To determine the impact of switching from the predictive low glucose suspend (PLGS) system to the advanced hybrid Tandem Control-IQ system on glucometrics and glycosylated haemoglobin (HbA1c) at one year. To assess the impact on the quality of life perceived by parents.
MethodProspective study in 71 patients aged 6–18 years with type 1 diabetes (DM1), in treatment with PLGS, who switched to an advanced hybrid system. Glucometric data were collected before the change, at 4 and 8 weeks, and at one year of use; HbA1c before the change and after one year. The Diabetes Impact and Devices Satisfaction (DIDS) questionnaire was used at weeks 4 and 8.
ResultsAn increase in time in range (TIR) was observed with a median of 76% (P<.001) at 4 weeks, which was maintained after one year (+8% in the total group). Overall, 73.24% of patients achieved a TIR above 70%. The subgroup with an initial TIR of less than 56% increased it by 14.4%. After one year there was a 0.3% reduction in HbA1c. Level 1 hypoglycaemia, level 1 and level 2 hyperglycaemia, mean glucose (GM) and coefficient of variation (CV) decreased.
Auto mode stayed on 97% of the time and no dropouts occurred.
Caregivers had a perception of better glycaemic control and less need to monitor blood glucose variations during the night. None of them would switch back to the previous system and they feel safe with the new system.
ConclusionsThe Tandem Control-IQ advanced hybrid system was shown to be effective one year after its implementation with improvement in all glucometric parameters and HbA1c, as well as night-time rest in caregivers.
Determinar el impacto del cambio del sistema PLGS (parada por predicción de hipoglucemia) al sistema híbrido avanzado Tandem Control-IQ sobre la glucométrica y la hemoglobina glucosilada (HbA1c) al año. Valorar el impacto sobre la calidad de vida percibida en los padres.
MétodoEstudio prospectivo en 71 pacientes entre 6 y 18 años con diabetes tipo 1 (DM1), en tratamiento con PLGS, que cambiaron a sistema híbrido avanzado. Se recogen glucometrías antes del cambio, a las 4 y 8 semanas y al año de uso; HbA1c antes del cambio y al año. Se aplica el cuestionario Diabetes Impact and Devices Satisfaction (DIDS) a las 4 y 8 semanas.
ResultadosSe objetivó un aumento del tiempo en rango (TIR) con un 76% de mediana (P<,001) a las 4 semanas, que se mantiene tras un año (+8% en grupo total). El 73,24% de pacientes alcanzan un TIR por encima del 70%. El subgrupo con TIR inicial menor al 56% lo incrementan un 14,4%. Al año se reduce un 0,3% en HbA1c. Disminuyen las hipoglucemias de nivel 1, hiperglucemias de nivel 1 y 2, glucosa media (GM) y coeficiente de variación (CV).
El modo automático se mantiene en el 97% del tiempo y no se producen abandonos.
Los cuidadores tienen una percepción de mejor control glucémico y menor necesidad de vigilar las variaciones de glucemia durante la noche. Ninguno cambiaría al sistema previo y se sienten seguros con el nuevo sistema.
ConclusionesEl sistema híbrido avanzado Tandem Control-IQ se mostró eficaz al año de su implantación con mejoría de todos los parámetros glucométricos y la HbA1c, así como el descanso nocturno de los cuidadores.
It is necessary to achieve glycaemic control targets in type 1 diabetes mellitus (DM1) in children and adolescents, although it is complex, since microvascular complications1 can present early2 and continued exposure to hyperglycaemia has shown a detrimental effect on the development of the central nervous system (CNS).3
The use of continuous interstitial glucose monitoring (CGM) in the majority of the DM1 population has made it possible to move from a static control target, glycosylated haemoglobin (HbA1c), to dynamic and agreed targets for control also at this stage of life.4,5 Until now, the improvements in the studies of integrated pump-sensor systems in the paediatric population6 were associated with a long time of use.7 This time was conditioned by invasive, imprecise systems, requiring repeated calibrations and continuous pump-sensor signal losses, among other factors. The high number of nocturnal alerts, many not linked to glycaemic events, causes the quality of life perceived by caregivers and patients to be compromised.8 In addition, as the study by Foster et al.9 shows, only 20% of the population under 15 years managed to achieve glycaemic control objectives, caused by the complexity of managing DM1. In this age group, caregivers' fear of hypoglycaemia and the need for constant commitment to control DM1, which usually declines during adolescence, lead to the frequent abandonment of these therapeutic options.
