Less than one third of patients with type 1 diabetes mellitus (T1DM) achieve the cut-off value proposed as good metabolic control by most guidelines, HbA1c <7 %. However, HbA1c reductions and prevention of severe hypoglycemia (SH) have shown clinically relevant benefits. The study objective therefore was to assess the effectiveness of continuous subcutaneous insulin infusion (CSII) therapy at 5 years of follow-up in a cohort of patients attending a specialized unit using HbA1c reduction and SH absence as combined goals.
MethodsA retrospective, observational study on 178 patients with T1DM who started CSII treatment at Hospital Clinic of Barcelona between 2003 and 2008. HbA1c levels at baseline and after 5 years of treatment with CSII and presence or absence of SH were recorded. The combined variables calculated included: a) HbA1c reduction by ≥0.5 points and absence of SH in the last 2 years; b) HbA1c at 5 years <7.5 % and no SH in the last 2 years; c) HbA1c <8.5 % and no HG in the last 2 years; d) HbA1c reduction by ≥0.5 points and/or HbA1c <7.5 % at 5 years with no SH in the last 2 years of follow-up.
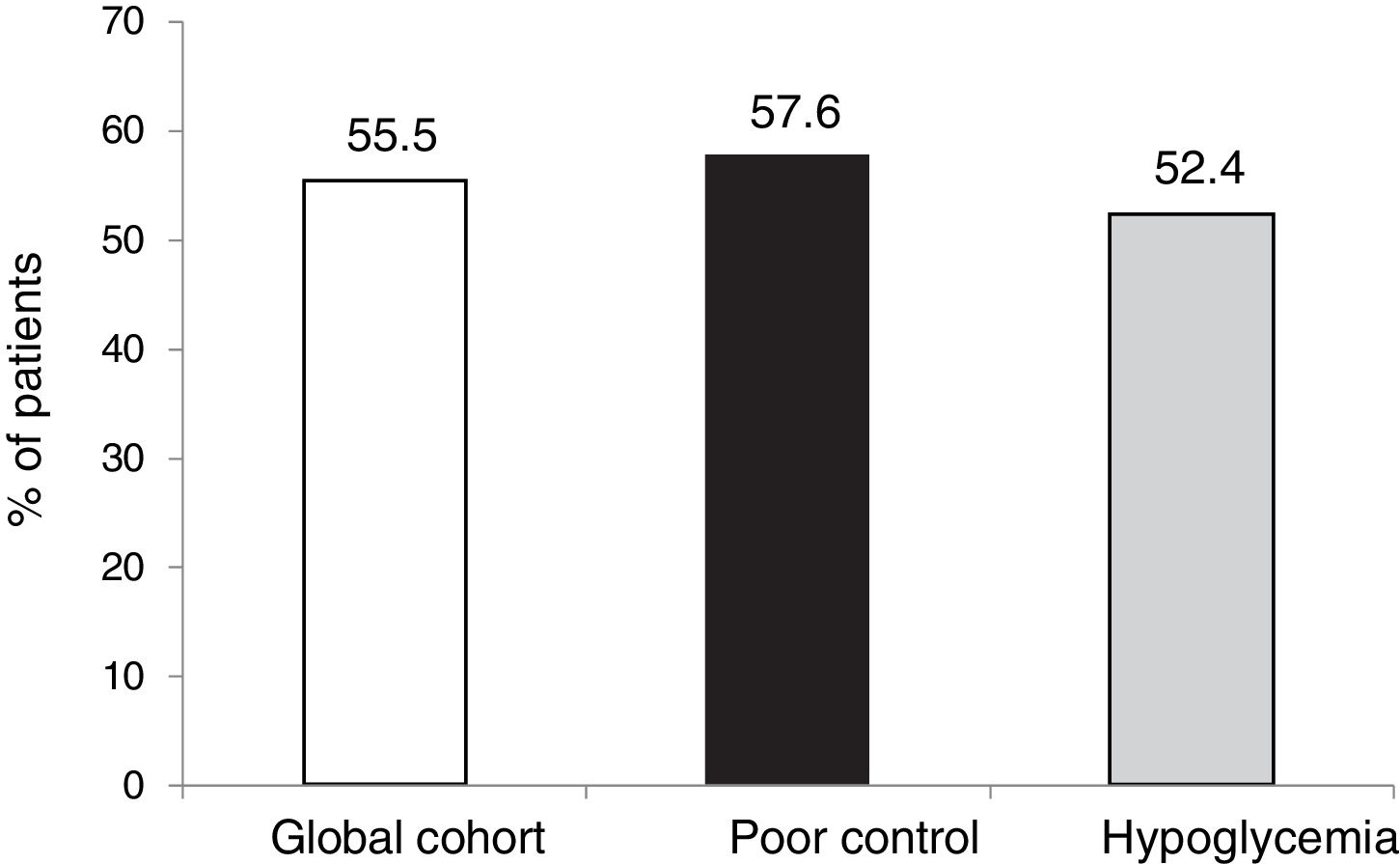
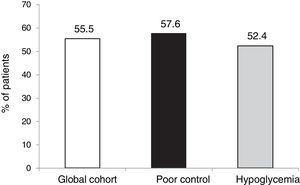
ResultsTwenty-seven of the 178 patients were excluded due to loss to follow-up or CSII discontinuation. A total of 151 patients (aged 37.4 ± 10.5 years, 64 % women, diabetes duration of 19.2 ± 10.7 years) were therefore analyzed. The two main reasons for starting CSII were suboptimal metabolic control (SMC, 60.9 %) and severe hypoglycemia/hypoglycemia unawareness (25.5 %). HbA1c levels in total cohort and in patients with SMC were 8.0 ± 1.2 and 8.4 ± 1.1 at CSII start and 7.8 ± 1.2 and 8.0 ± 1.3 at 5 years of treatment (p = 0.104 and p = 0.016) respectively. In the overall cohort, 55.5 % of patients achieved at 5 years the combined goal of HbA1c <7.5 % and/or HbA1c reductions ≥ 0.5 % without SH.
