The global increase in the prevalence rates of overweight or obesity has also affected patients with type 1 diabetes (T1D), where this disease had traditionally been associated with a lean phenotype. On the other hand, the effect of obesity on new glycemic control metrics obtained from continuous glucose monitoring (CGM) in T1D is poorly understood. We wanted to assess whether there is any relationship between BMI (body mass index) and the different CGM metrics or HbA1c.
MethodsTwo hundred and twenty-five patients with T1D (47.1% ♀, mean age 42.9±14.7 years) with a CGM for a minimum of 6 months were analysed by downloading their CGM and collecting clinical and anthropometric variables.
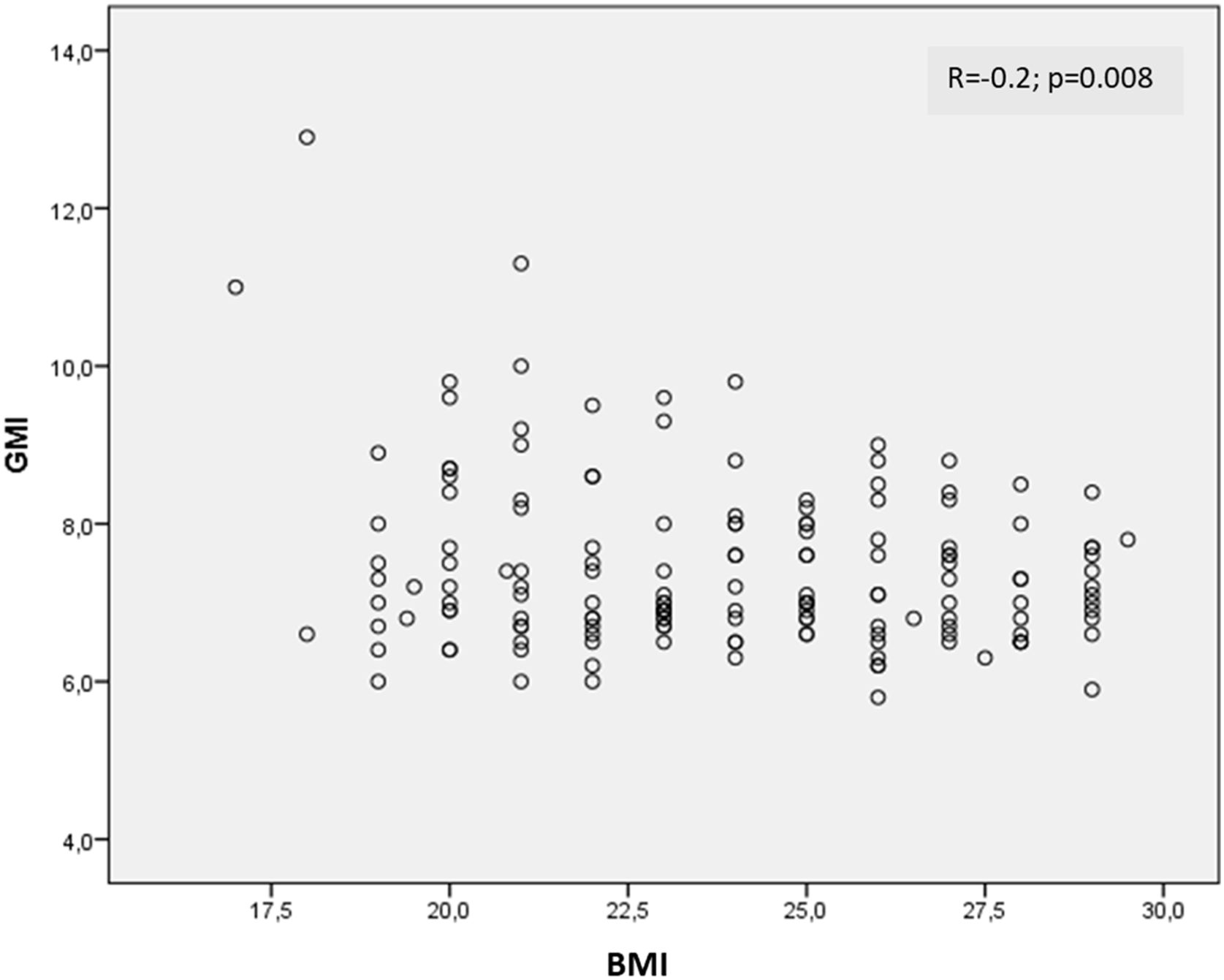
Results35.1% (79/225) of the T1D patients had overweight and 17.3% (39/225) lived with obesity, while the remaining 47.6% had a normal weight. A negative correlation was found between GMI (glucose management indicator) and BMI (−0.2; p=0.008) and HbA1c (−0.2; p=0.01). In contrast, a positive correlation was observed between the total dose of insulin and the BMI (0.3; p<0.0001). No significant correlations were found between BMI and other CGM metrics.