Advanced hybrid closed-loop (AHCL) systems adjust basal insulin delivery and correction boluses based on glycaemic trend, and although they maintain the need for preprandial boluses, sensor calibration and consumable replacement with the necessary frequency, they have reduced the need for intervention by the user or their caregivers.10,11
Different AHCL systems have been developed with different adjustment algorithms and linked to two CGM systems. The t:slim X2 with Control-IQ (Tandem Inc., San Diego, CA)12 system linked to the Dexcom G6 (Dexcom Inc., San Diego, CA) has been approved for use in children over the age of 6.13,14 This system acts against hyperglycaemia, in addition to continuing to prevent hypoglycaemia like its predecessor, the predictive low-glucose suspend (PLGS) Tandem Basal-IQ15,16 system. Like this one, it maintains the option of programming different personal profiles, including the baseline and other parameters of the bolus calculator, which makes it easy to individualise insulin settings based on needs.
Material and methodsIn the paediatric department of a tertiary hospital, 225 patients under 18 years with DM1 were followed up, of whom 127 are on continuous insulin infusion therapy. To initiate this therapy in our department, we followed the 2007 recommendations on the use of insulin pump therapy in the paediatric age group: consensus statement of the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society and the International Society Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes.
A prospective, non-randomised, non-blind study was carried out in patients between 6 and 18 years of age receiving treatment with the PLGS system for at least three months prior to the change, with a DM1 progression time of at least one year, who weighed at least 25kg, had a total daily insulin dose of more than 10 IU, and knew the mechanics of downloading the system to the Tidepool v1.44.1 platform.
Once the first data download was verified and the informed consent was signed, the link was sent for the training course on updating to Tandem Control-IQ using the Tandem Device Updater (version 4.2.2.8b0550b; UDI 00850006613410; 2020 Tandem Diabetes Care, Inc.). Those who had technical problems were seen in person, while doubts about downloading data were mostly resolved by telephone.
For the update, it was recommended to reduce the correction factor and carbohydrate ratio by 20% for those who had a lower previous TIR.17 Data were evaluated 24h after the update and weekly for the first four weeks. Glucose data were collected at the fourth and eighth weeks and one year after the update. HbA1c was collected at baseline and one year after the update. The programmed controls were maintained every three months, and as much as possible were carried out in person.
To assess the perceived quality of control, changes in sleep quality and overall satisfaction with the system, it was decided to use a translated version of the validated Diabetes Impact and Devices Satisfaction (DIDS)18 questionnaire in its final format, used by Pinsker et al.19 in their study with Control-IQ and which consisted of closed, multiple-choice questions, in which a single answer could be selected. The questionnaire was sent to one of the guardians, in Google Forms format, at four and eight weeks. Only one survey could be completed per upgraded system serial number.
The main objectives of the study were to evaluate if, after updating the system, the percentage of time in range (TIR) increases from 70 to 180mg/dl measured by CGM, if there are changes in HbA1c, and to assess the degree of satisfaction, the less need for intervention during the night and the improvement of the quality of sleep with the use of the new hybrid system.
As secondary objectives, the decrease in hyperglycaemia times, greater than 180mg/dl (TAR) and greater than 250mg/dl (TAR 250mg/dl), was assessed; those of hypoglycaemia, less than 70mg/dl (TBR) and less than 54mg/dl (TBR 54mg/dl); mean glycaemia (MG), coefficient of variation (CV), and glycaemic management indicator (GMI) on CGM.
Descriptive statistics include mean with standard deviation (SD), median with interquartile range (IQR), depending on the data distribution, and comparison was made at baseline, at 4 and 8 weeks, and one year after update, with the program SPSS26.0.0. A P-value of <.05 was considered statistically significant. The percentages of the responses issued are shown from the survey.