ConclusionsAfter 5 years of CSII therapy, more than half the patients achieved the combined goal of significant HbA1c reduction and absence of SH. Use of combined goals offers the opportunity to assess the effectiveness of T1DM treatment from a viewpoint closer to its clinical meaning.
Menos de un tercio de los pacientes con Diabetes tipo 1 (DT1) consiguen el objetivo de punto de corte establecido como control metabólico óptimo (HbA1c<7%). Sin embargo, reducciones porcentuales de HbA1c y la prevención de hipoglucemias graves (HG) han demostrado beneficios clínicamente relevantes. Por ello, el objetivo de este estudio ha sido evaluar la efectividad de la terapia ISCI a los 5 años de seguimiento en una cohorte de pacientes de una unidad especializada mediante objetivos combinados de descenso de HbA1c y ausencia de HG.
Material y métodosEstudio observacional retrospectivo que incluye a 178 pacientes que iniciaron terapia ISCI de manera consecutiva entre los años 2003 y 2008. Se han analizado las características basales de los individuos, la HbA1c inicial y a los 5 años de tratamiento con ISCI y la presencia o no de HG. Se calcularon las variables combinadas: a) descenso de al menos 0,5 puntos de HbA1c y ausencia de HG en los últimos 2 años; b) HbA1c a los 5 años <7.5% sin HG en los últimos 2 años; c) HbA1c <8.5% sin HG en los últimos 2 años; d) descenso de ≥ 0,5 puntos y/o HbA1c <7.5% a los 5 años sin presencia de HG en los 2 últimos años de seguimiento.
Resultados27 de los 178 pacientes fueron excluidos debido a pérdida del seguimiento o abandono de la terapia ISCI. 151 pacientes (edad 37,4 ± 10,5 años, 64% mujeres, 19,2 ± 10,7 años de evolución de la DT1) fueron analizados. Las 2 indicaciones principales para el inicio de ISCI fueron: control metabólico subóptimo (CMS, 60.9%) e HG/desapercibida (28.5%). Las HbA1c de la cohorte total / CMS fueron de 8,0 ± 1,2 y 8,4 ± 1,1 al inicio de la terapia ISCI y de 7,8 ± 1,2 y 8,0 ± 1,3 a los 5 años (p = 0,104 y p = 0,016) respectivamente. En la cohorte global un 55.5% de los pacientes alcanzaron a los 5 años el objetivo combinado HbA1c<7.5% y/o descenso ≥ 0.5% sin HG.