ConclusionsOverweight or obesity do not imply worse glycemic control in patients with T1D or less use of CGM. Possibly, and in order to achieve a good glycemic control, more units of insulin are necessary in these patients which, in turn, makes weight control more difficult.
El aumento global de la prevalencia de sobrepeso u obesidad también ha afectado a los pacientes con diabetes tipo 1 (DT1), cuando esta enfermedad tradicionalmente se había asociado con un fenotipo delgado. Por otro lado, el efecto de la obesidad en las nuevas métricas de control glucémico obtenidas a partir de la monitorización continua de glucosa (MCG) en la DT1 es poco conocido. Nuestro objetivo era evaluar si existe alguna relación entre el índice de masa corporal (IMC) y las diferentes métricas de la MCG o la hemoglobina glicosilada (HbA1c).
MétodosFueron analizados 225 pacientes con DT1 (47,1% ♀, edad media 42,9±14,7 años) con MCG durante un mínimo de seis meses, descargando su MCG y recogiendo variables clínicas y antropométricas.
ResultadosDe los pacientes con DT1, el 35,1% (79/225) tenía sobrepeso y el 17,3% (39/225) vivía con obesidad, mientras que el 47,6% restante tenía un normopeso. Se evidenció una correlación negativa entre el indicador de manejo de glucosa (GMI) y el IMC (−0,2; p=0,008) y la HbA1c (−0,2; p=0,01). Por el contrario, se observó una correlación positiva entre la dosis total de insulina y el IMC (0,3; p<0,0001). No se encontraron correlaciones significativas entre el IMC y otras métricas de la MCG.
ConclusionesEl sobrepeso y la obesidad no implican un peor control glucémico en pacientes con DT1 ni un menor uso de MCG. Posiblemente, y para conseguir un buen control glucémico, sean necesarias más unidades de insulina en estos pacientes lo que, a su vez, dificulta el control del peso.
Obesity is a complex, chronic, relapsing disease with a continually increasing incidence worldwide. Obesity can lead to numerous different comorbidities, such as cancer, cardiometabolic disease and psychological problems, with the consequent negative effects on health.1,2 In Spain, in the adult population, the prevalence of obesity is estimated to be 20.5% among women, rising to 22.8% in men.3 Although once rare among patients with type 1 diabetes (T1D), obesity is now becoming a growing and dangerous associated condition.4,5 With the incidence of T1D in Spain being medium-to-high, at 15 cases per 1000population/year,6 as both disorders are so common, we decided to investigate their coexistence and its consequences.
The prevalence of obesity among patients with T1D is still open to debate because of the high degree of heterogeneity among the published studies; it ranges from 2% in Asian populations to 28.4% in American cohorts.7–13 These variations could be explained by differences between ethnicities and regions in both conditions. Recent data from the DCCT study showed a significant increase in obesity among adults with T1D which could not be explained by ageing of the cohort and which occurred more rapidly than the rise in obesity in the general population.14 Therefore, it is important to have regional data.
Mechanisms that predispose individuals with T1D to obesity go beyond the effects of exogenous hyperinsulinaemia, which result in an imbalance between the hepatic and peripheral effects of insulin. These mechanisms would be much more complex and include genetics, epigenetics, enteroendocrine hormonal system microbiota and psychological problems, where multiple organs might be affected.5
People with T1D and obesity may have greater difficulty in achieving proper metabolic control, an increase in cardiovascular risk factors and a consequent a rise in cardiovascular complications.5
There is still debate about whether people with T1D and concomitant obesity have worse blood glucose control. Studies conducted in the paediatric population have shown contradictory results regarding HbA1c levels among patients with T1D with or without obesity.15,16 The few studies conducted in the adult population have also shown inconsistent results.17–19 To our knowledge, all the studies published to date have assessed blood glucose control by using HbA1c, without considering all the new metrics obtained by continuous glucose monitoring (CGM), such as time in range and blood glucose variability.
We aimed to study whether the coexistence of obesity among patients with T1D is associated with worse blood glucose control in terms of not only HbA1c but also CGM metrics.
