There are situations of short stature, with a normal stimulus test for GH, but decreased nocturnal secretion in which there could be a benefit with GH treatment.
ObjetivesTo assess adult height and height gain in patients with neurosecretory dysfunction diagnosis treated with growth hormone.
Material y methodsLongitudinal, retrospective and observational study including 61 patients treated with growth hormone after diagnosis of neurosecretory dysfunction who have already reached adult height. Variables such as adult height gain, growth rate, growth prognosis variation and IGF-I and IGFBP-3 were evaluated. Variables related to a good response in the first year have also been calculated, using the Index of responsiveness (IoR).
ResultsGH treatment produces an improvement in growth rate and height, observing an increase in adult height with respect to initial height of 1.15±0.60 SD, height with respect to genetic height of -0.015±0.62 SD and adult height with respect to the initial growth prognosis 0,74±1,13 DE. The IoR in the first year is associated with a greater increase in height in the first year (p=0.000), with a greater adult height (p=0.000) and with a greater gain in adult height compared to its initial height (p=0.039).
ConclusionsPatients with growth delay due to neurosecretory dysfunction of GH show a good response to treatment with rhGH, observing a significant height gain in their genetic size and improving their initial growth prognosis.
Existen situaciones de talla baja, con test de estímulo para GH normal, pero secreción nocturna disminuida en la que podría existir un beneficio con el tratamiento con GH.
ObjetivoEvaluar la talla adulta y la ganancia de talla en pacientes con diagnóstico de disfunción neurosecretora tratados con hormona del crecimiento.
Material y métodosEstudio longitudinal, retrospectivo y observacional con 61 pacientes tratados con GH tras diagnóstico de disfunción neurosecretora que han alcanzado la talla adulta. Se evaluaron variables como la ganancia de talla adulta, la velocidad de crecimiento, la variación del pronóstico de crecimiento y la evolución de IGF-I e IGFBP-3. También han sido calculadas variables de buena respuesta en el primer año, mediante el uso del Index of responsiveness (IoR).
ResultadosHubo una mejoría en la talla y en la velocidad de crecimiento, observándose un incremento de talla adulta respecto a talla inicial de 1,15±0,60DE, talla respecto a talla genética de -0,015±0,62DE y talla adulta respecto a pronóstico de crecimiento inicial 0,74±1,13 DE. El IoR en el primer año se asocia con un mayor incremento de talla en el primer año (p=0.000), una mayor talla adulta (p=0,000), y una mayor ganancia de talla adulta respecto a su talla inicial (p=0,039).
ConclusionesLos pacientes diagnosticados de disfunción neurosecretora muestran una buena respuesta al tratamiento con GH observándose una ganancia de talla similar a su talla genética y mejorando su pronóstico de crecimiento inicial.
Short stature is a very common reason for consultation and concern in paediatrics. One of the causes may be neurosecretory dysfunction, characterised by obtaining a normal result in growth hormone (GH) stimulation tests, normal or high IGF-1 levels, but with abnormal nocturnal secretion test results (mean of 21 determinations); a mean ≤3ng/mL1 is a diagnostic criterion.
Growth is a complex process involving genetic, endocrine and autocrine-paracrine factors, as well as permissive factors such as nutrition, psychosocial situation and other environmental factors.2 It is therefore a process sensitive to unfavourable conditions, making it a good indicator of health.3 GH secretion has a pulsatile pattern and is predominantly nocturnal, but it is still a largely unknown physiological and pathophysiological process in terms of the pattern of secretion and the pathways through which the different factors modify and regulate it. In the case of neurosecretory dysfunction, there is an altered nocturnal secretion pattern at the expense of a reduction in the number and amplitude of the secretion pulses.
