG) | p | |
|---|---|---|---|
| n=27 | n=27 | ||
| Gender (male/female) | 7 (26%)/20 (74%) | 5 (19%)/22 (81%) | ns |
| Age (mean and σ) | 41±10.8 | 45.6±8.2 | ns |
| Weight (kg) (mean andσ) | 121.8±11.8 | 117.5±18.5 | ns |
| Height (cm) (mean and σ) | 165.7±7.7 | 161.8±9.1 | ns |
| BMI (kg/height2) (mean and σ) | 46.1±4.1 | 44.8±4.9 | ns |
| Ideal weight (kg) (mean and σ) | 61.8±5.7 | 58.5±6.9 | ns |
| Comorbidities | |||
| Type 2 DM | 4 (14.8%) | 12 (44.4%) | 0.01 |
| AHT | 11 (40.7%) | 11 (40.7%) | ns |
| OSAS | 11 (40.7%) | 20 (74.1%) | 0.01 |
| Hypercholesterolemia | 9 (33.3%) | 12 (44.4%) | ns |
| Hypertriglyceridemia | 3 (11.1%) | 6 (22.2%) | ns |
cm: centimeters; DM: diabetes mellitus; AHT: arterial hypertension; BMI: body mass index; kg: kilogram; kg/m2: kilograms/meter squared; ns: nonsignificant; OSAS: obstructive sleep apnea syndrome.
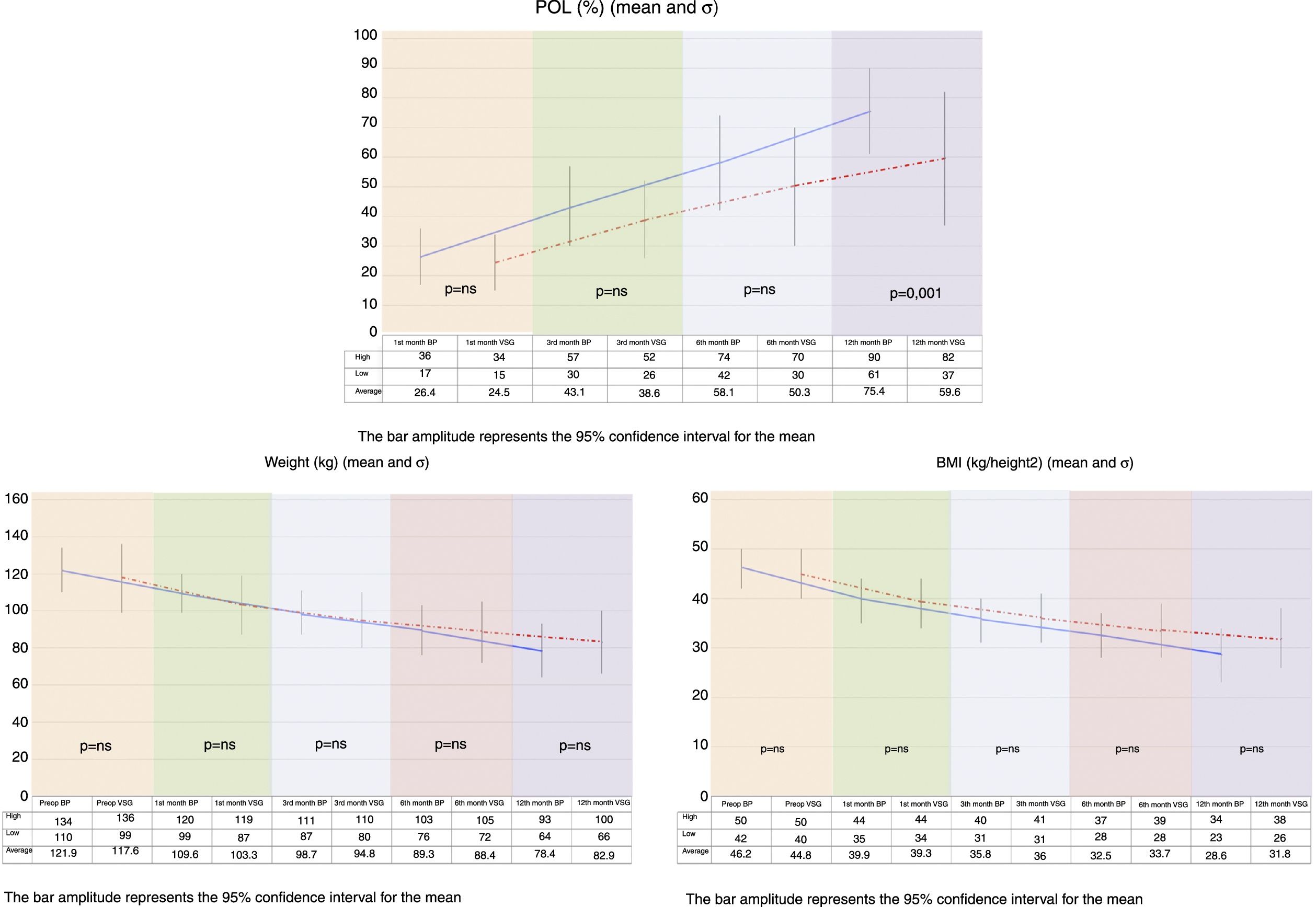
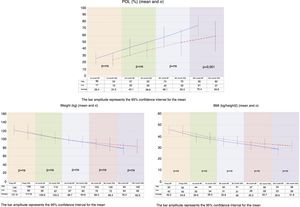
On comparing the two groups, BMI reduction was found to be greater in the BP group than in the VSG group (p=0.038) one year after surgery (Fig. 1).