The study was approved by the Ethics Committee of our hospital. Informed consent was requested from the parents or guardians before starting the study, as well as from those older than 15 years. The study was conducted with a commitment to respect the updated Declaration of Helsinki on ethical principles for medical research. Personal data was handled anonymously, always in accordance with the data protection principles contained in the new legislation of the European data protection regulation of 25 May 2018.
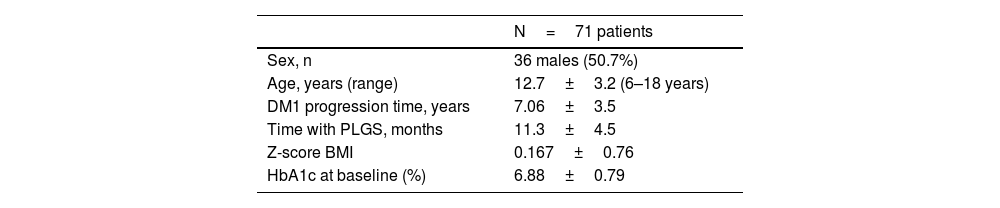
ResultsThe clinical characteristics of the study population are shown in Table 1. There was a loss to follow-up due to transfer of residence after turning 18 years of age. There were no dropouts during the follow-up time.
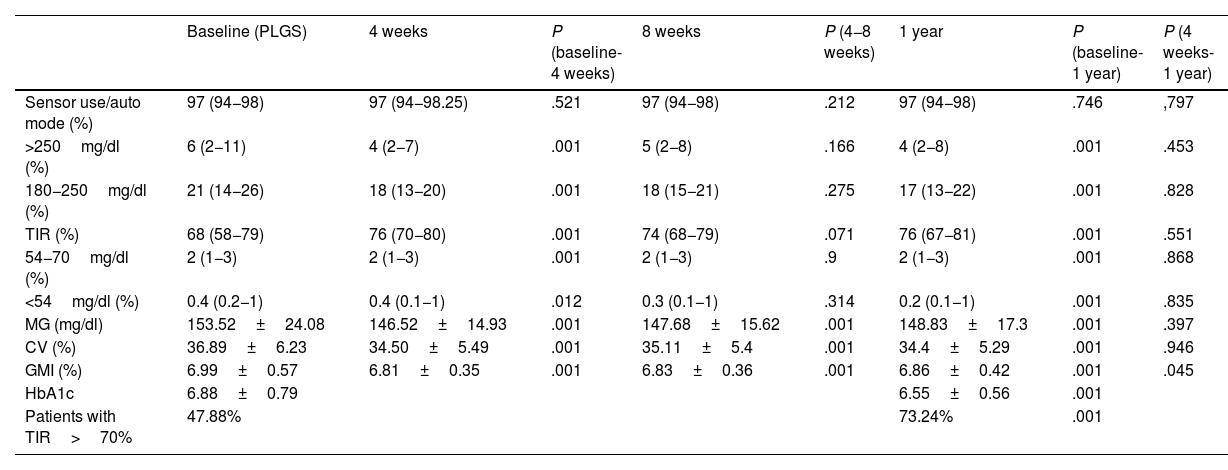
Table 2 shows the evolution of blood glucose levels at baseline, at 4 and 8 weeks, and at one year. Results are compared from baseline to 4 weeks, from baseline to 8 weeks, and from baseline to one year after the update.
Summary of global data expressed as mean (standard deviation) or median (25/75 quartiles) at baseline, at 4 and 8 weeks, and 1 year after update.
