Diabetic peripheral neuropathy (DPN) is considered to be a risk factor for development of sarcopenia. Therefore, our study aimed to detect the association between peripheral neuropathy with skeletal muscle mass and function in type two diabetes mellitus (T2DM) patients.
MethodsA total of 176 participants, ≥45 years were included in the study. Out of 176, 60 were healthy volunteers, 60 had T2DM without neuropathy, 56 had T2DM with neuropathy. In all the participants peripheral nerve function was assessed by nerve conduction studies (Common peroneal and Sural nerve) and sarcopenia parameters were evaluated according to the Asian Working Group for Sarcopenia (AWGS) criteria.
ResultsThe present study suggested that diabetic peripheral neuropathy (DPN) was associated with decline in muscle mass, which was found only in men. Our study showed a positive correlation between appendicular skeletal muscle index (ASMI) and common peroneal nerve amplitude and sural nerve amplitude with r=0.527, p<0.05; r=0.847, p<0.001 respectively. Furthermore, in multiple linear regression analyses, we found a positive relationship between ASMI and sural nerve amplitude after adjustment for confounders like age, duration of diabetes, and HbA1C (B=0.739; p<0.001).
ConclusionAs DPN patients are more prone to developing sarcopenia, and periodic assessment of skeletal muscle mass and function is warranted to initiate early lifestyle interventions in these patients, which will improve their quality of life.
La neuropatía diabética periférica se considera un factor de riesgo para el desarrollo de «sarcopenia». Nuestro estudio tuvo como objetivo detectar la asociación entre la neuropatía periférica con la masa muscular esquelética y función en pacientes con diabetes mellitus tipo 2.
MétodosUn total de 176 participantes, ≥45 años fueron incluidos en el estudio. De 176, 60 eran voluntarios sanos, 60 tenían diabetes mellitus tipo 2 sin neuropatía y 56 tenían neuropatía. En todos los participantes, la función nerviosa periférica se evaluó mediante estudios de conducción nerviosa (nervio peroneo común y sural) y los parámetros de sarcopenia se evaluaron de acuerdo con el grupo de trabajo asiático para los criterios de sarcopenia.
ResultadosEl presente estudio sugirió que la neuropatía periférica diabética se asoció con la disminución de la masa muscular, que se encontró sólo en los hombres. Nuestro estudio mostró una correlación positiva entre el índice del músculo esquelético apendicular y la amplitud del nervio peroneo común y la amplitud del nervio sural, con r=0,527, p<0,05; r=0,847, p<0,001, respectivamente. Es más, en el análisis de regresión lineal múltiple encontramos una relación positiva entre el índice del músculo esquelético apendicular y la amplitud del nervio sural después del ajuste por factores de confusión como años, duración de la diabetes y hemoglobina glucosilada (B=0,739; p<0,001).
ConclusiónComo los pacientes diabéticos con neuropatía periférica son más propensos a desarrollar sarcopenia, se justifica la evaluación periódica de la masa y la función del músculo esquelético para iniciar intervenciones tempranas en el estilo de vida en estos pacientes que mejorarán la calidad de vida.
