Hypoglycemia is a frequent adverse effect of the treatment of diabetes, but it is very rare in people without diabetes. The secretion of blood glucose-lowering substances by beta-cell tumors (insulinomas) or tumors corresponding to other cell lines,1 is one of the possible non-diabetic causes of hypoglycemia.
We present the case of an 18-year-old male diagnosed four years previously with adrenal gland carcinoma subjected to surgery, different chemotherapy treatment schemes and mitotane. The patient had required the removal of liver and lung metastases, and was referred to the Department of Oncology of our center for the start of fourth line chemotherapy with streptozocin due to the progression of the disease.
Some months before admission, he had developed clinical manifestations consistent with hypoglycemia (perspiration, dizziness and blurred vision), with low basal glycemia values (28–63mg/dl) and persistent hypopotassemia (1.7–3mmol/l). Upon admission he was treated with mitotane 1500mg/12h p.o. (mitotane levels of 8.4mg/l [therapeutic range 14–20]) and hydrocortisone (20–10–10mg p.o.). A physical examination revealed normal blood pressure values, no signs of Cushing's disease, and grade 1 obesity.
Evaluation by the Department of Endocrinology discarded endogenous hyperinsulinism (venous glycemia 28.8mg/dl, insulin<1.39pmol/l [17.80–173], peptide C 16.22pmol/l [370–1470]). Proinsulin determination was not requested, since the presence of tumor disease, lowered peptide C and refractory hypopotassemia were regarded as making a diagnosis of proinsulinoma unlikely. Hypothyroidism and adrenal gland insufficiency were discarded (TSH 1.18mIU/l [0.30–5.00], ACTH<0.2pmol/l [1.6–13.9], cortisol 413.3nmol/l [171–680]). Although mitotane can cause central hypothyroidism and primary adrenal gland insufficiency, measurement of the free T4 levels