Not all authors find a correlation between height or growth rate and spontaneous GH secretion. This topic has been studied extensively in the literature.1,4,5 Back in 1984, in patients with acute lymphoblastic leukaemia who had received treatment with prophylactic cranial radiotherapy, Blatt et al. found an alteration in spontaneous GH secretion and a normal response to stimulation tests, with a reduction in growth rate. This was similar to what occurred in non-irradiated patients with impaired growth, and the idea that there could be abnormalities in the neuroregulation of GH secretion was mooted.6
Short stature takes on particular importance in the population: it is the leading cause of consultation in endocrinology departments because of the associated psychological consequences. Short stature is defined as: (1) height below the 3rd percentile or −2 SD with respect to the normal curve for the same age and gender of the population to which the person belongs; (2) with a growth rate less than P25 (−1 SD) for age and gender, maintained for at least two years, regardless of current height; (3) height which, being within ±2 SD of the curve corresponding to the person's population, is below −2 SD with respect to their target height; and (4) a prediction of adult height below −2 SD with respect to the person's target height.7
Short stature is classified into two fundamental groups, idiopathic short stature and pathological short stature (primary growth or disease-related disorders, for example gastrointestinal, oncological and chronic kidney diseases, etc).8,9 Idiopathic short stature (80% of cases) encompasses all short stature conditions in which the cause is unknown and in which the following criteria are also met: (1) normal length and weight at birth for gestational age; (2) normal body proportions; (3) absence of endocrine disease, chromosomal disease, severe psycho-affective disorders, chronic organic disease; (4) adequate nutrition; and (5) normal or slow maturation time. In other words, idiopathic short stature is a diagnosis of exclusion.10
An appropriate diagnostic approach is necessary from the initial medical consultations, with a detailed previous medical history taken, followed by a thorough physical examination. On the basis of these data, the appropriate investigations will be carried out to rule out causes of pathological short stature and, only if they are ruled out, to consider idiopathic short stature.
These investigations include GH stimulation tests, although they have limitations in terms of reproducibility, which questions their real validity as an instrument for the study of pituitary GH secretion. Despite that, they continue to be used in most countries for the diagnosis of GH deficiency and resistance. However, the value of these tests should, when appropriate, be considered in the clinical context of auxological, imaging and molecular studies.11
As has already been said, the use of GH stimulation tests continues to be a controversial issue, as no defined limits of normality have been established, and moreover they are poorly reproducible. Values considered normal are often found in patients with severe short stature and low growth rates.
The objective of this study was to evaluate the response to GH treatment in patients diagnosed with neurosecretory dysfunction, with normal GH in the stimulus test, treated until they reach adult height.
Material and methodsThis was a longitudinal, retrospective, observational study which included 61 patients being followed up by the paediatric endocrinology clinic of a tertiary hospital, treated with GH after the diagnosis of neurosecretory dysfunction and who had reached adult height at the time of the study. The use of GH was funded by the regional health service. This research project was approved by the region's Independent Ethics Committee.