Sarcopenia is becoming an evolving clinical entity around the World. Under ICD10 it was recognised as a distinct clinical condition.1 According to the International Diabetes Federation, 463 million people had diabetes in the year 2019 and by the year 2030 the numbers may increase to 578 million and approximately 90 percent of these individuals will have type two diabetes mellitus (T2DM).2 T2DM has been considered a risk factor for the development of sarcopenia.3 Muscle mass declines by 3–8% per year after 30 years of age and this rate of decline further increases after 60 years.4 Earlier the Health, Aging, and Body Composition Study (Health ABC study) stated that elderly individuals with T2DM are more prone to decline in skeletal muscle mass compared to age-matched normoglycaemic individuals.5
Skeletal muscle is responsible for 80% of glucose uptake following food intake.6 Therefore, the preservation of muscle mass plays a crucial role in glucose homeostasis. Skeletal muscle loss is found to be three times more in diabetic individuals in comparison to non-diabetic individuals.7 Skeletal muscle dysfunction in T2DM is associated with various mechanisms like hyperglycaemia, insulin resistance, muscle fat infiltration, and peripheral neuropathy. Peripheral neuropathy may be directly linked to muscle loss as muscles are innervated by peripheral nerves regulating their activity.8 Diabetes mellitus is the leading cause of neuropathy, approximately affecting 463 million individuals worldwide.2 After an initial diagnosis of diabetic peripheral neuropathy (DPN), 70% of individuals show increased mortality over 10 years.9,10 It has been also hypothesised that reduced skeletal muscle function in DPN patients among elderly individuals is associated with a greater incidence of falls, fractures, foot ulcers, and mortality.11 An observational study by Resnick et al., on 39 patients aged between 70 and 79 years reported reduced muscle performance in patients with DPN in comparison to non-diabetic individuals.12 Another study on 170 elderly participants demonstrated a correlation between higher DPN questionnaire scores and sarcopenia.13 However, the study regarding the association between nerve conduction parameters and skeletal muscle mass and function in diabetic peripheral neuropathy is limited. Hence our study aimed to assess muscle mass, strength, and physical performance in T2DM individuals and to evaluate their association with peripheral nerve functions.
Materials and methodsStudy designThis was a cross-sectional study conducted in patients with T2DM to evaluate the association between peripheral neuropathy and skeletal muscle mass and function in comparison to age and gender-matched healthy volunteers. A total of 176 participants aged ≥45 years were included in the study. Out of 176, 56 had T2DM with neuropathy (28 male and 28 female), 60 had T2DM without neuropathy (30 male and 30 female), and 60 were age and gender healthy volunteers (30 male and 30 female). T2DM patients were recruited from the outpatient Department of Endocrinology. Written informed consent was taken from all the participants. This study was undertaken in the Electrophysiology Laboratory, Department of Physiology. Inclusion criteria: T2DM patients on oral hypoglycaemic drugs of either gender with age ≥45 years. Exclusion criteria: Type 1 DM, gestational diabetes mellitus, patients with self-reported mobility-related difficulty, and those on steroids. The approval was obtained from the Institute Research Council and Institute Ethics Committee for human studies before the commencement of the study.
Medical history and biochemical measurementsA thorough medical history regarding age, sex, height, weight, waist/hip ratio, duration of diabetes, intake of drugs including oral hypoglycaemic and other drugs, use of insulin, and smoking history was collected. Height and weight were measured by a stadiometer and weighing machine respectively in patients without shoes and with light clothes. Hip and waist circumference was measured by measuring tape. BMI was calculated by Quetelet's index. Systolic and diastolic blood pressure were recorded in a sitting position from the brachial artery after 15min of rest. After overnight fasting for no fewer than 8h, blood samples were collected. Then, the serum levels of fasting blood sugar, postprandial blood sugar, haemoglobin, glycated haemoglobin, triglycerides, high-density lipoprotein, and creatinine were evaluated.
Assessment of peripheral neuropathyTo assess peripheral nerve function, a nerve conduction study was done in all the participants. The procedure was done in the morning for all the subjects. The room temperature was maintained at 26°C. Patients were informed about the procedure to alleviate anxiety. The study was done using NIHON KOHDEN-NEURO PACK EP/EMG machine. The subjects were asked to lie comfortably on a bed. The subject was asked to lie supine and skin over the examination area of the lower limbs was cleaned with spirit. A motor nerve conduction study was done in the common peroneal nerve and a sensory nerve conduction study was done in the sural nerve. The motor nerve conduction study was done to obtain compound muscle action potential (CMAP) and the parameters like latency, amplitude, and velocity were measured. By the sensory nerve conduction study, sensory nerve action potential (SNAP) was recorded and parameters like latency and amplitude were measured. Neuropathy was diagnosed if there was an increase in latency or decrease in amplitude or velocity based on the reference values.14
Assessment of sarcopenia parametersSkeletal muscle mass and function were evaluated according to the Asian Working Group for Sarcopenia (AWGS) criteria.