Both procedures achieved substantial weight loss, but the BP group showed significantly greater POL than the VSG group at 12 months postsurgery. The VSG group failed to achieve 65% PSP at one year of follow-up (Fig. 1).
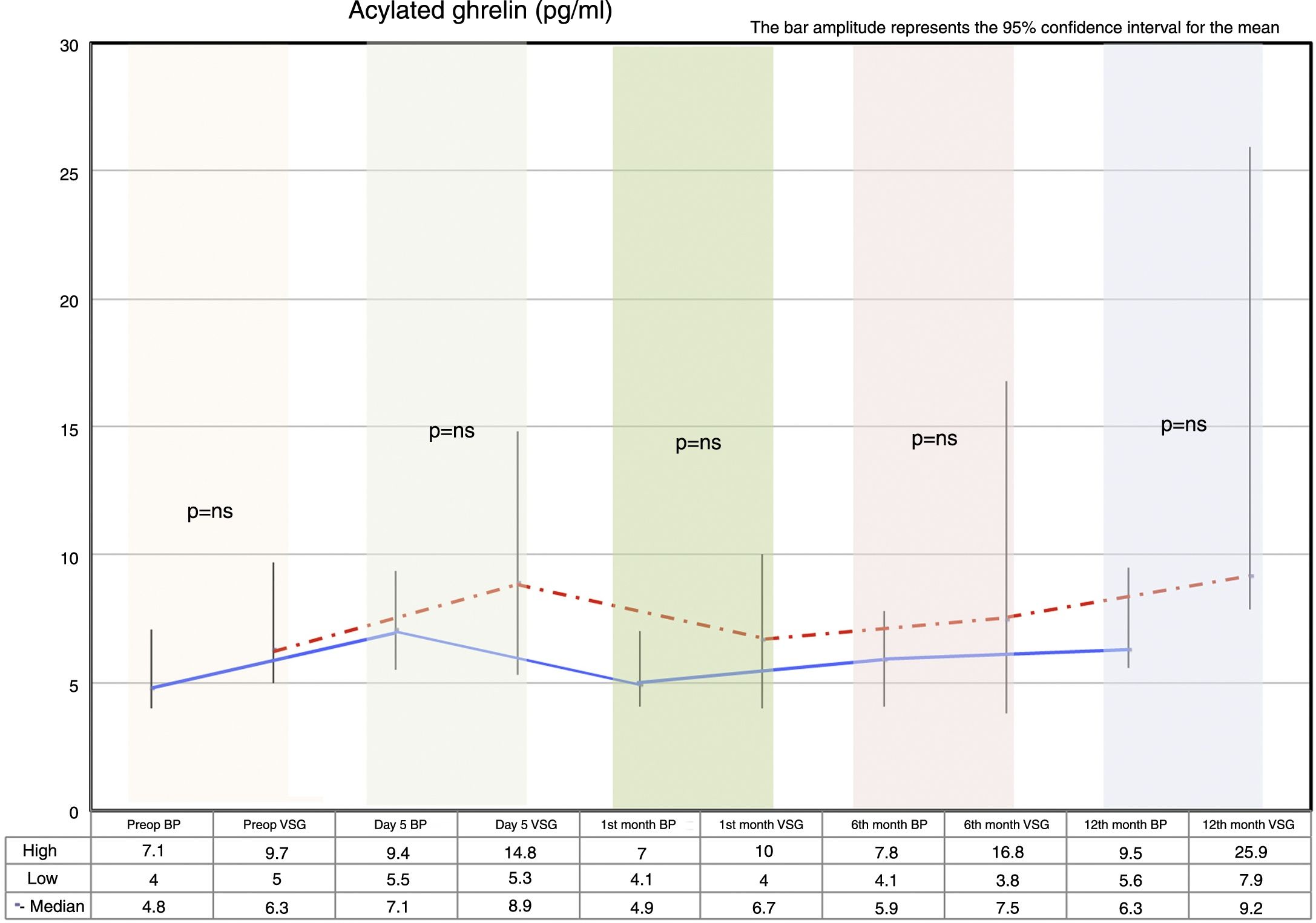
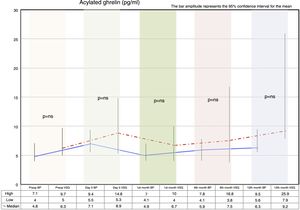
Fasting ghrelin behaviorAcylated ghrelin was seen to increase with both procedures on day 5 after surgery. In the first month, a decrease in ghrelin with respect to day 5 after surgery was observed in both groups, followed by a gradual increase over time until values higher than the preoperative values were reached at 12 months postsurgery.
The VSG group showed higher acylated ghrelin values at 12 months than the BP group, though the difference failed to reach statistical significance (Fig. 2).
Correlation between fasting ghrelin and the body mass index preoperatively and after 12 monthsAcylated ghrelin and the BMI measured before surgery exhibited a positive linear relationship (r=0.13; R2=0.00), though without reaching statistical significance (p=0.35). In other words, no correlation was found between ghrelin and BMI as determined before surgery.
Acylated ghrelin and the BMI measured 12 months after surgery showed a negative linear relationship (r=−0.01; R2=0.00), that likewise failed to reach statistical significance (p=0.92). In other words, no correlation was found between ghrelin and the BMI as determined 12 months after surgery.
DiscussionIn the present study we observed an increase in acylated ghrelin levels after one year of follow-up, with no significant differences between BP and VSG. By contrast, comparatively greater weight loss was recorded in the BP group at 12 months, with no significant correlation between the variations in ghrelin levels and the BMI.
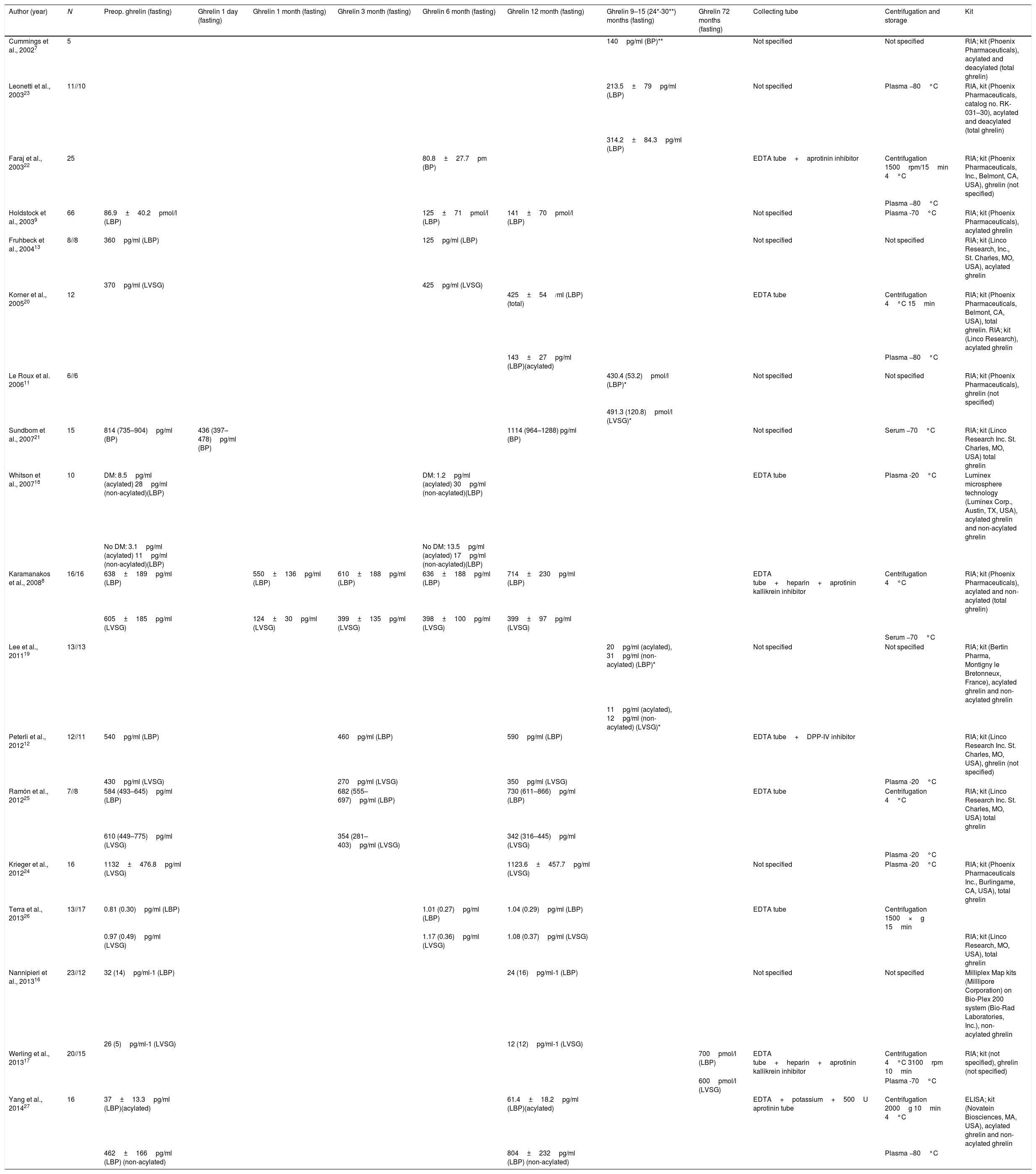
In order to compare our results with those published to date, we conducted a literature review, compiling a table of the different bariatric surgery case series, focusing mainly on BP and VSG. Table 2 shows the author and year of publication, fasting ghrelin values, and the timing of measurements, as well as the characteristics described by the authors in terms of collection, storage and kit used to perform the analyses.
