Gestational diabetes mellitus (GDM) plays an important role in the later onset of cardiovascular disease (CVD) in the postpartum period,1 leading to an increased cardiovascular risk (CVR) in women with prior GDM. This CVR is mainly associated with insulin resistance (IR), widely recognized as a promoter of CVDs.2 IR can be associated with inflammation markers as well as with abdominal obesity (AO) and excess weight (EW), and act as a triggering factor for the development of CVDs.3 Therefore, the postpartum identification of these set of risk factors in women with prior GDM is imperative to prevent the development of CVDs and estimation of CVR.4
The initial identification of these women starts with GDM screening. In this regard, GDM diagnostic criteria have changed recently. Based on the data provided by the HAPO study, the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) consensus panel established new criteria (IADPSGc) for diagnosing GDM. These new screening criteria have entailed a 3.5-fold increase of GDM in our setting5; however, some of these women seem to be considered a low-risk group because IADPSGc are less stringent than Carpenter–Coustan criteria (CCc). Thus, examining them in the postpartum period presents a perfect opportunity to establish whether they are indeed a low risk group, or whether using this new criterion enables targeting more women at risk of developing future CVDs. While the association between GDM and CVDs has been established in women with prior GDM diagnosed by CCc,1 the identification of women with CVR in those diagnosed with GDM by IADPSGc has yet to be established and seems worthwhile.
We seek to determine and compare the presence of 12-weeks-postpartum insulin-resistance syndrome (IRS) in women with prior GDM identified by CCc and IADPSGc, using IRS as a predictor of CVR. Universal and centralized screening is performed since 2002 at the Central Laboratory of the St. Carlos Hospital. Women diagnosed with GDM receive special healthcare and follow-up at the Diabetes and Pregnancy Unit of the hospital. Up until 2012, the CCc was used to identify GDM between 24 and 28 weeks of gestation and from April 2012 the new IADPSGc was implemented. Women included in our database were those diagnosed with GDM between 2007 and 2013 and who attended their recall for testing around the third month postpartum. Women with multiple gestation and/or in vitro fertilization were excluded.
A total of 1361 out of the 1620 eligible women attended postpartum screening. 791 of these women were diagnosed with GDM by CCc, and 570 were diagnosed by the IADPSGc. Postpartum attendance rates were similar in both groups; 86.5% and 81% in the groups of Coustan–Carpenter and IADPSG, respectively. Comparing attendees versus non-attendees, we found no significant differences in any of the analyzed variables.6 Analysis of pregestational and postgestational weight and body mass index (BMI), waist circumference (WC), HOMA-IR, protein and lipid profile was performed. We assessed the presence of IRS, determined by HOMA-IR ≥3.8, WC ≥89.5cm and BMI ≥25kg/m2. The WC and HOMA-IR cut-off points used are specific to the Spanish population.7,8 The statistical analysis was performed with SPSS version 15.0 software for Windows. Continuous variables are expressed as Mean±SDM and categorical values as numbers and percentages. To establish significant differences student t test and chi-square test were used.