ConclusionesTras cinco años de terapia ISCI más de la mitad de nuestros pacientes consiguen el objetivo combinado de reducción significativa de Hb1c y ausencia de HG. La utilización de objetivos combinados nos ofrece la posibilidad de evaluar la efectividad de las terapias en la DT1 desde un punto de vista más cercano a su significado clínico.
People with type 1 diabetes mellitus (T1DM) have higher all-cause and cardiovascular disease mortality than those without T1DM. Furthermore, epidemiological studies have shown that this relative risk increases as glycosylated hemoglobin (HbA1c) levels rise.1
Data from the Diabetes Control and Complications Trial (DCCT) have shown that intensive blood glucose control in patients with T1DM has significant benefits in terms of the occurrence of both microvascular and macrovascular complications.2,3 As a result, most clinical guides now establish HbA1c < 7 % as the glycemic control target. However, not only reaching this concrete HbA1c target, but also obtaining a percentage decrease in HbA1c has demonstrated benefits in these clinical trials. For example, in the DCCT a 10 % relative decrease in HbA1c was associated with a 44 % reduction in the risk of the progression of retinopathy and a 30 % decrease in clinical neuropathy.4–6
However, data from multiple cohorts of T1DM patients have shown that this control objective or target is not reached in most cases,7,8 and the occurrence of hypoglycemia episodes is one of the main reasons behind this.9 In addition, the presence of severe hypoglycemia (SH) has been associated in some studies with an increase in both mortality and cardiovascular disease in patients with T1DM.10–13
The effectiveness of continuous subcutaneous insulin infusion (CSII) therapy has been widely evaluated in both clinical trials and in standard clinical practice, with highly variable results due to multiple differences in study design (the characteristics of the included patients, the types of devices used, the duration of follow-up, etc.).14 Nevertheless, when used in appropriate patients, CSII has shown long-term benefits, with reductions in hypoglycemia and improvements in the levels of HbA1c.15,16
However, to date no studies have examined the long-term effectiveness of CSII therapy with objectives combining reductions in HbA1c and SH. The present study was therefore carried out to assess the effectiveness of CSII at 5 years of follow-up in a cohort of patients from a specialized unit based on the combined goals of lowered HbA1c and the absence of SH, with a view to analyzing the effectiveness of such therapy from an eminently clinical perspective.
Patients and methodsThe data compiled in a previously published retrospective, single-center observational study were reanalyzed.15 A review was made of both the case histories and the databases of all the patients with T1DM followed-up on at a specialist unit who had started insulin pump treatment during the years 2003–2008. Accordingly, all the patients underwent follow-up covering at least 5 years of CSII treatment.
The patients had started SCII following the criteria of the Catalan Health Service (Servei Català de la Salut): a) suboptimum control (intensified therapy with multiple dose insulin [MDI] being unable to secure HbA1c < 7.5 % without recurrent hypoglycemia episodes); b) recurrent SH episodes; c) major difficulties with nocturnal glycemic control; d) the need to optimize control in the context of pregnancy or the planning of pregnancy; e) the rapid progression of the disease and early microvascular disorders; f) insulin allergy or severe lipoatrophy; and g) variable patient working hours. In addition, the existence of contraindications to CSII was ruled out in all patients (e.g., the inability to maintain self-control of diabetes with MDI, poor adherence to treatment and to the visits established with the treating team, or evidence of psychiatric disorders).
The patients attended a session in which information was provided about the treatment prior to it being started. Subsequently, the patients participated in the specific therapeutic education program established at our center, involving both educational nurses and endocrinologists. This program consists of four weekly visits in groups of four people during one month; individual visits every one or two months as required in the first 6 months; and reassessment visits after 6 and 12 months.
The study was approved by the Ethics Committee of Hospital Clínic i Universitari de Barcelona (Barcelona, Spain), and all subjects gave their written informed consent.
Before CSII was started, the following data were collected: gender, disease duration, indication for starting CSII, HbA1c, and the number of SH episodes in the previous two years. We also recorded the most recent HbA1c value (Tosoh G8 Automated HPLC Analyzer, normal range 4–6%) available after 5 years of follow-up and the hypoglycemia data at that same timepoint. Severe hypoglycemia was defined as hypoglycemia episodes in which the patient required the help of a third person to deal with the situation due to major neuroglycopenia symptoms.