Materials and methodsSubjectsWe retrospectively analysed data from 225 patients with T1D who had been using intermittent CGM (FreeStyle Libre flash 2, Abbott Diabetes Care, Witney, United Kingdom) for a minimum follow-up of six months. Exclusion criteria were as follows: (a) patients who did not have >70% use of CGM over the previous 14 days20; (b) subjects who refused to sign the informed consent form. This study was conducted according to the World Medical Association Declaration of Helsinki. The study was approved by the Ethics Committee of the Hospital Universitari Son Llàtzer. Written informed consent was obtained from all subjects prior to study participation.
Data collectionClinical (gender, age, mean T1D duration, units of basal and prandial insulin) and anthropometrical (body mass index or BMI) variables were collected from computerised medical records. The most recent CGM reports were downloaded from the Libreview website.
BMI and its categories were classified according to WHO criteria.3
Statistical analysisThe primary endpoint in this study was to assess the effect of weight on blood glucose control, by using CGM for a minimum of six months.
Statistical analyses were performed using IBM® SPSS® Statistics Version 23.0. Initial analyses were descriptive and included calculation of mean, median and standard deviation for continuous variables and frequencies for categorical variables. The distribution of the sample was analysed by the Kolmogorov–Smirnoff test. Comparison between the two groups was analysed by an unpaired Student's test for variables with a normal distribution and the Wilcoxon test for the variables without a normal distribution. A p value <0.05 on the two-tailed test was considered to indicate statistical significance.
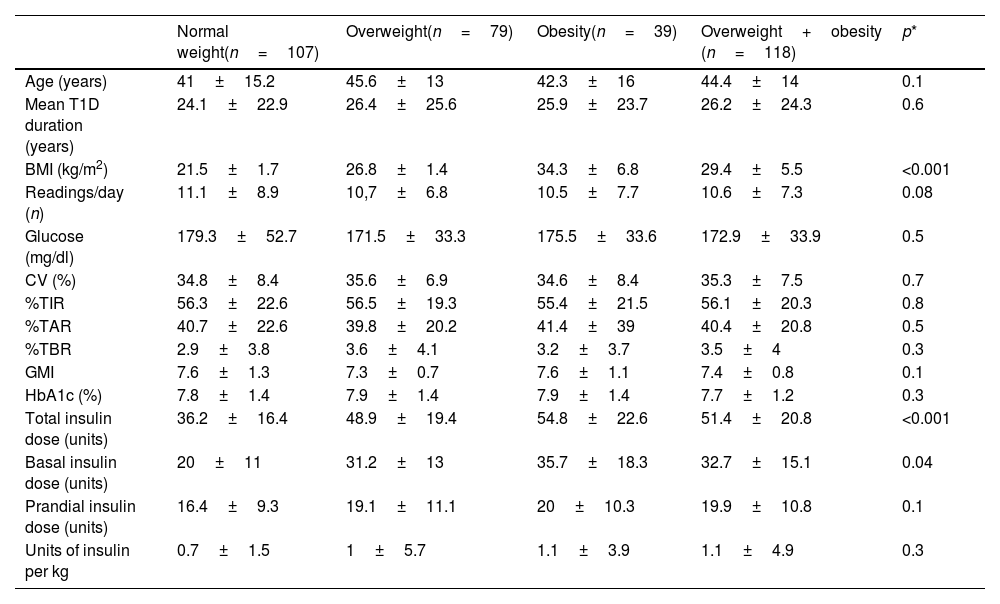
ResultsOf the 225 patients with T1D included, 35.1% (79/225) were overweight and 17.3% (39/225) lived with obesity, while the remaining 47.6% had a normal weight. All groups were comparable in terms of gender, age and mean T1D duration.
The mean number of CGM readings per day was comparable between subjects with normal weight and patients living with overweight or obesity (PwOO) (11.1±8.9 vs 10.6±7.3readings/day; p=0.08). No significant differences were found when comparing mean interstitial glucose (179.3±52.7 vs 172.9±33.9mg/dl; p=0.5) and blood glucose variability (34.8±8.4 vs 35.3±7.5%; p=0.7) between patients with normal weight and PwOO. Not only time in range (%TIR) (56.3±22.6 vs 56.1±20.3%; p=0.8), but also time above range (%TAR) (40.7±22.6 vs 40.4±20.8%; p=0.5) and time below range (%TBR) (2.9±3.8 vs 3.5±4%; p=0.3) were comparable between groups.
Glucose management indicator (GMI) (7.6±1.3 vs 7.4±0.8; p=0.1) and HbA1c (7.8±1.4 vs 7.7±1.2%; p=0.3) were similar between patients with normal BMI and PwOO.