The inclusion criteria were as follows:
- a)
Height less than −2 SD at diagnosis.
- b)
GH stimulation test >10ng/mL.
- c)
Nocturnal secretion test: mean of 21 determinations less than 3ng/mL.
- d)
Have received replacement therapy with rhGH.
- e)
Have been born with an appropriate weight and length for the gestational age.
- f)
Have reached adult height (the height reached at the last consultation, a Tanner stage V and bone age >14 years in females and >16 years in males).
The exclusion criteria were as follows:
- a)
Existence of intercurrent diseases that affect growth (for example, being born small for the gestational age, syndromes and bone dysplasias).
- b)
Lack of continuity of treatment during follow-up.
- c)
Not have completed growth at the time of the study.
- d)
Have received concomitant treatment with gonadotropin-releasing hormone analogues (GnRH).
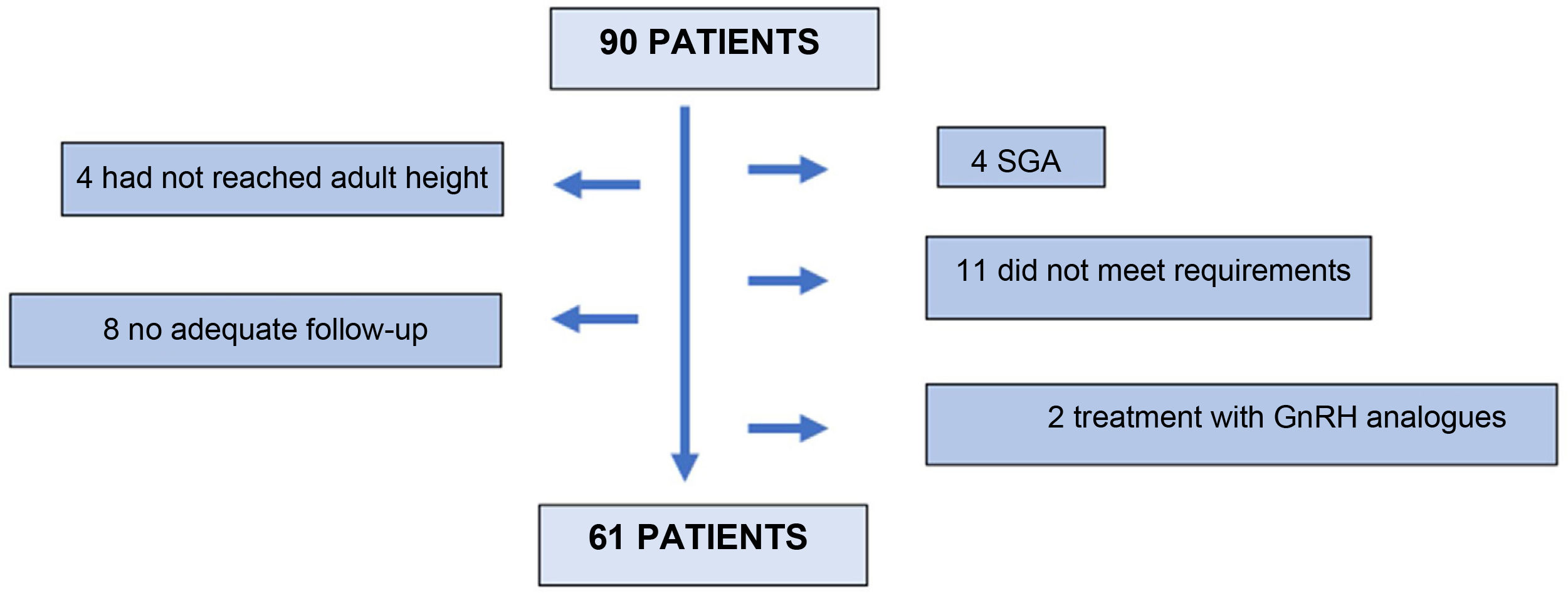
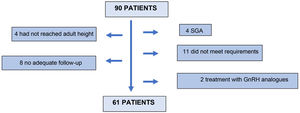
Ninety (90) patients were screened, 61 of whom finally entered the study. Twenty-nine (29) patients were excluded: 4 for not having reached adult height at the last visit; 8 for inadequate follow-up; 4 patients for being small for gestational age; 11 for not meeting the inclusion criteria in the stimulus or nocturnal secretion test; and 2 for receiving concomitant treatment with GnRH analogues (Fig. 1).
Anthropometric and analytical variables were evaluated at different times during the study (prior to treatment, at the beginning of treatment, after 4 months of treatment, at the beginning of puberty, after 1year of treatment, after 2 years of treatment and at the last consultation). One of the parameters studied was the index responsiveness (IoR), which is used in patients with GH deficiency to estimate whether or not they will be good responders to GH therapy. The IoR is calculated using the following formula:
First year IoR=(growth rate in first year 12.41 – 0.36 × age when started GH+0.47 × birth-weight SD+1.54 × (log(3 × dose at start of GH (mg/kg/week))) – 0.6 × (height at first year SD – genetic height SD) + 0.28 × weight first year SD))/1.72.
Second year IoR=(growth rate in second year – (5.69 – 0.09 × age when started GH+0.63 × (log(3 × dose at start of (mg/kg/week))) + 0.24 × weight at second year SD+0.31 × speed of growth in first year))/1.19.
Parameters of good response in the first year of treatment were also analysed: height gain ≥0.5 SD compared to previous; height gain ≥0.3 SD compared to previous; increase in growth rate >3cm/year compared to previous; and growth rate >1 SD compared to previous.
Final response variables were evaluated, such as the difference between adult height and height at the start of GH treatment. The difference between adult height and genetic height and the difference between adult height and growth prognosis at the start of treatment were also calculated.
A descriptive statistical study was then carried out at the different points of the study. For each variable, the central and dispersion measures (arithmetic mean, standard deviation, maximum and minimum) were calculated for the distributions that fulfilled normal distribution. The Kolmogorov-Smirnov test was applied to check the normality of each quantitative variable. Student's t-test for paired samples was used to compare means of quantitative variables. The degree of correlation between two quantitative variables was also determined by calculating the Pearson correlation coefficient. Finally, the data analysis was performed using the IBM SPSS Statistics version 25 statistical package; results in which p<0.05 were considered statistically significant.