We analyzed both the total patient cohort and two groups established according to the indication for starting CSII: poor metabolic control or recurrent SH episodes. In each of the groups an analysis was made of the percentage of patients who after 5 years had reached the combined goals of lowered HbA1c (with different cut-off points) and the absence of SH during the last two years of therapy. Likewise, we analyzed whether certain baseline characteristics could predict the long-term achievement of these goals.
Specifically, the following combined goals were analyzed: HbA1c after 5 years < 7.5 % without SH in the last two years; HbA1c after 5 years < 8.5 % without SH in the last two years; a reduction ≥ 0.5 points in HbA1c without SH in the last two years; a reduction ≥ 0.5 points and/or HbA1c < 7.5 % after 5 years without SH in the last two years.
The results are presented as the mean ± standard deviation (SD) or proportions. Comparisons between the data at baseline and after 5 years were made using the Student t-test for paired samples. Multiple regression analysis was used to analyze the possible relationship between the baseline variables and the results obtained at 5 years. Statistical significance was considered for p < 0.05.
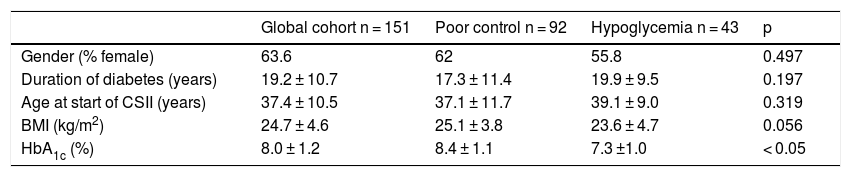
ResultsA total of 178 patients started CSII therapy between 2003 and 2008, but 27 of them were not analyzed because they did not complete the 5 years of follow-up for different reasons (7 due to a lack of resolution of the main indication i.e., treatment failure, 6 due to patient decision, and 14 for unknown reasons). Of the 151 patients included in the study, 63.6 % were women, and the mean age was 37.4 ± 10.5 years, with a duration of T1DM of 19.2 ± 10.7 years, and a mean HbA1c concentration of 8.0 ± 1.2 % (the baseline characteristics of the subgroups are described in Table 1). The main reasons for starting CSII therapy were: 1) suboptimal metabolic control (92 patients, 60.9 %); 2) repeated hypoglycemia or episodes of SH (43 patients, 25.5 %); 3) pregnancy or planned pregnancy (10 women, 6.6 %); and 4) other indications (6 patients, 7 %). The initial HbA1c level of the entire cohort was 8.0 ± 1.2 %, while at 5 years it was 7.8 ± 1.2 % (p = 0.1). However, in the group of patients who had started therapy because of poor metabolic control, HbA1c was significantly reduced (from 8.4 ± 1.1–8.0 ± 1.3 %; p < 0.005).
Baseline characteristics of the patients included in the study.
| Global cohort n = 151 | Poor control n = 92 | Hypoglycemia n = 43 | p | |
|---|---|---|---|---|
| Gender (% female) | 63.6 | 62 | 55.8 | 0.497 |
| Duration of diabetes (years) | 19.2 ± 10.7 | 17.3 ± 11.4 | 19.9 ± 9.5 | 0.197 |
| Age at start of CSII (years) | 37.4 ± 10.5 | 37.1 ± 11.7 | 39.1 ± 9.0 | 0.319 |
| BMI (kg/m2) | 24.7 ± 4.6 | 25.1 ± 3.8 | 23.6 ± 4.7 | 0.056 |
| HbA1c (%) | 8.0 ± 1.2 | 8.4 ± 1.1 | 7.3 ±1.0 | < 0.05 |
Source: Quirós et al.15
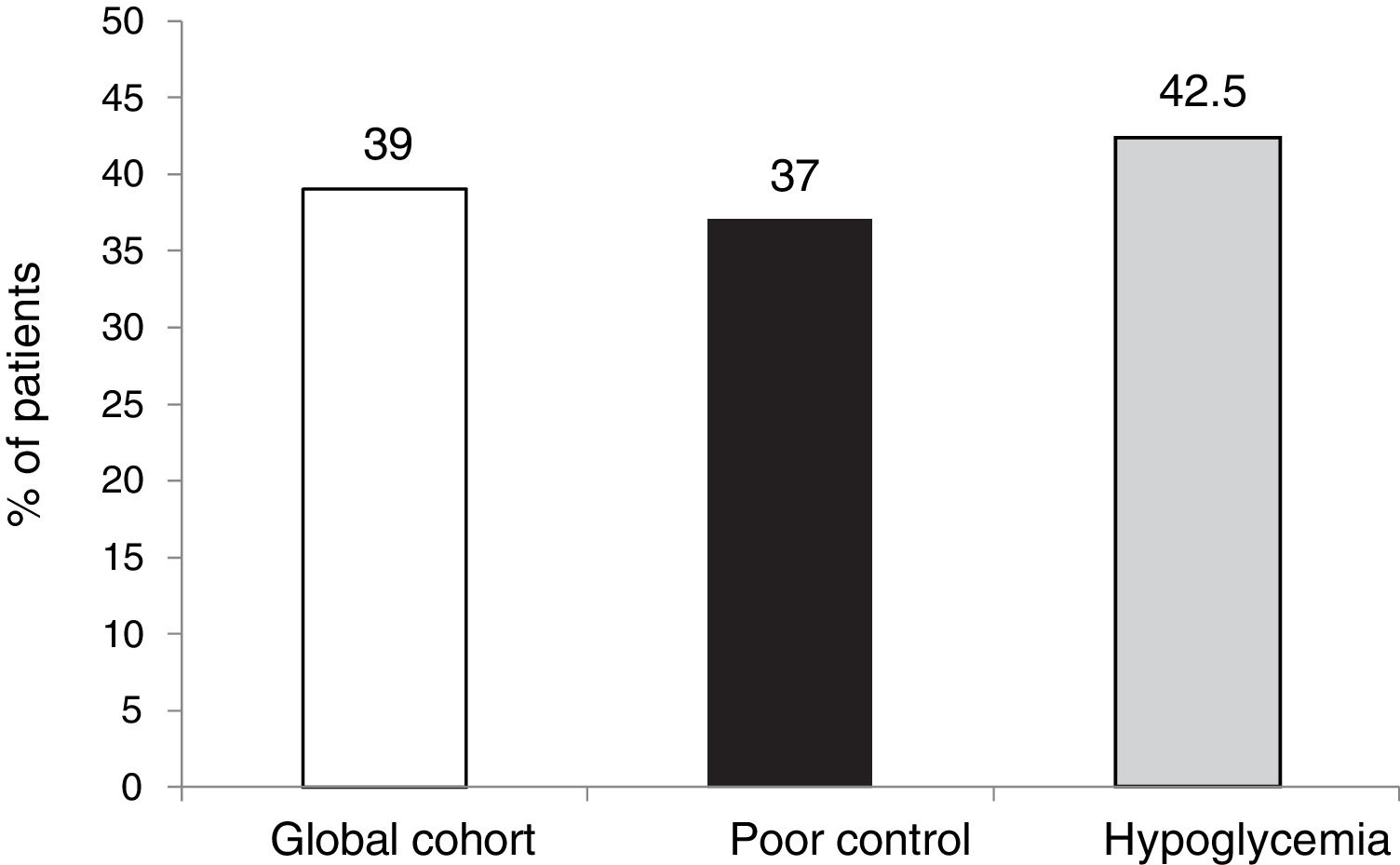
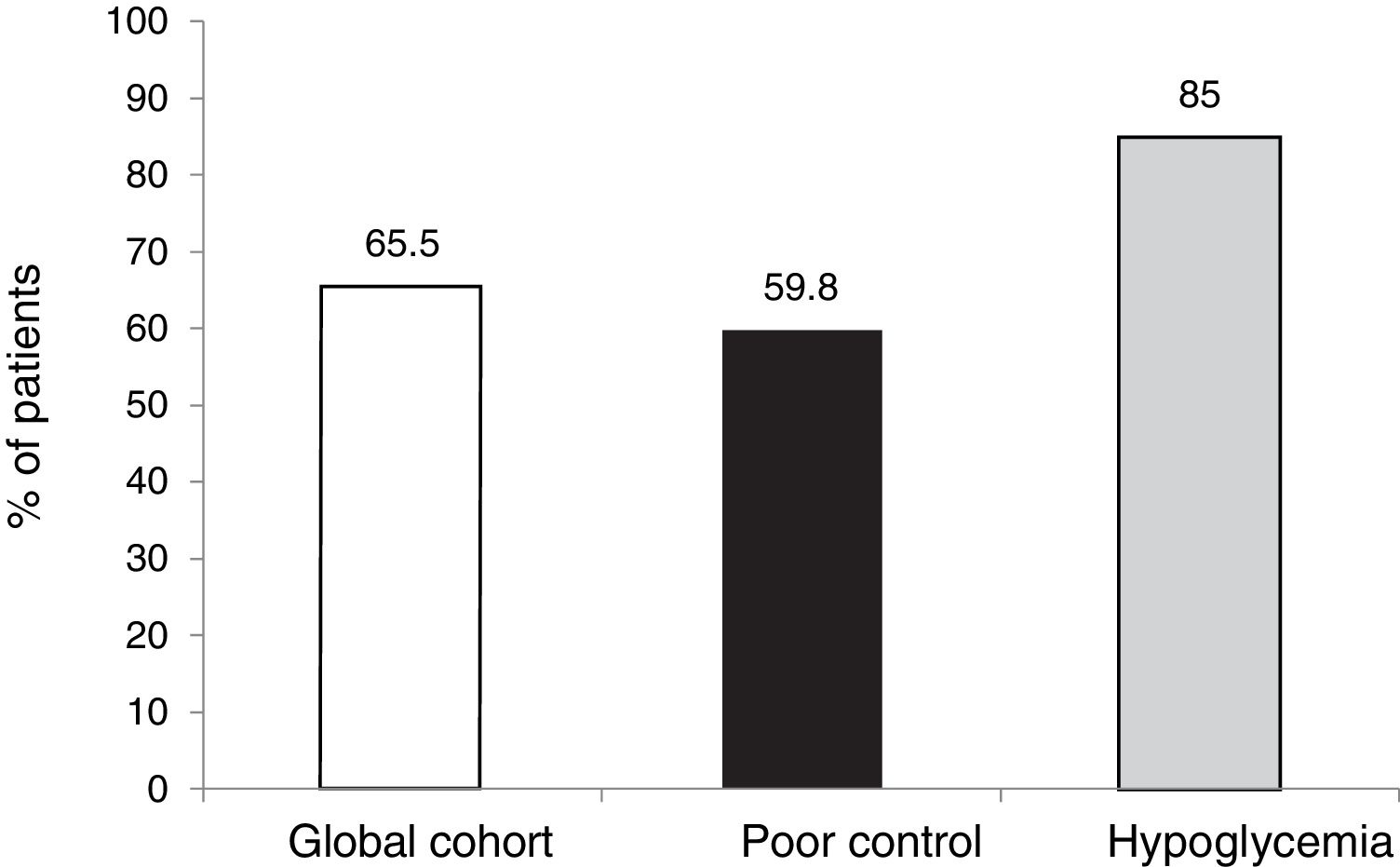
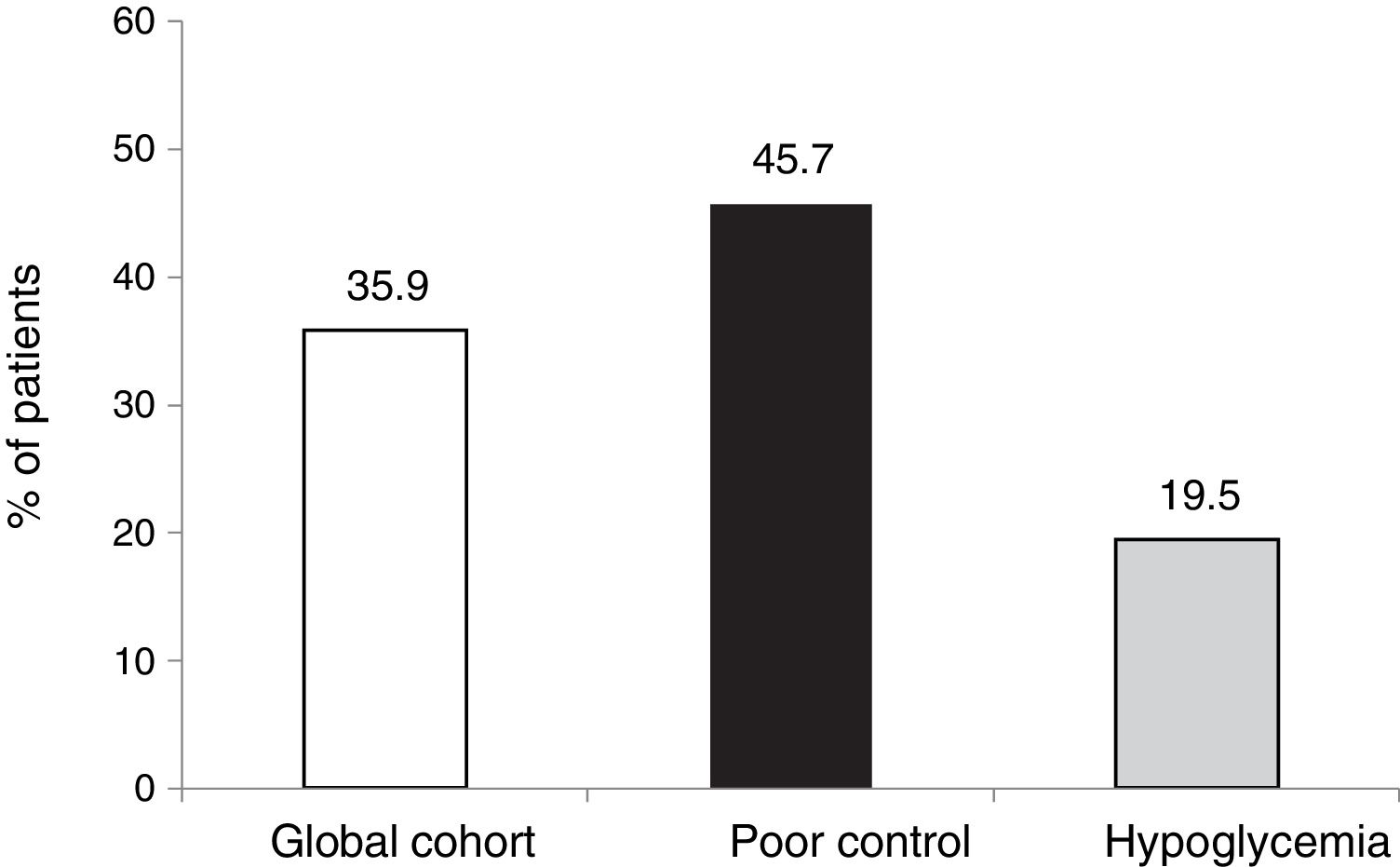
The outcomes in terms of the achievement of the combined goals of HbA1c reduction with different cut-off points and a lowering of the incidence of hypoglycemia episodes in both the global cohort and in the different groups according to treatment indication are shown in Figs. 1–4.
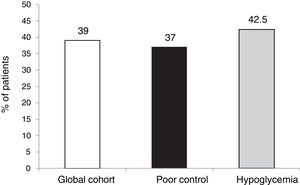