| Baseline (PLGS) | 4 weeks | P (baseline-4 weeks) | 8 weeks | P (4−8 weeks) | 1 year | P (baseline-1 year) | P (4 weeks-1 year) | |
|---|---|---|---|---|---|---|---|---|
| Sensor use/auto mode (%) | 97 (94−98) | 97 (94−98.25) | .521 | 97 (94−98) | .212 | 97 (94−98) | .746 | ,797 |
| >250mg/dl (%) | 6 (2−11) | 4 (2−7) | .001 | 5 (2−8) | .166 | 4 (2−8) | .001 | .453 |
| 180−250mg/dl (%) | 21 (14−26) | 18 (13−20) | .001 | 18 (15−21) | .275 | 17 (13−22) | .001 | .828 |
| TIR (%) | 68 (58−79) | 76 (70−80) | .001 | 74 (68−79) | .071 | 76 (67−81) | .001 | .551 |
| 54−70mg/dl (%) | 2 (1−3) | 2 (1−3) | .001 | 2 (1−3) | .9 | 2 (1−3) | .001 | .868 |
| <54mg/dl (%) | 0.4 (0.2−1) | 0.4 (0.1−1) | .012 | 0.3 (0.1−1) | .314 | 0.2 (0.1−1) | .001 | .835 |
| MG (mg/dl) | 153.52±24.08 | 146.52±14.93 | .001 | 147.68±15.62 | .001 | 148.83±17.3 | .001 | .397 |
| CV (%) | 36.89±6.23 | 34.50±5.49 | .001 | 35.11±5.4 | .001 | 34.4±5.29 | .001 | .946 |
| GMI (%) | 6.99±0.57 | 6.81±0.35 | .001 | 6.83±0.36 | .001 | 6.86±0.42 | .001 | .045 |
| HbA1c | 6.88±0.79 | 6.55±0.56 | .001 | |||||
| Patients with TIR>70% | 47.88% | 73.24% | .001 |
Analysis of differences from baseline to 4 weeks, from 4 to 8 weeks, from baseline to 1 year, and from 4 weeks to 1 year. Mean HbA1c before the change and one year after. Percentage of patients with TIR greater than 70% before the change and one year after the update. Statistical significance: P<.05.
It can be seen that glycaemic control improved with an 8% increase in TIR (from 68% to 76%) at 4 weeks, which is maintained one year after updating, and with a 0.33% decrease in HbA1c, both significant. Fig. 1 shows the evolution and Fig. 2 shows that the greatest changes occur in those patients with the worst initial TIR. From the data, it stands out that 47.88% of the patients had a TIR of 70% or higher at baseline, and after one year, 73.24% of the patients were in this range of values.