As expected, both the total (36.2±16.4 vs 51.4±20.8; p<0.001) and basal insulin doses (20±11 vs 32.7±15.1; p=0.04) were significantly higher among PwOO. Prandial insulin dose tended to be higher among PwOO, although not statistically significant (16.4±9.3 vs 19.9±10.8; p=0.1). Total units of insulin per kilo were comparable between the two groups (0.7±1.5 vs 1.1±4.9; p=0.3).
These data are shown in Table 1.
Differences between patients with T1D and normal weight and those with T1D and overweight and obesity.
| Normal weight(n=107) | Overweight(n=79) | Obesity(n=39) | Overweight+obesity (n=118) | p* | |
|---|---|---|---|---|---|
| Age (years) | 41±15.2 | 45.6±13 | 42.3±16 | 44.4±14 | 0.1 |
| Mean T1D duration (years) | 24.1±22.9 | 26.4±25.6 | 25.9±23.7 | 26.2±24.3 | 0.6 |
| BMI (kg/m2) | 21.5±1.7 | 26.8±1.4 | 34.3±6.8 | 29.4±5.5 | <0.001 |
| Readings/day (n) | 11.1±8.9 | 10,7±6.8 | 10.5±7.7 | 10.6±7.3 | 0.08 |
| Glucose (mg/dl) | 179.3±52.7 | 171.5±33.3 | 175.5±33.6 | 172.9±33.9 | 0.5 |
| CV (%) | 34.8±8.4 | 35.6±6.9 | 34.6±8.4 | 35.3±7.5 | 0.7 |
| %TIR | 56.3±22.6 | 56.5±19.3 | 55.4±21.5 | 56.1±20.3 | 0.8 |
| %TAR | 40.7±22.6 | 39.8±20.2 | 41.4±39 | 40.4±20.8 | 0.5 |
| %TBR | 2.9±3.8 | 3.6±4.1 | 3.2±3.7 | 3.5±4 | 0.3 |
| GMI | 7.6±1.3 | 7.3±0.7 | 7.6±1.1 | 7.4±0.8 | 0.1 |
| HbA1c (%) | 7.8±1.4 | 7.9±1.4 | 7.9±1.4 | 7.7±1.2 | 0.3 |
| Total insulin dose (units) | 36.2±16.4 | 48.9±19.4 | 54.8±22.6 | 51.4±20.8 | <0.001 |
| Basal insulin dose (units) | 20±11 | 31.2±13 | 35.7±18.3 | 32.7±15.1 | 0.04 |
| Prandial insulin dose (units) | 16.4±9.3 | 19.1±11.1 | 20±10.3 | 19.9±10.8 | 0.1 |
| Units of insulin per kg | 0.7±1.5 | 1±5.7 | 1.1±3.9 | 1.1±4.9 | 0.3 |
All data are expressed as mean±standard deviation (SD) or percentages (%). CV: blood glucose variability; TIR: time in range; TAR: time above range; TBR: time below range; GMI: glucose management indicator; HbA1c: glycated haemoglobin.
A negative correlation was found between GMI and BMI (r=−0.2; p=0.008) (Fig. 1) and HbA1c (r=−0.2; p=0.01). Conversely, a positive correlation was observed between the total dose of insulin and the BMI (r=0.3; p<0.0001) and age and BMI (0.2; p=0.008). No significant correlations were found between BMI and TIR, TAR, TBR, CV, mean interstitial glucose or average daily readings.
DiscussionWe found that the prevalence of obesity among patients with T1D from a Mediterranean sample was 17.3%. However, the presence of obesity among patients with T1D did not mean worse blood glucose control measured not only by HbA1c, but also by CGM metrics. Furthermore, despite not being very powerful, we found a negative correlation between BMI and both GMI and HbA1c.
The frequency of obesity observed in our sample is in line with that reported by Genua et al. in a population-based cohort study in Catalonia, also a Mediterranean area, with a similar mean age, where the prevalence was 18%.17 Therefore, rates of obesity among patients with T1D are similar, or even a little bit lower, than the 23% in the general Spanish population.3 Both figures are in line with studies in other European countries, like Belgium, with a prevalence of 17%,9 or Germany, where a prevalence of 15.3% was reported.8 Obesity rates would therefore seem to increase among patients with T1D in parallel with the general population. However, non-European countries, with a greater prevalence of obesity in the general population, reported a marked lower prevalence of obesity among patients with T1D.7 In fact, a study of temporal trends has shown an increase in obesity among adults with T1D, from 3.4% at baseline (1986–1988) to 22.7% during follow-up (2004–2007), which cannot be explained by the ageing of the cohort and developed more rapidly than the increases in the general population.21 In the light of all these data, we have to accept that T1D is no longer a “disease of lean people”, and be aware of all the comorbidities associated with obesity.