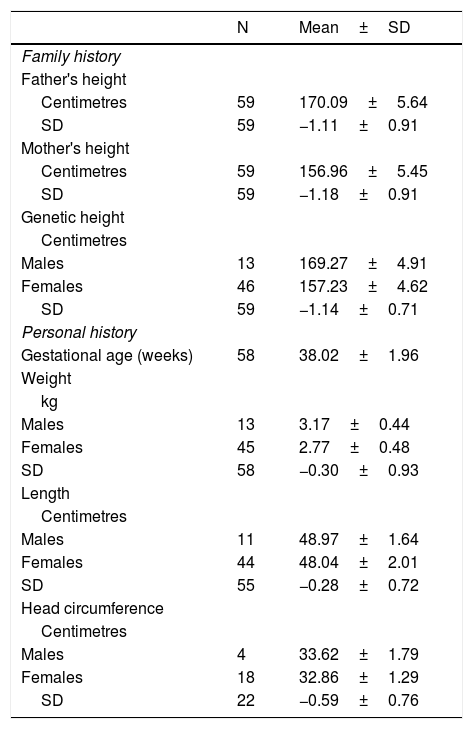
ResultsThe study included 61 patients diagnosed with neurosecretory dysfunction (78.7% females, 21.3% males) treated with GH who had reached adult height. All of them had a GH stimulation test and an mean of 12.55±8.16ng/mL was obtained. Once GH deficiency had been ruled out, the nocturnal secretion test was performed, with a mean of 1.98±0.62ng/mL. The analysis of the patients' personal history showed that our sample had normal weight, length and head circumference for their gestational age. There was no family history of short stature and genetic height was −1.14±0.71 SD (Table 1).
Family and personal history and target height.
| N | Mean±SD | |
|---|---|---|
| Family history | ||
| Father's height | ||
| Centimetres | 59 | 170.09±5.64 |
| SD | 59 | −1.11±0.91 |
| Mother's height | ||
| Centimetres | 59 | 156.96±5.45 |
| SD | 59 | −1.18±0.91 |
| Genetic height | ||
| Centimetres | ||
| Males | 13 | 169.27±4.91 |
| Females | 46 | 157.23±4.62 |
| SD | 59 | −1.14±0.71 |
| Personal history | ||
| Gestational age (weeks) | 58 | 38.02±1.96 |
| Weight | ||
| kg | ||
| Males | 13 | 3.17±0.44 |
| Females | 45 | 2.77±0.48 |
| SD | 58 | −0.30±0.93 |
| Length | ||
| Centimetres | ||
| Males | 11 | 48.97±1.64 |