Author, year of publication and ghrelin series.
| Author (year) | N | Preop. ghrelin (fasting) | Ghrelin 1 day (fasting) | Ghrelin 1 month (fasting) | Ghrelin 3 month (fasting) | Ghrelin 6 month (fasting) | Ghrelin 12 month (fasting) | Ghrelin 9–15 (24*-30**) months (fasting) | Ghrelin 72 months (fasting) | Collecting tube | Centrifugation and storage | Kit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cummings et al., 20027 | 5 | 140pg/ml (BP)** | Not specified | Not specified | RIA; kit (Phoenix Pharmaceuticals), acylated and deacylated (total ghrelin) | |||||||
| Leonetti et al., 200323 | 11//10 | 213.5±79pg/ml (LBP) | Not specified | Plasma −80°C | RIA, kit (Phoenix Pharmaceuticals, catalog no. RK-031–30), acylated and deacylated (total ghrelin) | |||||||
| 314.2±84.3pg/ml (LBP) | ||||||||||||
| Faraj et al., 200322 | 25 | 80.8±27.7pm (BP) | EDTA tube+aprotinin inhibitor | Centrifugation 1500rpm/15min 4°C | RIA; kit (Phoenix Pharmaceuticals, Inc., Belmont, CA, USA), ghrelin (not specified) | |||||||
| Plasma −80°C | ||||||||||||
| Holdstock et al., 20039 | 66 | 86.9±40.2pmol/l (LBP) | 125±71pmol/l (LBP) | 141±70pmol/l (LBP) | Not specified | Plasma -70°C | RIA; kit (Phoenix Pharmaceuticals), acylated ghrelin | |||||
| Fruhbeck et al., 200413 | 8//8 | 360pg/ml (LBP) | 125pg/ml (LBP) | Not specified | Not specified | RIA; kit (Linco Research, Inc., St. Charles, MO, USA), acylated ghrelin | ||||||
| 370pg/ml (LVSG) | 425pg/ml (LVSG) | |||||||||||
| Korner et al., 200520 | 12 | 425±54/ml (LBP) (total) | EDTA tube | Centrifugation 4°C 15min | RIA; kit (Phoenix Pharmaceuticals, Belmont, CA, USA), total ghrelin. RIA; kit (Linco Research), acylated ghrelin | |||||||
| 143±27pg/ml (LBP)(acylated) | Plasma −80°C | |||||||||||
| Le Roux et al. 200611 | 6//6 | 430.4 (53.2)pmol/l (LBP)* | Not specified | Not specified | RIA; kit (Phoenix Pharmaceuticals), ghrelin (not specified) | |||||||
| 491.3 (120.8)pmol/l (LVSG)* | ||||||||||||
| Sundbom et al., 200721 | 15 | 814 (735–904)pg/ml (BP) | 436 (397–478)pg/ml (BP) | 1114 (964–1288) pg/ml (BP) | Not specified | Serum −70°C | RIA; kit (Linco Research Inc. St. Charles, MO, USA) total ghrelin | |||||