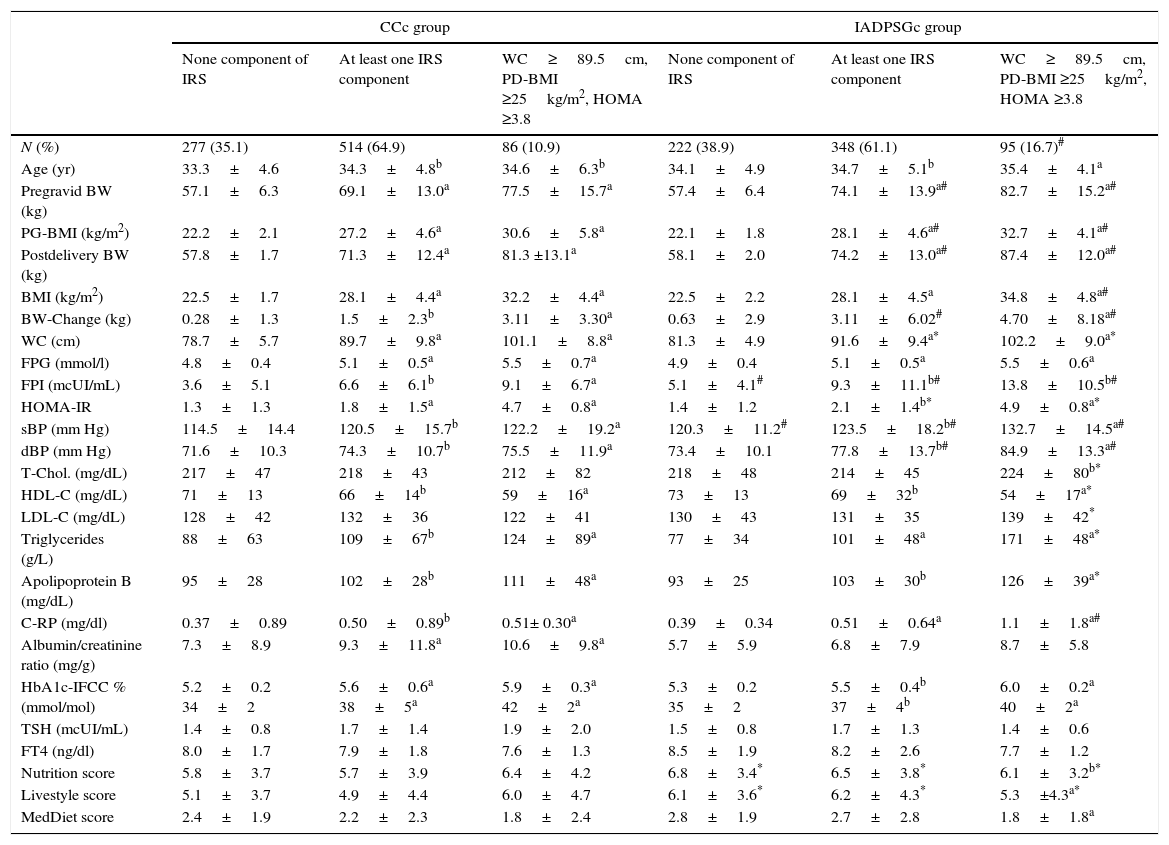
When comparing CCc and IADPSGc cohorts, rates of having one or two components of IRS (WC≥89.5cm or BMI≥25kg/m2 and WC≥89.5cm) in the postpartum period were similar in both groups (36.2% vs. 35.4% and 29.8% vs. 30% respectively). However, IRS was significantly higher in the IADPSGc group compared to the CCc group (16.7% vs. 10.9%; p<0.001), odd ratio 1.335 (IC 95% 1.202–1.788; p<0.001). When comparing the IRS group in both cohorts, women with IRS in the IADPSGc group had significantly less favourable cardiovascular profile in terms of fasting plasma insulin (FPI), systolic blood pressure (sBp), diastolic blood pressure (dBp), HDL, Triglycerides, Apoliprotein B and C-Reactive Protein (C-RP) (all p<0.05, Table 1).
Clinical and biochemical post-delivery data found in the CCc group and the IADPSGc group sorted by IRS components.
| CCc group | IADPSGc group | |||||
|---|---|---|---|---|---|---|
| None component of IRS | At least one IRS component | WC≥89.5cm, PD-BMI ≥25kg/m2, HOMA ≥3.8 | None component of IRS | At least one IRS component | WC≥89.5cm, PD-BMI ≥25kg/m2, HOMA ≥3.8 | |
| N (%) | 277 (35.1) | 514 (64.9) | 86 (10.9) | 222 (38.9) | 348 (61.1) | 95 (16.7)# |
| Age (yr) | 33.3±4.6 | 34.3±4.8b | 34.6±6.3b | 34.1±4.9 | 34.7±5.1b | 35.4±4.1a |
| Pregravid BW (kg) | 57.1±6.3 | 69.1±13.0a | 77.5±15.7a | 57.4±6.4 | 74.1±13.9a# | 82.7±15.2a# |
| PG-BMI (kg/m2) | 22.2±2.1 | 27.2±4.6a | 30.6±5.8a | 22.1±1.8 | 28.1±4.6a# | 32.7±4.1a# |
| Postdelivery BW (kg) | 57.8±1.7 | 71.3±12.4a | 81.3 ±13.1a | 58.1±2.0 | 74.2±13.0a# | 87.4±12.0a# |
| BMI (kg/m2) | 22.5±1.7 | 28.1±4.4a | 32.2±4.4a | 22.5±2.2 | 28.1±4.5a | 34.8±4.8a# |
| BW-Change (kg) | 0.28±1.3 | 1.5±2.3b | 3.11±3.30a | 0.63±2.9 | 3.11±6.02# | 4.70±8.18a# |
| WC (cm) | 78.7±5.7 | 89.7±9.8a | 101.1±8.8a | 81.3±4.9 | 91.6±9.4a* | 102.2±9.0a* |
| FPG (mmol/l) | 4.8±0.4 | 5.1±0.5a | 5.5±0.7a | 4.9±0.4 | 5.1±0.5a | 5.5±0.6a |
| FPI (mcUI/mL) | 3.6±5.1 | 6.6±6.1b | 9.1±6.7a | 5.1±4.1# | 9.3±11.1b# | 13.8±10.5b# |
| HOMA-IR | 1.3±1.3 | 1.8±1.5a | 4.7±0.8a | 1.4±1.2 | 2.1±1.4b* | 4.9±0.8a* |
| sBP (mm Hg) | 114.5±14.4 | 120.5±15.7b | 122.2±19.2a | 120.3±11.2# | 123.5±18.2b# | 132.7±14.5a# |
| dBP (mm Hg) | 71.6±10.3 | 74.3±10.7b | 75.5±11.9a | 73.4±10.1 | 77.8±13.7b# | 84.9±13.3a# |
| T-Chol. (mg/dL) | 217±47 | 218±43 | 212±82 | 218±48 | 214±45 | 224±80b* |
| HDL-C (mg/dL) | 71±13 | 66±14b | 59±16a | 73±13 | 69±32b | 54±17a* |
| LDL-C (mg/dL) | 128±42 | 132±36 | 122±41 | 130±43 | 131±35 | 139±42* |
| Triglycerides (g/L) | 88±63 | 109±67b | 124±89a | 77±34 | 101±48a | 171±48a* |
| Apolipoprotein B (mg/dL) | 95±28 | 102±28b | 111±48a | 93±25 | 103±30b | 126±39a* |
| C-RP (mg/dl) | 0.37±0.89 | 0.50±0.89b | 0.51± 0.30a | 0.39±0.34 | 0.51±0.64a | 1.1±1.8a# |
| Albumin/creatinine ratio (mg/g) | 7.3±8.9 | 9.3±11.8a | 10.6±9.8a | 5.7±5.9 | 6.8±7.9 | 8.7±5.8 |