| Females | 44 | 48.04±2.01 |
| SD | 55 | −0.28±0.72 |
| Head circumference | ||
| Centimetres | ||
| Males | 4 | 33.62±1.79 |
| Females | 18 | 32.86±1.29 |
| SD | 22 | −0.59±0.76 |
SD, standard deviation; kg, kilograms; N, number of patients.
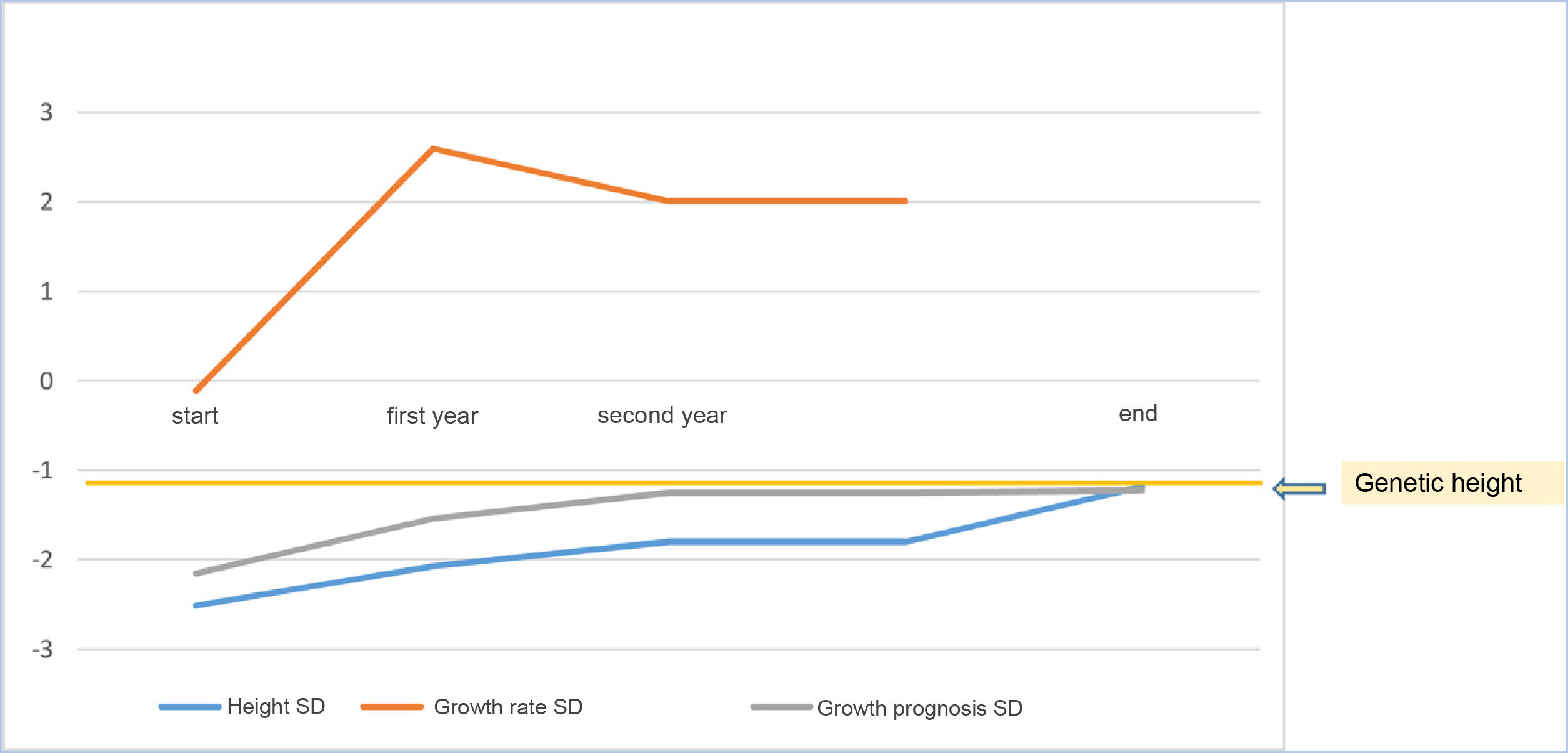
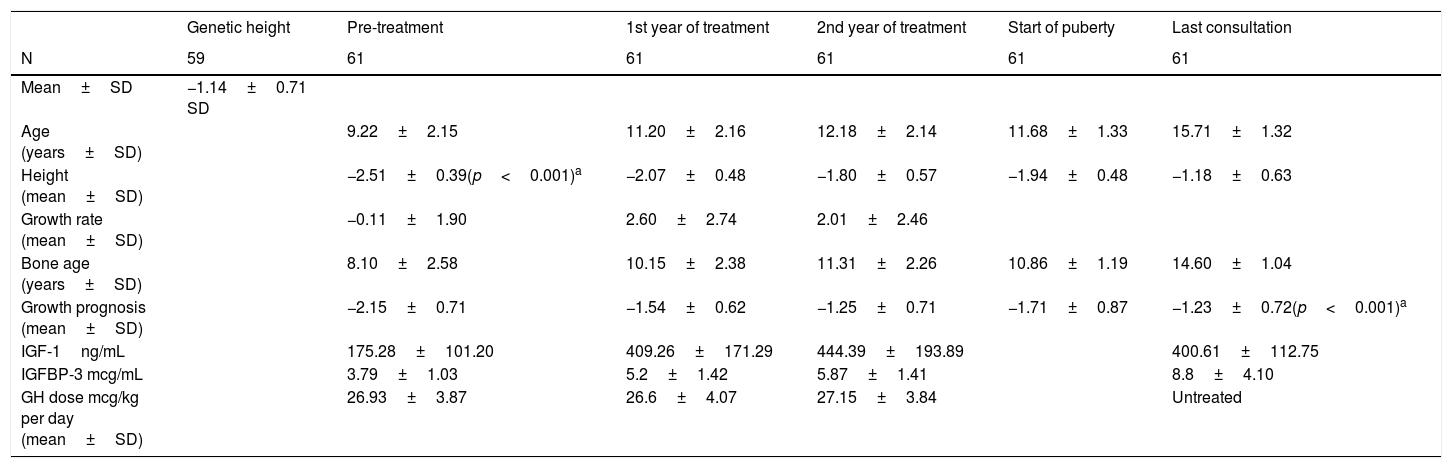
At the start of treatment, the mean chronological age of the patients was 10.17±2.13 years (males 10±2.61years and females 10.09±2.01years) with a height of −2.53±0.39 SD, and a growth prognosis of −2.15±0.71 SD. When pubertal stage at the start was analysed, 65.6% were prepubescent (Tanner I), while 34.4% had started puberty (24.6% Tanner II and 9.8% Tanner III).
The mean age in the first year of treatment was 11.20±2.16 years, with a height of −2.07±0.48 SD and a growth rate of 2.60±2.74 SD. Good response data were analysed in the first year and 78.7% were found to increase their height in SD≥0.5; 80.3% showed an increase in height in SD≥0.3; 37.7% had an increase in their growth rate >3cm/year and 72.1% increased their growth rate >1 SD.
At two years of treatment, the mean age was 12.18±2.14 years, with a height of −1.80±0.57 SD, a growth rate of 2.01±2.46 SD and a growth prognosis of −1.25±0.71 SD.