| Whitson et al., 200718 | 10 | DM: 8.5pg/ml (acylated) 28pg/ml (non-acylated)(LBP) | DM: 1.2pg/ml (acylated) 30pg/ml (non-acylated)(LBP) | EDTA tube | Plasma -20°C | Luminex microsphere technology (Luminex Corp., Austin, TX, USA), acylated ghrelin and non-acylated ghrelin | ||||||
| No DM: 3.1pg/ml (acylated) 11pg/ml (non-acylated)(LBP) | No DM: 13.5pg/ml (acylated) 17pg/ml (non-acylated)(LBP) | |||||||||||
| Karamanakos et al., 20088 | 16/16 | 638±189pg/ml (LBP) | 550±136pg/ml (LBP) | 610±188pg/ml (LBP) | 636±188pg/ml (LBP) | 714±230pg/ml (LBP) | EDTA tube+heparin+aprotinin kallikrein inhibitor | Centrifugation 4°C | RIA; kit (Phoenix Pharmaceuticals), acylated and non-acylated (total ghrelin) | |||
| 605±185pg/ml (LVSG) | 124±30pg/ml (LVSG) | 399±135pg/ml (LVSG) | 398±100pg/ml (LVSG) | 399±97pg/ml (LVSG) | ||||||||
| Serum −70°C | ||||||||||||
| Lee et al., 201119 | 13//13 | 20pg/ml (acylated), 31pg/ml (non-acylated) (LBP)* | Not specified | Not specified | RIA; kit (Bertin Pharma, Montigny le Bretonneux, France), acylated ghrelin and non-acylated ghrelin | |||||||
| 11pg/ml (acylated), 12pg/ml (non-acylated) (LVSG)* | ||||||||||||
| Peterli et al., 201212 | 12//11 | 540pg/ml (LBP) | 460pg/ml (LBP) | 590pg/ml (LBP) | EDTA tube+DPP-IV inhibitor | RIA; kit (Linco Research Inc. St. Charles, MO, USA), ghrelin (not specified) | ||||||
| 430pg/ml (LVSG) | 270pg/ml (LVSG) | 350pg/ml (LVSG) | Plasma -20°C | |||||||||
| Ramón et al., 201225 | 7//8 | 584 (493–645)pg/ml (LBP) | 682 (555–697)pg/ml (LBP) | 730 (611–866)pg/ml (LBP) | EDTA tube | Centrifugation 4°C | RIA; kit (Linco Research Inc. St. Charles, MO, USA) total ghrelin | |||||
| 610 (449–775)pg/ml (LVSG) | 354 (281–403)pg/ml (LVSG) | 342 (316–445)pg/ml (LVSG) | ||||||||||
| Plasma -20°C | ||||||||||||
| Krieger et al., 201224 | 16 | 1132±476.8pg/ml (LVSG) | 1123.6±457.7pg/ml (LVSG) | Not specified | Plasma -20°C | RIA; kit (Phoenix Pharmaceuticals Inc., Burlingame, CA, USA), total ghrelin | ||||||
| Terra et al., 201326 | 13//17 | 0.81 (0.30)pg/ml (LBP) | 1.01 (0.27)pg/ml (LBP) | 1.04 (0.29)pg/ml (LBP) | EDTA tube | Centrifugation 1500×g 15min | ||||||