| HbA1c-IFCC % (mmol/mol) | 5.2±0.2 34±2 | 5.6±0.6a 38±5a | 5.9±0.3a 42±2a | 5.3±0.2 35±2 | 5.5±0.4b 37±4b | 6.0±0.2a 40±2a |
| TSH (mcUI/mL) | 1.4±0.8 | 1.7±1.4 | 1.9±2.0 | 1.5±0.8 | 1.7±1.3 | 1.4±0.6 |
| FT4 (ng/dl) | 8.0±1.7 | 7.9±1.8 | 7.6±1.3 | 8.5±1.9 | 8.2±2.6 | 7.7±1.2 |
| Nutrition score | 5.8±3.7 | 5.7±3.9 | 6.4±4.2 | 6.8±3.4* | 6.5±3.8* | 6.1±3.2b* |
| Livestyle score | 5.1±3.7 | 4.9±4.4 | 6.0±4.7 | 6.1±3.6* | 6.2±4.3* | 5.3±4.3a* |
| MedDiet score | 2.4±1.9 | 2.2±2.3 | 1.8±2.4 | 2.8±1.9 | 2.7±2.8 | 1.8±1.8a |
Data are mean±SDM or number (%).
PG, pregravid; PD, postdelivery; WC, waist circumference; BMI, body mass index; FPG, fasting plasma glucose; FPI, fasting plasma insulin; sBP, systolic blood pressure; dBP; diastolic blood pressure; C-RP, C-reactive protein. MedDiet, MEDAS score: 14-point Mediterranean Diet Adherence Screener (MEDAS).9 Nutrition and Livestyle Score, 15- and 18-point Diabetes Nutrition and Complications Trial (DNCT).5
Changing GDM diagnostic criteria allows the identification of a higher number of women with IRS postpartum, which could be because IADPSGc identify a higher proportion of women with IR, associated with higher fasting plasma glucose (FPG) values. In fact, this significant difference is established when introducing impaired HOMA-IR variable which was more prevalent in the IADPSGc group. Results from our previous studies suggested that these criteria seemed to be more effective than CCc in identifying women with impaired FPG5 which is an indicator of IR. This fact is also supported by the results obtained in this study, where FPI was significantly higher in the group of IRS in IADPSGc cohort than in the CCc cohort. Women with IRS in the IADPSGc group had less favourable cardiovascular profile than those in CCc group, and therefore have a more compromised health postpartum. In fact, the inflammatory profile of women with IRS in the IADPSGc cohort was also less favourable than in the CCc cohort. This could be attributed to the fact that women in the IADPSGc cohort tend to be more insulin resistant. Our study is not without limitations. It has been proven that breastfeeding can reduce insulin resistance.10 Unfortunately, information of all participants regarding this matter is lacking. The database only contains this information of women in the IADPSG criteria group but not those in the Coustan–Carpenter criteria group. A total of 408 (72%) breastfed exclusively and 88 (15%) gave mixed breastfeeding in the IADPSG criteria group. Even though data regarding the Coustan–Carpenter criteria group is unknown, the rate of breastfeeding could not be great enough to justify a reduction in the rate of IRS in this group.
In conclusion, using IADPSGc for GDM screening the identification of a higher proportion of women with CVR is possible, enabling the development and early application of preventive strategies.
FundingFundación para Estudios Endocrinometabolicos, and IdISSC Hospital Clinico San Carlos, Madrid, and the Instituto de Salud Carlos III of Spain (PI14/01563). CAB was supported by a grant from the Fundación para Estudios Endocrinometabólicos.
Conflict of interestNone.