Finally, once adult height had been reached, the mean age was 15.71±1.32 years, with a height of −1.18±0.63 SD (Table 2), being at their genetic height (−1.14±0.71 SD; 169.27±4.91cm for males 157.23±4.62cm in females) (Table 2).
Changes in anthropometric data during the study.
| Genetic height | Pre-treatment | 1st year of treatment | 2nd year of treatment | Start of puberty | Last consultation | |
|---|---|---|---|---|---|---|
| N | 59 | 61 | 61 | 61 | 61 | 61 |
| Mean±SD | −1.14±0.71 SD | |||||
| Age (years±SD) | 9.22±2.15 | 11.20±2.16 | 12.18±2.14 | 11.68±1.33 | 15.71±1.32 | |
| Height (mean±SD) | −2.51±0.39(p<0.001)a | −2.07±0.48 | −1.80±0.57 | −1.94±0.48 | −1.18±0.63 | |
| Growth rate (mean±SD) | −0.11±1.90 | 2.60±2.74 | 2.01±2.46 | |||
| Bone age (years±SD) | 8.10±2.58 | 10.15±2.38 | 11.31±2.26 | 10.86±1.19 | 14.60±1.04 | |
| Growth prognosis (mean±SD) | −2.15±0.71 | −1.54±0.62 | −1.25±0.71 | −1.71±0.87 | −1.23±0.72(p<0.001)a | |
| IGF-1ng/mL | 175.28±101.20 | 409.26±171.29 | 444.39±193.89 | 400.61±112.75 | ||
| IGFBP-3 mcg/mL | 3.79±1.03 | 5.2±1.42 | 5.87±1.41 | 8.8±4.10 | ||
| GH dose mcg/kg per day (mean±SD) | 26.93±3.87 | 26.6±4.07 | 27.15±3.84 | Untreated |
SD, standard deviation; N, sample size.
Patients received GH therapy for a mean of 4.71±2.15 years, with a stable mean GH dose throughout the years of treatment (start 26.93±3.87 mcg/kg per day; first year 26.6±4.07 mcg/kg per day; second year 27.15±3.84 mcg/kg per day).
The IoR in the first year correlated positively with the adult height in SD (r=0.454; p<0.001) and with the height gain in SD in the first year (r = 0.482; p<0.001). It also correlated positively with the difference in adult height in SD with respect to height at the start of treatment (r = 0.27; p= 0.039). However, it was not associated with the difference in adult height SD compared to genetic height in SD or with the difference in adult height with respect to growth prognosis at baseline in SD or with the increase in growth rate in the first year (Table 3).
Calculation of the index of responsiveness in the first year.
| IoR | |||
|---|---|---|---|
| N | r | p | |
| Adult height SD | 61 | 0.454 | <0.001 |
| Difference in adult height SD compared to height at the start of treatment SD | 61 | 0.277 | 0.039 |
| Difference in adult height SD compared to genetic height SD | 61 | 0.099 | 0.466 |
| Difference in adult height SD compared to growth prognosis at the start SD | 49 | −0.253 | 0.08 |
| Height gain in SD in the first year | 58 | 0.482 | <0.001 |
| Increase in growth rate in cm/year in the first year | 59 | 0.171 | 0.196 |
| Increase in growth rate in SD in the first year | 59 | 0.487 | <0.001 |
SD, standard deviation; IoR, index of responsiveness; N, sample size; r, Pearson's correlation coefficient.
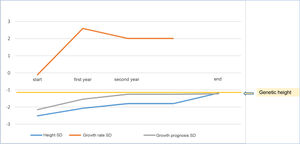
As a mean, patients remained at −0.015±0.62 SD of their target height, improved 1.15±0.60 SD in height compared to their height at the start of treatment, 0.74±1.13 SD compared to the growth prognosis prior to the start of the treatment and 0.78±0.63 SD compared to the growth prognosis at the onset of puberty (Fig. 2).
Biochemically, both IGF-1 and IGFBP-3 levels increased in the first two years (always within normal ranges) and then diminished following the completion of treatment.
No major adverse effects were recorded in the study. Only one of the 61 patients analysed reported headache, with the eye examination showing a normal fundus, requiring temporary suspension of GH, although it was subsequently resumed without any incidents.
DiscussionThe study included 61 patients (78.7% female) being followed up by the paediatric endocrinology clinic of a tertiary hospital, treated with GH after the diagnosis of neurosecretory dysfunction, who had reached adult height at the time of the study; 65.6% were prepubescent at the start of treatment. The patients managed to achieve their genetic height and improved their initial growth prognosis.