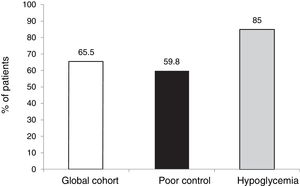
The targets referring to the lowering of HbA1c to below 7.5 % and 8.5 %, eradicating episodes of SH, were reached in 39 % and 65.5 % of the cases, respectively, in the global cohort, both of these percentages being higher in the group of patients that started SCII due to hypoglycemia. Cut-off points of 7.5 % and 8.5 % were selected because they represent the HbA1c value above which the Catalan Health Service and the National Institute for Health and Care Excellence recommend starting CSII therapy due to poor metabolic control with MDI.
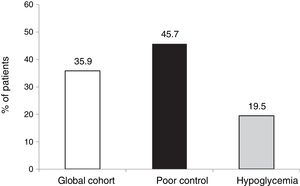
The objective or goal of reducing HbA1c by at least 0.5 points without SH was achieved by 35.9 % of the patients in our cohort, the proportion being lower in those patients who started therapy due to hypoglycemia, since in this group the initial HbA1c value was clearly lower (7.3 %).
However, in terms of the objective combining both a decrease of ≥ 0.5 points and HbA1c < 7.5 % after 5 years without SH, the proportion that reached this objective was similar in both groups (55.5 %).