It is still uncertain whether patients with T1D and obesity have poorer control. In fact the majority of studies have focused on the paediatric population. The T1D Exchange Clinic Registry found a positive association between better blood glucose control and risk of obesity.15 Conversely, the SWEET registry concluded that paediatric patients with T1D and obesity had higher levels of HbA1c.16 Genua et al. analysed more than 6000 adults with T1D retrospectively. They found that obesity did not have a detrimental effect on blood glucose control. However, other cardiovascular risk factors were more prevalent and more poorly controlled.17 Another study using the Swedish National Diabetes Registry cohort found that weight gain was associated with an increased risk of mortality, cardiovascular disease and heart failure.18 In another study from Australia, despite similar levels of HbA1c, the authors concluded that a BMI of 30kg/m2 or above was the predominant risk factor for retinopathy and cardiovascular disease.19 However, to date, all the studies have used HbA1c to determine blood glucose control, and none of them provide any data on other glucose metrics often involved in cardiovascular events and microvascular complications, such as blood glucose variability and TIR.22,23 We found a discrete negative correlation between either GMI or HbA1c and BMI, which might suggest that better blood glucose control could contribute to weight gain or maintenance by preventing glycosuria.
The potential mechanisms involved in the development of obesity among people with T1D remain unclear. As expected, we found that insulin needs were greater among subjects with T1D living with obesity compared to patients with normal weight. Data from the DCCT trial showed that weight gain in the intensive treatment group was greater than the conventional and control groups in the first 1.3 years from baseline. Afterwards, the rate of weight gain was slower but continued at a higher rate among patients in the intensive group. However, a larger number of units of insulin or a higher risk of hypoglycaemia could not explain these differences in weight gain per se, suggesting genetic and environmental causes as promoting factors.14
Some genes that predispose to obesity and type 2 diabetes are also connected in T1D. Also, in the DCCT trial, individuals on intensive treatment who had a family history of type 2 diabetes gained more weight.24,25 The increased risk of T1D in children of mothers who lived with obesity suggests that epigenetics also plays an important role.26 Epigenetics could also play a role in energy balance and metabolism in patients with T1D, especially in scenarios where obesity coexists. Mitochondrial dysfunction is a hallmark of obesity, T1D and type 2 diabetes which would benefit from further research. In conclusion, there are still many gaps in the obesity-T1D interaction and a multidisciplinary research strategy is needed which includes lifestyle, genetics, epigenetics, molecular mechanisms and neuropsychology.5
Although not the objective of our study, it is worth noting that the degree of control of our patients was suboptimal, regardless of their weight. Our patients were in a “comfortable” situation, that is, without risk of hypoglycaemia. However, although probably asymptomatic, they had high glucose levels, which would increase the risk of chronic complications in the medium-to-long term. This shows the importance of proper education for these patients.
We acknowledge our study has several limitations. We did not assess body composition or waist circumference and we therefore did not know either the amount or distribution of fat mass. However, most of the previous studies also relied on BMI. Our study was performed in a Mediterranean area, with a specific lifestyle pattern, and could not be generalised to another regions or ethnicities. We did not record other diseases or treatments which may have influenced obesity or blood glucose control. However, this is the first study to have used CGM to assess whether or not obesity impacts blood glucose control. Further studies are necessary to confirm our results.
Obesity is a chronic and relapsing disease associated with multiple complications whose incidence is increasing alarmingly. It seems that the prevalence of obesity is also on the rise among patients with T1D and, although it may not have a negative effect on blood glucose control, early intervention is essential to control other cardiovascular risk factors and preserve quality of life. We would like to stress the importance of including patients with T1D and obesity in anti-obesity medication studies, to address not only blood glucose control but other cardiovascular risk factors, such as obesity, as it appears that T1D is no longer a disease associated with thinness.
In conclusion, obesity does not mean worse blood glucose control in patients with T1D or less use of CGM. To achieve good blood glucose control, more units of insulin may be necessary in these patients and this, in turn, makes weight control more difficult.
Authors’ contributionsJN wrote the manuscript, researched data, and gave approval of the final version. IR, AR and MP researched data and helped in writing the article. PS performed the statistics and reviewed the manuscript. LM contributed to the discussion, reviewed the manuscript, and gave approval of the final version. All authors have approved the final article.
Ethical approvalThe study was approved by the hospital's Ethics Committee. Written informed consent was obtained from all patients prior to study participation.
Conflicts of interestAll authors declare no conflicts of interest regarding this manuscript.