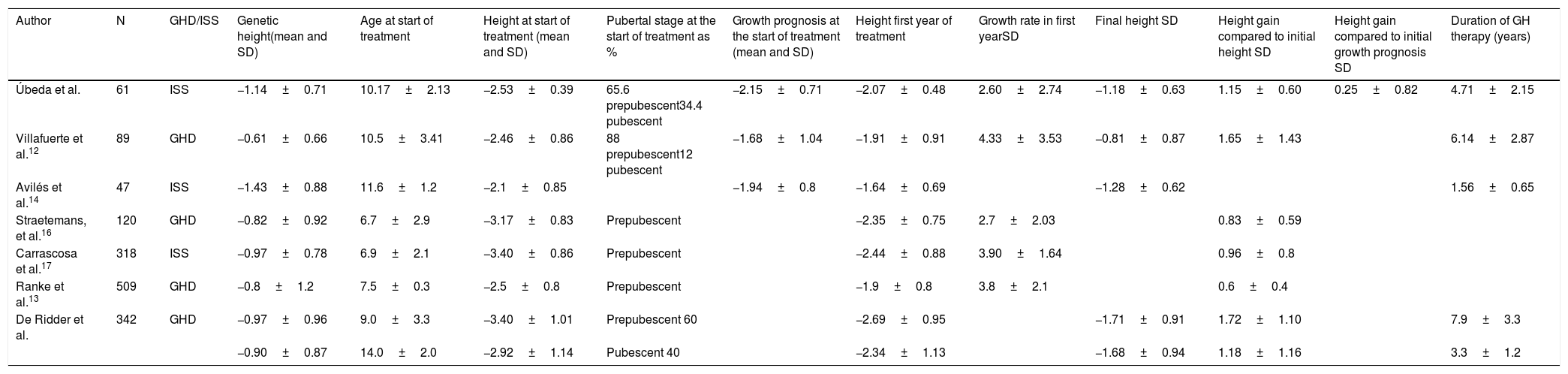
At the start of the treatment, the mean chronological age of the patients was 10.17±2.13 years, with a height of −2.53±0.39 SD. The start of the treatment may seem late, but this may be because neurosecretory dysfunction tends to appear around puberty. This may explain why we found a higher percentage of pubescent patients in our sample compared to previous studies, such as the one by Villafuerte et al.,12 a retrospective study of patients with GH deficiency, in which only 6.3% had started puberty at the start of treatment. This may explain why the predictions of adult height are slightly more favourable in that study, since when they started treatment they had a greater growth potential. Comparing the data, we see that the growth prognosis prior to the start of treatment with GH in our sample was −2.15±0.71 SD (164.19±5.37cm in males and 150.90±4cm in females), while in the aforementioned study the growth prognosis was −1.74±0.99 SD. The growth rate prior to starting treatment was −0.11±1.9 SD. Previous studies report similar data regarding treatment initiation age, height in SD at the start of the study and adult height prognosis in SD.13–17
After the first year of treatment, the patients had a height of −2.07±0.48 SD with a growth prognosis of −1.54±0.62 SD and a growth rate of 2.60±2.74 SD. Similar data in the first year of treatment were reported by Avilés et al.15,18 with regard to the height attained in the first year in patients with idiopathic short stature and with regard to growth velocity. Spiliotis et al.19 had previously compared patients with GH deficiency and patients with neurosecretory dysfunction, finding an increase in growth rate which doubled in the first year, similar to our sample.
The assessment of the good response data in the first year of treatment with GH showed that 78.7% increased their height in SD≥0.5; 80.3% showed an increase in height in SD≥0.3; 37.7% had an increase in their growth rate >3cm/year and 72.1% increased their growth rate >1 SD. The data obtained in different studies18–23 carried out in patients diagnosed with GH deficiency show a similar percentage of good responders to those obtained in our sample. In the above study, 74% had height gain in SD>0.5; 88% had an increase in height >0.3 SD; and 81% had an increase in growth rate >1 SD, which suggests that the response in this condition may be similar to the response in patients with GH deficiency.
Several studies12,14 show similar data for the final height reached, while others show a better final height than that obtained in our sample. In these studies, the population studied was younger at the start of the study, with a higher percentage of prepubescent children and also a greater genetic height.15 The mean GH treatment time was somewhat longer, with a mean of 5.4 years,12,15,24 which may be related to the earlier start of treatment (Table 4).