| 0.97 (0.49)pg/ml (LVSG) | 1.17 (0.36)pg/ml (LVSG) | 1.08 (0.37)pg/ml (LVSG) | RIA; kit (Linco Research, MO, USA), total ghrelin | |||||||||
| Nannipieri et al., 201316 | 23//12 | 32 (14)pg/ml-1 (LBP) | 24 (16)pg/ml-1 (LBP) | Not specified | Not specified | Milliplex Map kits (Milllipore Corporation) on Bio-Plex 200 system (Bio-Rad Laboratories, Inc.), non-acylated ghrelin | ||||||
| 26 (5)pg/ml-1 (LVSG) | 12 (12)pg/ml-1 (LVSG) | |||||||||||
| Werling et al., 201317 | 20//15 | 700pmol/l (LBP) | EDTA tube+heparin+aprotinin kallikrein inhibitor | Centrifugation 4°C 3100rpm 10min | RIA; kit (not specified), ghrelin (not specified) | |||||||
| 600pmol/l (LVSG) | Plasma -70°C | |||||||||||
| Yang et al., 201427 | 16 | 37±13.3pg/ml (LBP)(acylated) | 61.4±18.2pg/ml (LBP)(acylated) | EDTA+potassium+500U aprotinin tube | Centrifugation 2000g 10min 4°C | ELISA; kit (Novatein Biosciences, MA, USA), acylated ghrelin and non-acylated ghrelin | ||||||
| 462±166pg/ml (LBP) (non-acylated) | 804±232pg/ml (LBP) (non-acylated) | Plasma −80°C |
BP: open gastric bypass; LBP: laparoscopic gastric bypass; DM: diabetes mellitus; DPP-IV: dipeptidyl peptidase-IV; LVSG: laparoscopic vertical sleeve gastrectomy; RIA: radioimmunoassay.
The case series were small, and radioimmunoassay was the most widely used procedure. One-half of the studies compared BP versus VSG via the laparoscopic route. The largest series comprised 23 BP versus 12 VSG procedures16 and 20 BP versus 15 VSG cases.17 It is difficult to establish comparisons with our own study. Many authors do not specify the type of ghrelin they measured.11,12 This is very important, because the active form of ghrelin is the acylated form. Comparisons are further complicated by the fact that different units of measure were used, with few measurements over time. Of the 19 studies included, only 6 measured the acylated ghrelin fraction,9,13,18–20,27 though comparisons with our results cannot be made because the test methods used were different, and many studies failed to report the sample processing method employed. Of the reviewed studies, only that published by Whitson et al.18 used Luminex methodology similar to our own, with acylated