We present the case of a 46-year-old man with no relevant medical history who was referred to the endocrinology clinic due to a recent swelling on the left side of the anterior aspect of his neck. On examination, a goitre was found in the left lobe of his thyroid gland. A neck ultrasound scan revealed a mass in the upper and middle segments of the left lobe of the patient's thyroid gland measuring 46 × 33 × 50 mm (L × AP × CC), hypoechoic, with lobulated borders and markedly vascularised, as well as lymphadenopathy on the left at levels II, III, V and VI. Taken the same time, fine needle aspiration biopsy showed a highly atypical cells, classified as Bethesda V, for which he was referred to endocrine surgery.
Two months later, he underwent total thyroidectomy and dissection of the central and left lateral cervical compartments. Pathology analysis of the surgical specimen revealed Hürthle cell follicular carcinoma with areas of carcinoma poorly differentiated in the left thyroid lobe and several areas of metastatic lymphadenopathy. The cancer had invaded the thyroid capsule and adjacent muscle tissue. There were also signs of extensive vascular invasion and the surgical margins were affected.
One month after surgery, an analysis was performed showing thyroglobulin (Tg) 244.9 ng/mL and thyroglobulin antibodies (TgAb) 37 IU/mL (the reference value at our hospital is less than 80 IU/mL). A whole body scan was carried out, revealing isotope retention at the left paratracheal, upper mediastinal and bilateral lung regions, consistent with tumour spread (pT3bN1M1). It was therefore decided to complete treatment with an ablative dose of 150 mCi of I-131. Subsequently, he was confirmed to be refractory to I-131, with a marked rise in Tg (592.4 ng/mL at three months and 1,071 ng/mL at six months).
The patient was referred to medical oncology, but before the first consultation, he was admitted due to superior vena cava syndrome related to disease progression, and it was decided to start lenvatinib. Two weeks later, new elevation of transaminases was found (AST 44 U/l [normal: 13–40] and ALT 172 U/l [normal: 7–40], the figure prior to starting lenvatinib being 36 U/l). As such, treatment was discontinued.
Two weeks after that, the patient was readmitted with a 24 -h history of epigastric pain, which he described as "stabbing", radiating around the waist, with amylase 96 U/l and lipase 472 U/l. Lenvatinib-induced acute pancreatitis was suspected. The CT performed that day revealed an acute haematoma in the tail of pancreas, which was classified as acute pancreatitis secondary to pancreatic haemorrhage. Any history of abdominal trauma was ruled out.
The following day he became haemodynamically unstable and repeat CT scan showed atraumatic splenic rupture with haemoperitoneum (Fig. 1A and B). In view of the patient's hypovolaemic shock, it was decided to operate, performing splenectomy and resection of the tail of pancreas, with histopathology ruling out tumour invasion in these organs. He subsequently went into hypovolaemic shock again, this time due to bleeding from the short gastric arteries, which required urgent embolisation (Fig. 1 C–G). He also developed a pulmonary embolism, but in view of his recent bleeding, he was not anticoagulated. At a later stage, the patient started having melaena, with worsening anaemia, requiring several transfusions and emergency sleeve gastrectomy after becoming haemodynamically unstable. Finally, the patient's pro-haemorrhagic state was brought under control and six weeks after admission we were able to discharge him home.