- •
The SARC-CalF questionnaire was provided to the participants to assess sarcopenia.15
- •
Appendicular skeletal muscle mass (ASM) was assessed by prediction equation (PE) [0.408*Weight(kg)]−[0.209*Waist circumference(cm)]+[0.072*Handgrip strength(kg)]+10.032 for males.
- •
0.007*age (years)+0.095*height(cm)+0.196*weight(kg)−0.061*waist circumference (cm)+0.087*Handgrip strength (kg)−7.896 for female.16 The appendicular skeletal muscle mass index (ASMI) was calculated as ASM (kg)/height(m2).
- •
Low ASMI was defined as values less than 7kg/m2 for males and 5.4kg/m2 for females.17
- •
Gait speed (GS) was measured by asking the patient to walk for a distance of four metres at their usual pace and the time taken for the distance was noted. While walking, walkers and canes could be used if required. Gait speed ≤0.8m/s was considered as poor physical performance.17
- •
Handgrip strength (HS) was assessed by a hand grip dynamometer “INCO”: Model-PP105. The test was performed on the right side three times and the highest value was recorded for analysis. Low muscle strength was explained as values <28kg for males and <18kg for females.17
SPSS version 28.0 was used for statistical analysis. The distribution of data was tested by the Kolmogorov–Smirnov test. Continuous data were expressed as mean±SD. Comparison of mean between the groups was done by one-way ANOVA followed by post hoc Bonferroni test. The Pearson correlation test was performed to correlate between nerve conduction parameters and skeletal muscle mass. A multiple linear regression model was used to analyse the relationship between ASMI (dependent variable) and nerve conduction parameters (independent variable) after adjusting for age, duration of diabetes, and HbA1C. A p-value of <0.05 was considered to be statistically significant.
ResultsWe recruited patients with T2DM aged ≥45 years. We stratified our study participants based on gender. Under each gender, we categorised T2DM patients into groups of those with neuropathy and those without neuropathy, and these patients were compared against age and gender-matched healthy volunteers. Table 1 shows the comparison of baseline characteristics between the groups. The p-value is significant for the duration of diabetes, fasting blood sugar (FBS), post-prandial blood sugar (PPBS), and glycated haemoglobin (HbA1C) among groups in males and females.
Comparison of anthropometric and biochemical parameters between groups.
| Parameters | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Healthy volunteers (n=30) | DM without neuropathy (n=30) | DM with neuropathy (n=28) | p value | Healthy volunteers (n=30) | DM without neuropathy (n=30) | DM with neuropathy (n=28) | p value | |
| Age (yrs) | 50.9±6.5 | 54.1±4.8 | 54.01±7.09 | 0.086 | 48.6±4.7 | 50.4±4.9 | 47.8±6.9 | 0.198 |
| Duration of diabetes (yrs)a | – | 7.3±3.4 | 11.8±6.9 | 0.006 | – | 6.03±2.8 | 12.2±6.1 | 0.001 |
| Height (cms) | 168.09±13.6 | 170.8±10.1 | 166.7±9.01 | 0.376 | 157.5±5.7 | 157.8±6.1 | 155.3±6.1 | 0.284 |
| Weight (kg) | 60.9±13.9 | 65.4±10.8 | 72.0±11.5 | 0.121 | 57.5±7.3 | 56.6±8.0 | 56.6±4.4 | 0.565 |
| BMI (kg/m2) | 21.08±4.1 | 22.3±0.8 | 22.05±1.17 | 0.063 | 22.3±2.7 | 21.1±3.4 | 22.5±3.4 | 0.205 |
| W/H ratio | 0.74±0.04 | 0.79±0.20 | 0.8±0.03 | 0.107 | 0.6±0.08 | 0.7±0.05 | 0.7±0.07 | 0.002 |
| SBP (mmHg) | 113.1±10.2 | 113.5±9.1 | 118.7±8.7 | 0.057 | 109.6±13.01 | 113.6±15.8 | 109.6±15.4 | 0.487 |