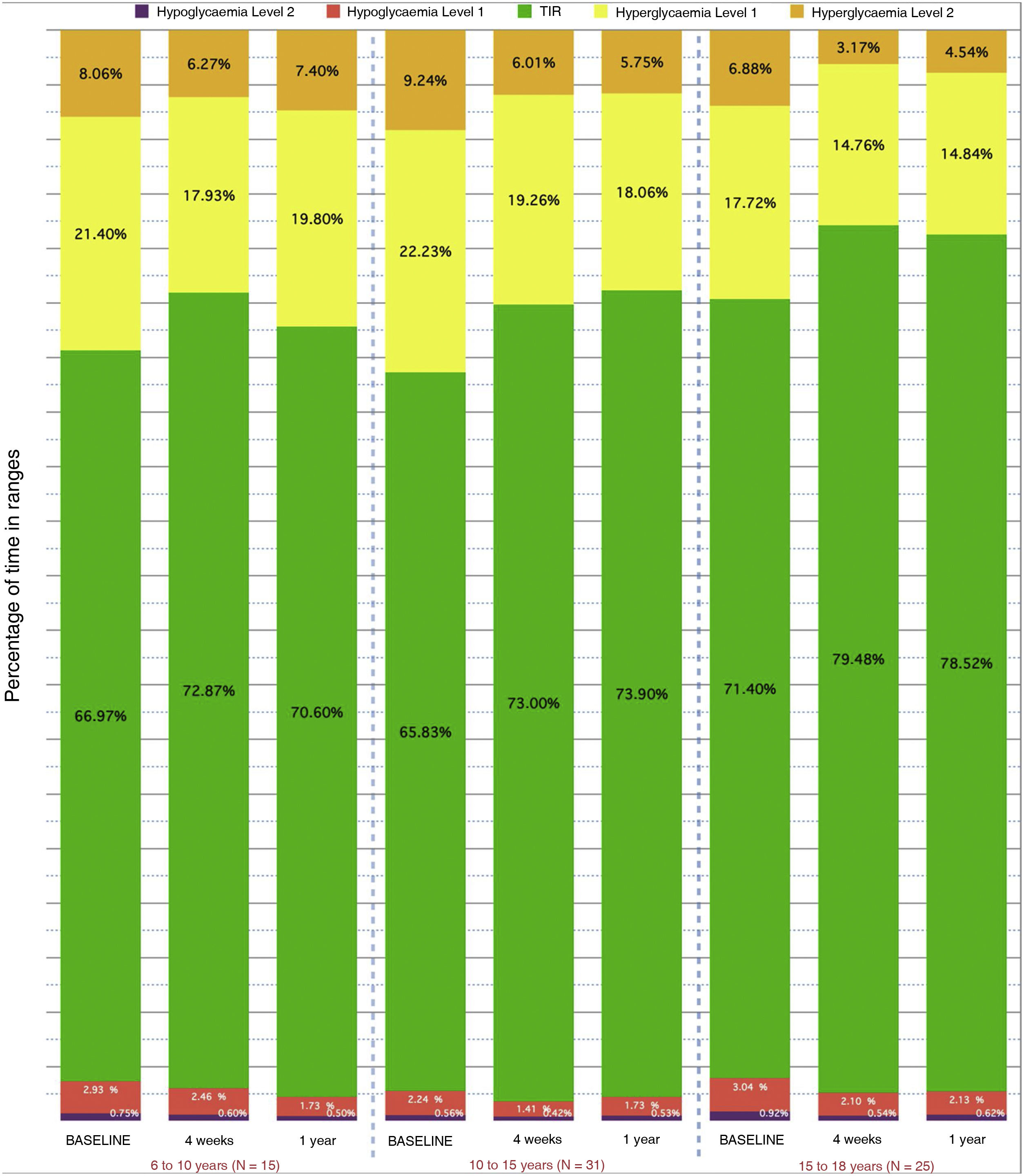
Fig. 3 shows the data broken down by age groups that show a greater decrease in TBR in the group under 10 years old (−1.2% compared to baseline) and a greater decrease in TAR+TAR 250 (−7.66%) in the group aged 10–15 years.
In the MG, at four weeks a decrease of 7mg/dl (P<.001) was obtained, as well as a decrease in the CV, which went from 36.8% (±6.23) to 34.50% (±5.49) (P<.001). All these changes were maintained at one year of follow-up.
A high time of use of the sensor was maintained, which did not differ significantly from the baseline.
During the follow-up period, no episode of severe hypoglycaemia or ketoacidosis was recorded. There were two problems when updating the system, both in the hospital clinic, apparently caused by the internet connection of our data network, which were solved after restarting the Tandem system.
Table 3 shows the percentage of responses to the survey in weeks 4 and 8. The increase in the subjective feeling of diabetes control stands out, as 93.3% of caregivers improved their night rest, with a 96.7% reduction in the need to attend to blood glucose variations during the night or to attend to system alarms.
Percentages grouped by type of response to surveys on system management and perceived improvement in quality of life at 4 and 8 weeks. Satisfaction with system, ease of use. Learning and feeling of security with the system at 8 weeks.