ghrelin values measured in pg/ml. These investigators performed measurements in BP patients divided into two populations (diabetic and non-diabetic), and recorded an increase in acylated ghrelin levels at 6 months in the non-diabetic group. This observation agrees with our own findings, and although we did not consider patient differentiation in terms of diabetes or non-diabetes, the BP group showed increased acylated ghrelin levels, and the proportion of diabetic patients was also low (14.8%). Further studies are needed to clarify whether this finding is of significance.
Some similarities can be found in the other studies included in Table 2. Holdstock et al.9 found the acylated ghrelin levels to be increased at 12 months, with the analysis of 66 BP procedures. In contrast, Fruhbeck et al.13 found the levels to be increased in the VSG group but decreased in the BP group, as determined 6 months after surgery. In our study, the acylated ghrelin levels increased with both surgical techniques 12 months after surgery. On the other hand, in the series published by Karamanakos et al.8 (16 BP cases and 16 VSG procedures), although total ghrelin was measured, a decrease in ghrelin was recorded in the first month versus the preoperative levels similar to that seen in our series, and with both surgical procedures. A ghrelin drop was also seen after surgery in the series published by Peterli et al.12 (12 BP cases and 11 VSG procedures), but measured in month three after surgery. In the open BP group reported by Sundbom et al.,21 an increase to above the preoperative levels was recorded at 12 months, though these authors measured total ghrelin. More data are available on acylated ghrelin levels measured in BP,7,9,18,27 with a general tendency to increase at one year of follow-up. By contrast, few results are available for comparison regarding VSG.11,13,19 In some series, acylated ghrelin clearly increased (Fruhbeck et al.13), though in other cases we have no preoperative data with which to establish comparisons (Le Roux et al.11 and Lee et al.19), the results being discordant. No studies similar to our own in terms of methodology and comparing BP with VSG were found.