was not considered necessary, since our patient never reached therapeutic levels. The low ACTH concentration with non-diminished cortisol levels could be explained by the prescribed hydrocortisone. Renal function was normal, and despite the existence of liver metastases and a cytolytic and cholestatic pattern, the normal coagulation parameters and the absence of encephalopathy meant that severe liver failure could be discarded.
The study was completed with the determination of GH 0.7μg/l (0–8), IGF1 24.96μg/l (233–512) and IGF2 331μg/l (350–1000μg/l), the IGF2/IGF1 ratio being 13.26 (<3). Aberrant IGF2 levels were not measured, since the technique was not available in our laboratory. Due to the type of tumor and the high IGF2/IGF1 ratio, hypopituitarism was not regarded as a possible cause.
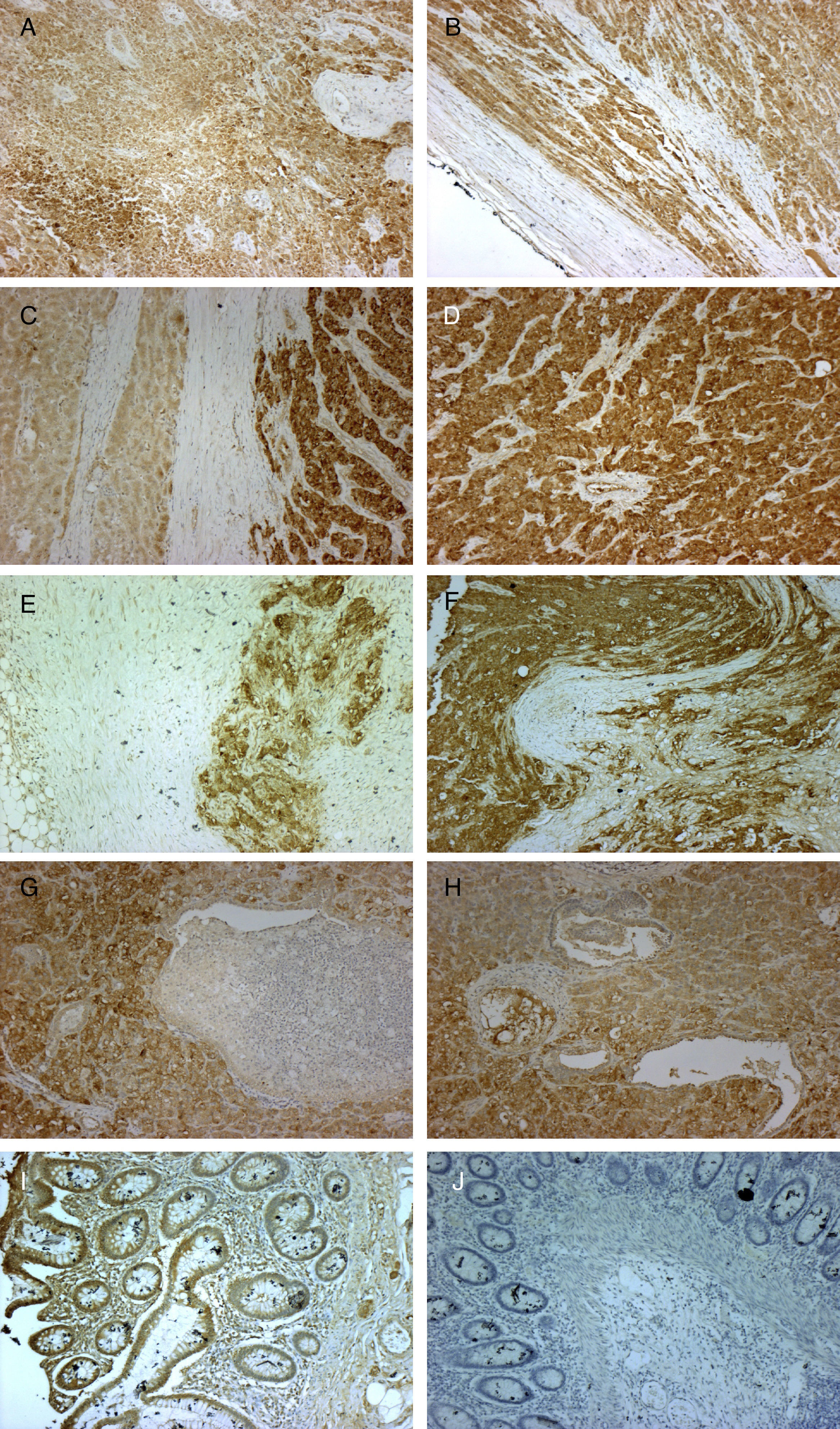
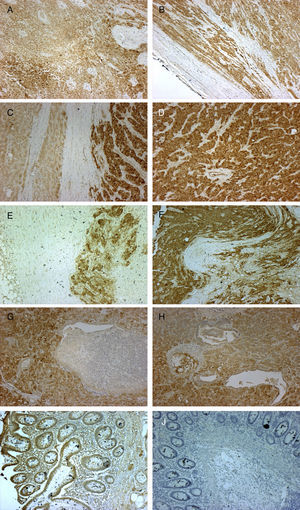
Immunohistochemical testing revealed the expression of IGF2 mRNA in both the primary tumor and in the lymphatic, hepatic and pulmonary metastatic tissues (Fig. 1).
Micrographs showing immunohistochemical staining with anti-IGF2 primary monoclonal antibody (MA5-17096 Thermo Fisher Scientific Pierce; 3,3-diaminobenzidine was used as reaction chromogen, and counterstaining was performed with Mayer hematoxylin for 30s, followed by mounting with VectaMount Permanent Mounting Medium; Vector Laboratories) in histological sections of the primary adrenal gland carcinoma (A, B, 10×), liver metastases (C, D, 10×), lymph node metastases (E, F, 10×), and lung metastases (G, H, 10×). Colon adenocarcinoma was used as positive (I, 10×) and negative control (J, 10×). Staining can be seen to be positive, indicating IGF2 expression in all cases except in the negative control, where the adenocarcinoma tissue was incubated with non-immune mouse serum instead of the primary antibody (J, 10×).