| DBP (mmHg) | 76.1±5.7 | 75.2±9.4 | 76.2±12.8 | 0.909 | 69.1±12.9 | 72.03±9.1 | 74.5±15.9 | 0.279 |
| HR (beats/min) | 79.5±9.7 | 80.2±7.6 | 80.8±9.1 | 0.855 | 74.7±8.1 | 78.9±8.2 | 77.6±11.6 | 0.227 |
| Hb (g/dL) | 12.8±2.5 | 12.8±1.8 | 12.5±1.9 | 0.849 | 12.3±1.4 | 11.7±1.7 | 12.6±1.2 | 0.103 |
| SHDL (mg/dL) | 39.9±9.7 | 41.07±7.5 | 43.1±7.1 | 0.341 | 42.2±7.5 | 45.4±10.5 | 41.3±4.8 | 0.122 |
| Triglyceride (mg/dL) | 144.2±10.05 | 145.5±12.06 | 153.5±29.3 | 0.136 | 146.9±24.4 | 160.7±29.1 | 170.7±58.3 | 0.078 |
| FBS (mg/dL) | 94.2±9.7 | 125.2±27.1* | 148.2±25.5*,# | <0.001 | 94.3±10.9 | 103.4±21.6 | 104.4±22.8 | 0.090 |
| PPBS (mg/dL) | 108.8±8.6 | 180.06±39.1* | 150.4±41.8*,# | <0.001 | 175.2±41.2 | 177.8±54.4 | 188.4±66.4 | 0.627 |
| HbA1c (%) | 5.1±0.2 | 7.2±1.0 | 7.7±2.7* | <0.001 | 5.2±0.3 | 6.3±2.4 | 7.7±2.2*,# | <0.001 |
| S.Cr (mg/dl) | 0.6±0.1 | 0.7±0.03 | 0.6±0.3 | 0.165 | 0.8±0.17 | 0.9±0.29 | 1.01±0.38 | 0.137 |
Data expressed as mean±SD; data analysed by One way ANOVA followed by post hoc Bonferroni test.
Indicates statistically significant difference in comparison with diabetes without neuropathy group; p<0.05 is considered statistically significant.
Abbreviations: BMI, body mass index; W/H ratio, waist–hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; SHDL, serum high density lipoprotein; FBS, fasting blood sugar; PPBS, post-prandial blood sugar; HbA1C, glycated haemoglobin; S. Cr, serum creatinine.
Table 2 shows the comparison of nerve conduction parameters between the groups in males and females, there was a significant difference in the latency, amplitude, and velocity of the common peroneal and sural nerve on both sides between the three groups (p<0.001).
Comparison of nerve conduction parameters between groups.
| Nerve conduction parameters | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Healthy volunteers (n=30) | DM without neuropathy (n=30) | DM with neuropathy (n=28) | p value | Healthy volunteers (n=30) | DM without neuropathy (n=30) | DM with neuropathy (n=28) | p value | |
| Right common peroneal latency (ms) | 3.3±1.09 | 3.7±0.8 | 7.4±2.6*,# | <0.001 | 3.7±1.3 | 3.7±1.6 | 8.2±1.4*,# | <0.001 |
| Left common peroneal latency (ms) | 3.6±1.1 | 3.5±0.9 | 7.7±3.3*,# | <0.001 | 2.9±1.3 | 4.3±1.7 | 6.8±2.5*,# | <0.001 |
| Right Common peroneal amplitude (mV) | 4.3±1.7 | 4.4±1.1 | 1.4±0.3*,# | <0.001 | 6.05±2.5 | 4.9±2.08 | 2.5±1.01*,# | <0.001 |
| Left common peroneal amplitude (mV) | 4.1±1.5 | 4.04±1.04 | 1.3±0.35*,# | <0.001 | 6.6±2.7 | 6.05±0.9 | 1.9±0.4*,# | <0.001 |
| Right common peroneal velocity (m/s) | 65.4±18.7 | 61.8±17.8 | 39.5±11.4*,# | <0.001 | 58.2±11.1 | 58.1±12.7 | 35.7±9.8*,# | <0.001 |
| Left common peroneal velocity (m/s) | 71.1±16.1 | 70.3±20.3 | 38.8±9.8*,# | <0.001 | 61.2±10.1 | 60.2±7.8 | 34.3±11.9*,# | <0.001 |
| Right sural latency (ms) | 3.6±0.3 | 3.9±0.9 | 6.8±2.1*,# | <0.001 | 3.3±0.9 | 3.1±0.6 | 5.9±1.8*,# | <0.001 |
| Left sural latency (ms) | 3.4±0.3 | 3.7±1.1 | 7.3±2.08*,# | <0.001 | 3.4±0.8 | 3.1±1.6 | 5.4±1.4*,# | <0.001 |
| Right Sural amplitude (μV) | 9.9±3.2 | 10.2±2.9 | 5.1±1.3*,# | <0.001 | 12.2±4.1 | 11.3±3.7 | 5.3±1.7*,# | <0.001 |
| Left sural amplitude(μV) | 12.8±4.1 | 12.04±2.1 | 4.2±1.3*,# | <0.001 | 12.8±3.3 | 11.6±4.2 | 5.1±0.8*,# | <0.001 |
Data expressed as mean±SD; data analysed by One way ANOVA followed by post hoc Bonferroni test.