| Questions | Responses at 4 and 8 weeks | |||||
|---|---|---|---|---|---|---|
| Easier | The same | More difficult | ||||
| Managing the infuser after the update is | 52.4% | 60% | 47.6% | 38.3% | 0% | 1.7% |
| Better | The same | Worse | ||||
|---|---|---|---|---|---|---|
| Since I have been using the Control IQ system I feel that, in general, my child's diabetes is | 76.2% | 90% | 14.3% | 10% | 9.5% | 0% |
| Very high values are | 81.0% | 86.7% | 9.5% | 13.3% | 9.5% | 0% |
| Very low values are | 76.2% | 81.7% | 19.0% | 15% | 4.8% | 3.3% |
| Since using the Control IQ system, my child's night sleep is | 61.9% | 75% | 33.3% | 25% | 4.8% | 0% |
| Since I have been using the Control IQ system, my parents' night rest is | 71.5% | 93.3% | 19.0% | 6.7% | 9.5% | 0% |
| Has decreased | Is the same | Has increased | ||||
|---|---|---|---|---|---|---|
| Since I have been using the Control IQ system, the need to deal with glucose fluctuations during the night | 81.0% | 96.7% | 14.2% | 3.3% | 4.8% | 0% |
| The number of nightly system alerts | 71.4% | 88.3% | 14.3% | 10% | 14.3% | 1.7% |
| Percentage at 8 weeks of satisfaction with the system of responses issued | ||
|---|---|---|
| Yes | No | |
| I would like to change the system | 0% | 100% |
| I think the system is easy to use | 98.3% | 1.7% |
| I would like more help to learn how the system works | 18.3% | 81.7% |
| I quickly learned how the system works | 95% | 5% |
| I feel safe with this new system | 96.7% | 3.3% |
Adequate control of blood glucose levels in DM1 reduces acute and chronic complications.1–3 The use of new technologies has made it possible to establish the therapeutic objectives of achieving a TIR greater than 70%, an HbA1c and a GMI less than 7% (adapted depending on the circumstances),4 a time in hypoglycaemia less than 5%, avoiding values less than 54mg/dl and a CV less than 36% during the paediatric age.20
The improvement, in our study group, occurred at the expense of significantly decreasing TBR (TBR 54mg/dl) and TAR (TAR 250mg/dl), with a lower level of significance for TBR 54mg/dl, probably because the starting point was already low. Patients with poorer baseline control would benefit more from upgrading to a hybrid system, as also concluded by Schoelwer et al.21
Improvement was already observed after four weeks, as has been shown in other studies with the same system.13
At our first cut-off point, there was an 8% improvement in the TIR, lower than that obtained by Breton et al.,22 who analysed the improvements of Tandem Control-IQ concerning a SAP and carried out suspensions in anticipation of hypoglycaemia. In their multicentre, randomised, controlled, non-blind study, with a sample of 101 patients aged between 6 and 13 years, they improved TIR by 11% at 16 weeks, starting from a TIR of 55%. The starting TIR of our series was higher (68%), which could explain the lower increase after the change. If we assess our subgroup, which starts at 55% at baseline, there is an increase of 14.4% in the TIR.
The AHCL systems have been shown to be superior to the first generation hybrid systems in the paediatric population, as shown when comparing our results with those of Forlenza et al.,23 who compared the use of the Minimed-670G in a population of 105 children between the ages of 7 and 13 years and achieved an 8.8% improvement in the TIR at three months, reaching a median of 65%, although with a 20% exit from automatic mode. In studies with Control-IQ, including ours, times in automatic mode are higher than 95%.22,24
In a population of 39 patients, aged between 14 and 24 years, Carlson et al.25 present an increase of 10.3% in the TIR with the use of the AHCL Minimed-780G model, with a mean of 72.7% (±5.6) (P<.001) at 45 days of follow-up. In our series, an average TIR of 78.52% was reached at one year, decreasing the TAR without increasing the TBR. Other series, which include the adult and paediatric population, show only percentages of improvement and the blood glucose results are presented together in an age range of 7–80 years and for a shorter duration in time.26 Even assuming that the mean baseline TIR is representative of the paediatric population, the final TIR would be similar to that obtained by our group.
AHCL systems significantly improve glycaemic control in both the paediatric and adult populations, and selecting one or the other may depend on the need for different adjustment profiles, depending on the activity carried out on different days of the week or even at different times of day. Or, if one of the objectives is to reduce the risk of hypoglycaemia, Control-IQ could be superior to other systems.24
The level of hypoglycaemia achieved in our group (2.2% median) is lower than in the studies by Forlenza et al.23 with the Minimed-670G system, which achieved a 1.7% reduction in hypoglycaemia, from a median starting point of 4.7%, and that achieved in the study by Breton et al.22 With the Minimed-780G, Carlson et al.25 achieved a reduction in hypoglycaemia of 0.9% (not significant), starting from a mean of 3.3%, also higher than that presented by our group of patients.