Forty-seven percent of the studies7,11,13,16,19,21,23,24 did not specify the characteristics of the tube used for sample collection, and 26% of the publications7,11,13,16,19 did not report sample storage and processing (centrifugation, temperature, time). The rest specified only some of the characteristics of the process. The kits used were different, with many manufacturers, and the units employed were also different. The variability of the ghrelin data can be explained in terms of the influence of the collection methodology used. A recent study has reported that a good practice for ghrelin collection is to use EDTA tubes that have been refrigerated, with immediate processing in order to preserve the active form, without freezing or thawing28 – though this method has not been validated. There are few descriptions in the literature on collection methods, and further studies are required in this regard.
It should also be taken into account that different surgical techniques may condition greater or lesser resection of the ghrelin-producing cell population in the gastric fundus.29 In our group of patients, the surgical procedure exerted no influence in this regard, and even in the VSG group, where the gastric fundus was resected, increased acylated ghrelin levels were recorded at 12 months. Based on these results, it is possible that the ghrelin variations are not related to greater or lesser resection of the gastric fundus, but to the negative energy balance found in patients undergoing bariatric surgery and who are losing weight.
A controversial issue in studies evaluating ghrelin is whether the patient was losing weight at the time of the measurement. Faraj et al.22 reported that ghrelin remained unchanged in patients with stable weight, while the levels increased in patients who were losing weight. In a situation of weight loss (dieting, anorexia, surgery), the ghrelin levels tend to increase, and some authors therefore consider that they should be measured in patients with stable weight,9,22 an inverse relationship being observed between the BMI and ghrelin. We found no significant differences between BP and VSG at 12 months. We interpret the observed increase in acylated ghrelin on day 5 in terms of a compensatory mechanism in response to prolonged fasting and surgical stress, since weight loss had not yet started. Although the levels subsequently decreased, the values reached at 12 months were higher than those recorded before either surgical procedure. This second increase could be explained by the fact that these are patients who are in the full process of weight loss, and the observed increase could act as a compensatory mechanism over the long term. However, the analysis is more complex, for although considerable weight loss occurred with both procedures, it was seen to be greater in the BP group, while slightly higher ghrelin levels were seen at 12 months in the VSG group, the difference failing to reach statistical significance, however. It may therefore be inferred that weight loss after bariatric surgery, in this case BP or VSG, is independent of the variations in ghrelin, as has been suggested in other studies.30,31
A limitation is the fact that we were working with a population of obese individuals, with hypertriglyceridemia, and some samples presented high lipid levels that interfered with the test method. The microspheres carry adhered antibody that binds to the analyte in the sample, and high lipid levels make adhesion difficult, thus causing difficulties in the analysis of some samples. The samples in which a very lipid-rich supernatant was observed after centrifugation were excluded from the study, since the most dispersed hormone values corresponded to samples of this kind.
This is the largest hormone comparison series reported in the literature to date with acylated ghrelin under fasting conditions, though the limited sample size does not allow the drawing of firm conclusions. We used a relatively novel analytical measurement system, allowing for the simultaneous analysis of several hormones with a single sample. In the present study we describe the ghrelin results, but GLP-1 and PYY levels were also measured. Few studies are available in the literature for comparison purposes, and further research is needed in this field. Specifically, larger randomized and long-term studies are needed in order that more consistent conclusions may be drawn.
ConclusionsWe recorded an increase in fasting acylated ghrelin levels after one year of follow-up with both surgical procedures (BP and VSG), when percentage weight loss was 30%. No negative linear correlation was found between acylated ghrelin and the BMI.
FundingResearch grant: Sub-program of Non-oriented Basic Research Projects (Proyectos de Investigación Fundamental no Orientada), call for 2012. Spanish Ministry of Economy and Competitiveness. Reference. BFU 2012-38103.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Thanks are due to the Morbid Obesity Unit of Hospital Universitario Santa Lucía in Cartagena for its active contribution to the preparation of this manuscript.
Thanks are also due to the Nutrition and Bromatology research group of the University of Murcia for collaboration in the hormone tests.
Please cite this article as: Navarro García MI, González-Costea Martínez R, Torregrosa Pérez N, Romera Barba E, Periago MJ, Vázquez Rojas JL. Comportamiento de ghrelina en ayunas después de bypass gástrico y gastrectomía vertical: estudio de cohortes analítico. Endocrinol Diabetes Nutr. 2020;67:89–101.