Comparative table. Studies analysed.
| Author | N | GHD/ISS | Genetic height(mean and SD) | Age at start of treatment | Height at start of treatment (mean and SD) | Pubertal stage at the start of treatment as % | Growth prognosis at the start of treatment (mean and SD) | Height first year of treatment | Growth rate in first yearSD | Final height SD | Height gain compared to initial height SD | Height gain compared to initial growth prognosis SD | Duration of GH therapy (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Úbeda et al. | 61 | ISS | −1.14±0.71 | 10.17±2.13 | −2.53±0.39 | 65.6 prepubescent34.4 pubescent | −2.15±0.71 | −2.07±0.48 | 2.60±2.74 | −1.18±0.63 | 1.15±0.60 | 0.25±0.82 | 4.71±2.15 |
| Villafuerte et al.12 | 89 | GHD | −0.61±0.66 | 10.5±3.41 | −2.46±0.86 | 88 prepubescent12 pubescent | −1.68±1.04 | −1.91±0.91 | 4.33±3.53 | −0.81±0.87 | 1.65±1.43 | 6.14±2.87 | |
| Avilés et al.14 | 47 | ISS | −1.43±0.88 | 11.6±1.2 | −2.1±0.85 | −1.94±0.8 | −1.64±0.69 | −1.28±0.62 | 1.56±0.65 | ||||
| Straetemans, et al.16 | 120 | GHD | −0.82±0.92 | 6.7±2.9 | −3.17±0.83 | Prepubescent | −2.35±0.75 | 2.7±2.03 | 0.83±0.59 | ||||
| Carrascosa et al.17 | 318 | ISS | −0.97±0.78 | 6.9±2.1 | −3.40±0.86 | Prepubescent | −2.44±0.88 | 3.90±1.64 | 0.96±0.8 | ||||
| Ranke et al.13 | 509 | GHD | −0.8±1.2 | 7.5±0.3 | −2.5±0.8 | Prepubescent | −1.9±0.8 | 3.8±2.1 | 0.6±0.4 | ||||
| De Ridder et al. | 342 | GHD | −0.97±0.96 | 9.0±3.3 | −3.40±1.01 | Prepubescent 60 | −2.69±0.95 | −1.71±0.91 | 1.72±1.10 | 7.9±3.3 | |||
| −0.90±0.87 | 14.0±2.0 | −2.92±1.14 | Pubescent 40 | −2.34±1.13 | −1.68±0.94 | 1.18±1.16 | 3.3±1.2 |
SD, standard deviation; GHD, growth hormone deficiency; N, sample size; ISS, idiopathic short stature.
During treatment with GH, the patients' heights improved by 1.15±0.60 SD compared to the start of treatment and 0.74±1.13 SD compared to the pre-treatment growth prognosis. The height gain versus initial height and versus the initial growth prognosis was similar to those of previous studies.12,13,15
With regard to the dose of GnRH, the dose of GH used in previous studies was higher, with a mean of 31 mcg/kg per day,15 while in our sample a stable mean dose of GH was used over the years of treatment (start 26.93±3.87 mcg/kg per day; first year 26.6±4.07 mcg/kg per day; second year 27.15±3.84 mcg/kg per day).
Finally, adverse effects are uncommon and few and far between.22 In our study, one patient required temporary discontinuation of GH due to headache. However, here eye fundus examination was normal and the patient was subsequently able to resume treatment.
On the basis of the evidence discussed above, patients treated with GnRH for a diagnosis of neurosecretory dysfunction have a good response, achieving a final height similar to that of patients treated for GH deficiency, and this good response can be detected as of the first year.22,23
As conclusions of the study, it should be noted that patients with growth retardation and normal GH stimulation test diagnosed with neurosecretory dysfunction present a good response to treatment with GnRH, similar to that of patients affected by GH deficiency, as they achieve their genetic height and improve their initial growth prognosis. As in GH deficiency, the IoR during the first year makes it possible to assess which patients will be good responders to treatment. The levels of IGF-1 and IGFBP-3 are normal before treatment with GnRH and then rise after starting, although they remain within normal limits at all times. For all the above reasons, we question the obligatory nature of two stimulus tests for the diagnosis of GH deficiency and the exclusion of patients with short stature and normal tests from treatment because, as this study has demonstrated, it can be beneficial.
Conflicts of interestThe authors declare that they have no conflicts of interest.
This work was presented as a poster in October 2020 at the Congress of the Sociedad Española de Endocrinológica Pediátrica (SEEP) [Spanish Society of Paediatric Endocrinology] in Zaragoza and as a Master's dissertation on Genetic, Nutritional and Environmental Conditioners of Growth and Development at the University of Zaragoza, in the 2019–2020 academic year.