Regarding the GMI, our study group showed a significant reduction (P<.001) of 0.18 points at four weeks and 0.13 at one year. In the studies with the Tandem Control-IQ, Forlenza et al.13 achieved a reduction of 0.01% (reaching 7.35%); Breton et al.,22 0.6% (starting from 7.6%); in the study with the Minimed-670G by Forlenza et al.,23 0.4% (from 7.9%), and with the Minimed-780G, Carlson et al.25 achieved a reduction of 0.5%, starting from a higher GMI (7.6%).
The automatic mode remains active as observed in other studies for Control-IQ,22,24,27 without the need to calibrate the sensor and making sensor changes every 10 days.
Technological advances have helped in the necessary ongoing decision-making that DM1 control requires at this stage of life, as well as serving to prevent the risks implicit in therapy. However, the lack of precision, the repeated alarms and the need to calibrate the glucose sensor induce fatigue with the use of technology that is combined with fatigue due to the disease, which leads to the abandonment of the technology on numerous occasions, especially in the age range of the population of this study.23,28 One of the fundamental improvements of the sensor used by the evaluated system is that it eliminates the obligation to calibrate.28 This becomes an option and the infuser-sensor connection is very stable, which significantly reduces the number of alarms issued.
Knowing the impression in the target population and that of their direct caregivers regarding the ease of use, the system alarms, the sensation of improvement and the repercussion on night rest29 was of interest after the update.
The results show a sensation of improvement in glycaemic control with decreased risk of severe hypoglycaemia and hyperglycaemia, decreased need to attend to nocturnal variations in blood glucose, with very satisfactory results regarding the overall experience and ease of use. In the same way as another study with AHCL systems30 concludes, which gives the vision that these systems are here to change, in addition to glycaemic control, the quality of life of caregivers and users.
Of the participants in our hospital, 100% said they would not return to the previous system.
One might think, as it is a system update, that users and/or their main caregivers might require a period of adaptation. However, as Breton and Kovatchev27 show in another real-life study in adults, the improvement can already be noted two weeks after using it and remains stable over time.
As limiting factors of our study, we could mention a possible selection bias, because it was the most motivated patients who had access to it. However, the group with a TIR of less than 65% was the one that benefited from the greatest changes.
The way in which the study was organised at our centre may mean that the results cannot be extrapolated.
ConclusionsThe AHCL Tandem Control-IQ system improves the TIR in patients between 6 and 18 years at four weeks and is maintained one year after the update. In all, 73.24% of patients have a TIR greater than 70% and meet the control criteria for paediatric age adapted by the International Society for Pediatric and Adolescent Diabetes (ISPAD). This increase is greater in those with worse initial control. The improvement is produced by reducing the times in hypoglycaemia and hyperglycaemia of level 1 and level 2. It is a safe system, which prevents severe hypoglycaemia and reduces mild hypoglycaemia better than others indicated in this age group.
In this study, the exits from automatic mode were reduced to the periods of change of the sensor, or when there was a loss of signal from it. Overall, 90.2% of patients were more than 94% in automatic mode at one year. There were no dropouts.
The fact that a system is upgradable reduces the financial costs for the healthcare system by being able to implement improvements without requiring hardware changes. The costs of consumables are variable depending on the autonomous community, as there is currently no centralised purchasing.
The choice of one system or another for the treatment of DM1 in the paediatric population will have an impact on the quality of life and the quality of sleep, both for caregivers and for the patients themselves. It decreases the need to interact with the system, maintaining or improving the degree of glycaemic control. All of the above must be taken into account to calculate the indirect costs of the therapy. Their usability will have an impact on the abandonment of the systems.
FundingThis study received no specific funding from public, private or non-profit organisations.
Conflicts of interestThe authors declare that they have no conflict of interest in relation to the preparation of this document.
To the participants and families for trusting us, once again, when carrying out this study. For their effort in downloading data from home, which in the end contributed to launching other means of communication.
To the resident doctors of the Paediatric Department of the Dr Balmis General University Hospital of Alicante, Àngela Vidal Bataller, Andrea Juan Gisbert and Gonzalo Fuente Lucas, who, during their rotation in Paediatric Endocrinology and Diabetes, helped in the collection of information for this study.