A fractionated diet was indicated, with fluid therapy in the form of a glucose saline solution. The addition of corticosteroids proved to be necessary (high-dose dexamethasone), followed by diazoxide (up to 500mg/day). At that point, fluid therapy was suspended and the dexamethasone dose lowered. Potassium levels were normalized with high-dose oral supplements (120meq/day p.o.) and spironolactone (100mg/day).
The patient was discharged without hypoglycemic episodes, but died shortly afterwards.
Hypoglycemia due to blood glucose-lowering substances secreted by extra-pancreatic tumors is a rare paraneoplastic phenomenon more commonly seen in mesenchymal or epithelial tumors. Its incidence, prevalence and prognosis are not known.1,2 Few cases of adrenal gland carcinoma associated with this condition have been described to date. Recently, Marchetti et al. reported the case of a woman with adrenal gland carcinoma initially manifesting with hypoinsulinic hypoglycemia and severe hypopotassemia, with lowered IGF2 levels and an increased IGF2/IGF1 ratio.3 Kim et al. in turn published the case of a young male with adrenal gland carcinoma presenting hypoglycemic episodes and undetectable insulinemia, with normal IGF2 levels but an elevated IGF2/IGF1 ratio.4
The typical clinical picture is fasting hypoglycemia, which represents the first symptom in 50% of all cases. Symptoms of neuroglycopenia are the most frequent manifestations,5 and are to be suspected in the presence of hypoinsulinic hypoglycemia with hypopotassemia, particularly in cancer patients.
The IGF system is composed of two ligands (IGF1 and IGF2) that are extensively (90%) bound to proteins (particularly IGFBP3). Both ligands are functionally and structurally related to insulin, and can interact with its receptor.6 The IGF2 gene is located in chromosome 11 together with tumor suppression genes (H19 and p5KIP2). These exhibit genomic imprinting (only the paternal allele is expressed for IGF2 and the maternal allele for H19 and p5KIP2), the expression of H19 and p5KIP2 being necessary to maintain it.1
When such imprinting is lost, over-expression of the IGF2 gene occurs, with an increase in the coded protein. This, in turn, overwhelms the enzymatic capacity of the cell, giving rise to partially processed IGF2, known as aberrant IGF2.1
Aberrant IGF2 forms complexes with IGFBP3 that cross the endothelial barrier, displacing IGF1 and IGF2, and thus increasing its bioavailability. Negative feedback reduces the concentrations of GH, giving rise to a vicious circle in which aberrant IGF2 further increases its bioavailability.1 This aberrant IGF2 interacts with the insulin receptor.1,5
A study should include the measurement of GH, IGF1 and IGFBP3 (which will be lowered), IGF2 (which may be in the normal range, or either low or high) and aberrant IGF2 (if the technique is available in the laboratory). An IGF2/IGF1 ratio of >10 (>20 according to other authors) is suggestive.5–7 Hypopotassemia secondary to potassium translocation into the cell is characteristic.1
The surgical piece can also be evaluated for IGF2 mRNA expression. When hypoglycemia is the first symptom, a tumor location study is indicated.
Curative treatment consists of surgical removal of the tumor.8 Symptomatic treatment includes a fractionated diet rich in carbohydrates, and fluid therapy with glucose.9 Corticosteroids are the most effective long-term management option.10 Somatostatin analogs have not been found to be effective, except in some cases as a continuous intravenous infusion. Recombinant GH is effective but is not used due to the possible stimulation of tumor growth.1 The use of diazoxide is controversial. In addition to activating the ATP-sensitive potassium channels, this drug inhibits the insulin receptor. A satisfactory response has been described in some cases.5,6
In conclusion, hypoglycemia due to tumors is a rare paraneoplastic phenomenon caused by the production of aberrant IGF2 with insulinic activity. Hypoinsulinic hypoglycemia with an increased IGF2/IGF1 ratio is suggestive of its diagnosis. The only curative treatment is surgical removal of the tumor, with corticosteroids being the most effective option for controlling the symptoms.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Morilla A, Gil P, Mato E, Chico A. Hipoglucemia de causa tumoral: a propósito de un caso. Endocrinol Nutr. 2017;64:398–400.