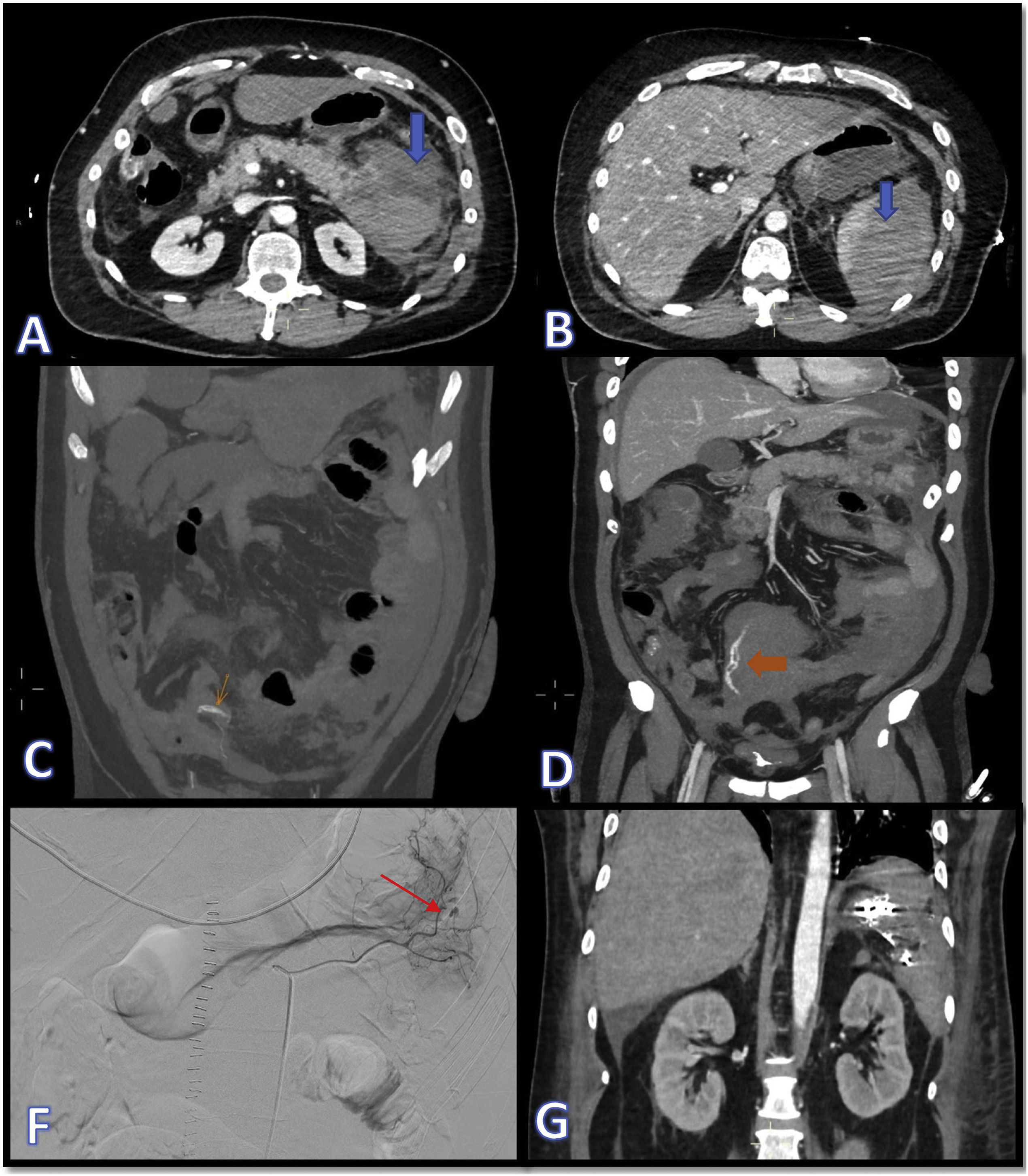
Images A and B show CT slices in the axial plane where dense fluid collections can be seen in relation to the pancreatic and splenic parenchyma, compatible with acute parenchymal haematomas (blue arrows). In images C and D, marked with orange arrows, signs of active bleeding can be seen, consistent with extravascular linear dense images, compatible with contrast extravasation. Images F and G show, respectively, the therapeutic arteriogram for control of the active bleeding (the red arrow indicates points of active bleeding) and the outcome by CT in the coronal plane with metallic embolisation material in the left hypochondrium.
Lenvatinib is an oral tyrosine kinase inhibitor which inhibits vascular endothelial growth factor (VEGF) receptors 1–3, fibroblast growth factor (FGF) receptors 1–4, platelet-derived growth factor receptor-α (PDGFRα), RET and KIT.1–3 It is indicated for treatment in adult patients with progressive, locally advanced or metastatic differentiated thyroid carcinoma refractory to radioactive iodine therapy.1
Lenvatinib demonstrated its clinical efficacy in the SELECT study, a multicentre, randomised, double-blind, placebo-controlled study conducted in 392 patients with radioactive iodine-refractory differentiated thyroid cancer. This study found a statistically significant increase in progression-free survival (PFS) in patients treated with lenvatinib compared to placebo (median PFS of 18.3 months with lenvatinib versus 3.6 months with placebo). A statistically significant increase was also seen in the response rate (64.8% in the lenvatinib group vs 1.5% in the placebo group).1,2
Lenvatinib is not without its side effects. One of the adverse reactions attributable to this treatment is liver failure, classified as rare (affects 1/100 to 1/1,000 cases).1 With the elevation of ALT, four times above the upper limit of normal, in our patient's blood tests two weeks after starting treatment, discontinuing the lenvatinib seemed prudent.
Bleeding as a side effect of lenvatinib is classified as very common, including epistaxis, gingival bleeding, haemoptysis, petechiae, haematuria, rectal bleeding and vaginal bleeding.1 We have found no reported cases of non-cancer-related lenvatinib-induced haemorrhagic pancreatitis.
Among the causes of atraumatic rupture of the spleen, we mainly found infectious diseases (such as mononucleosis or malaria) and haematological cancers in up to half of the cases, with cancer of the spleen being rare.4 We also found no reports in the literature of non-cancer-related spontaneous rupture of the spleen associated with this drug.
Some authors propose personalising the lenvatinib dose in cases of severe bleeding, in order to avoid stopping treatment, and there have even been reports of a significant clinical and radiological response with a dose of 14 mg every three days.5 Despite the serious adverse reactions our patient developed, we are considering reintroducing lenvatinib in an attempt to find an effective dose with no adverse effects.
FundingNo subsidies were received for the work.