Lastly, multiple regression analysis was performed to determine whether any of the recorded baseline characteristics were predictive of the achievement of the combined goal of a decrease of ≥ 0.5 points and/or HbA1c < 7.5 % after 5 years without SH in the last two years. None of the variables considered (gender, age, years since diabetes onset, the body mass index (BMI) or HbA1c at baseline) proved able to predict the achievement of this combined goal after 5 years of therapy.
DiscussionThe new analysis of the data of the previously published study15 shows that more than half of the patients who start CSII at a center of reference for this kind of therapy after MDI failure reach the combined goal of significant HbA1c reduction and the long-term eradication of SH.
As with all diabetes therapies, the degree by which HbA1c is lowered is directly related to the HbA1c value at baseline. Consequently, not only the achievement of optimum metabolic control (currently established as HbA1c ≤ 7 % by most clinical guides) is related to the obtaining of therapeutic benefit. Data from the DCCT have already shown that a sustained decrease of "only" 0.3 % in HbA1c is able to significantly reduce the progression of diabetic retinopathy.17 Likewise, long-term follow-up of the same patients shows that an increase in mortality only occurs from HbA1c > 9 %.18 Data from a Swedish population-based study point in the same direction, since global mortality among patients with HbA1c < 6.9 % did not differ from that of patients with HbA1c between 7 and 7.8%.1
In recent years both the lowering of the glycemic goals and the use of continuous glucose monitoring devices have shown hypoglycemia to be the most common acute complication of insulin therapy. The presence of mild hypoglycemia not only alters patient quality of life,19 but moreover poses one of the main barriers to optimizing HbA1c concentrations. Likewise, an increased frequency of mild hypoglycemia is the main determinant of the occurrence of unrecognized hypoglycemia, which in turn is the main determinant of the appearance of SH.20 Long-term follow-up data from the DCCT have shown that after 27 years of follow-up, the cause of death in 8 % of the patients with T1DM is hypoglycemia.21 Other population-based studies also support this relationship between SH and mortality.10,11
Due to the above, in recent years a number of authors and different clinical guides have advocated individualization of the treatment objectives.22 The patients included in our study started SCII treatment after MDI failure, due either to the persistence of elevated HbA1c or to the presence of hypoglycemia, despite adequate management of the therapy. Thus, these are T1DM patients with labile control where reaching HbA1c < 7 % may be a very complicated process, without the counterpart of a large number of hypoglycemia episodes.
Particularly in these cases, analysis of the outcomes of CSII therapy with combined goals, including the absence of SH with HbA1c improvement to different degrees, may be relevant from a clinical perspective. The fact that more than half of the patients achieved the combined goal of HbA1c lowering by ≥ 0.5 points and/or HbA1c < 7.5 % at 5 years without SH in the last two years, or that over two-thirds reached HbA1c < 8.5 % without SH in the last two years, appears to be a clinically relevant outcome, though room for improvement remains.
The present study clearly has a number of limitations: 1) The lack of a control group of patients with MDI did not allow us to compare the results obtained versus such treatment, though it is true that the included patients were individuals in which MDI had failed despite attempted control optimization; 2) As this was a retrospective study, the data referring to SH were collected from the reports of the treating physicians in the case history. However, given the nature of the episodes and the fact that medical teams collect such information as part of their routine practice, we consider the under-assessment of such episodes to be unlikely; 3) Since the study was carried out in a unit specializing in treatment of this kind, the results obtained might not be extrapolatable to other types of centers; and 4) Since long-term follow-up was carried out, the evaluated SCII devices might not be those currently used by most of the patients, though this is a limitation inherent to the long-term evaluation of any treatment involving a technological device.
In conclusion, given our current knowledge concerning the relationship among HbA1c levels, the presence of SH and long-term morbidity and mortality in patients with T1DM, the use of combined goals to assess the effectiveness of therapies for this disease is clinically relevant. The long-term effectiveness of CSII in patients with T1DM in which MDI has failed in a specialist unit assessed using combined goals is over 50 %.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Quirós C, Viñals C, Giménez M, Roca D, Conget I. Evaluación de la efectividad de la terapia ISCI a largo plazo mediante el objetivo combinado de descenso de HBA1c y ausencia de hipoglucemia grave. Endocrinol Diabetes Nutr. 2019;66:534–539.