Table 3 shows the comparison of sarcopenia parameters between the groups. In males, parameters like ASMI, and the SARC-CalF questionnaire score (SCS) showed a significant difference between groups (p<0.05). In females, we found a statistically significant difference only in the SCS parameter between the groups (p<0.05) whereas no significant differences were found in ASMI, gait speed, and handgrip strength.
Comparison of sarcopenia parameters between groups.
| Sarcopenia parameters | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Healthy volunteers (n=30) | DM without neuropathy (n=30) | DM with neuropathy (n=28) | p value | Healthy volunteers (n=30) | DM without neuropathy (n=30) | DM with neuropathy (n=28) | p value | |
| ASMI (kg/m2) | 7.2±1.3 | 7.1±0.5 | 5.7±2.2*,# | <0.001 | 5.8±1.2 | 6.09±2.4 | 6.04±2.2 | 0.930 |
| Gait speed (m/s) | 0.8±0.2 | 0.9±0.18 | 0.8±0.2 | 0.437 | 1.1±0.15 | 1.04±0.3 | 1.06±0.4 | 0.272 |
| Hand grip strength (kg) | 35.2±10.2 | 35.6±11.8 | 33.8±7.4 | 0.787 | 27.6±11.2 | 23.0±9.2 | 21.5±11.2 | 0.076 |
| SCS | 3.2±0.4 | 4.2±1.03 | 4.4±3.3* | 0.044 | 2.9±0.9 | 3.7±2.0 | 3.9±1.4* | 0.027 |
Data expressed as mean±SD; data analysed by One way ANOVA followed by post hoc Bonferroni test.
Table 4 shows correlation analyses between ASMI and nerve conduction parameters. In males, we found a positive correlation between ASMI and right common peroneal amplitude (r=0.527; p<0.01), left common peroneal amplitude (r=0.513; p<0.01), right sural amplitude (r=0.847; p<0.001), and left sural amplitude (r=0.791; p<0.001). We found a negative correlation with right common peroneal latency (r=−0.348), left common peroneal latency (r=−0.359), and left sural latency (r=−0.326) but without statistical significance and positive correlation with right common peroneal velocity (r=0.330) but without statistical significance. In females, there was no significant correlation between ASMI and nerve conduction parameters.
Correlation of ASMI with nerve conduction parameters in T2DM with neuropathy.
| Nerve conduction parameters | ASMI | |||
|---|---|---|---|---|
| Male | Female | |||
| r | p value | r | p value | |
| Right common peroneal latency | −0.348 | 0.06 | −0.325 | 0.091 |
| Left common peroneal latency | −0.359 | 0.06 | −0.284 | 0.143 |
| Right common peroneal amplitude | 0.527 | 0.004 | 0.336 | 0.08 |
| Left common peroneal amplitude | 0.513 | 0.005 | 0.376 | 0.052 |
| Right common peroneal velocity | 0.330 | 0.08 | 0.154 | 0.435 |
| Left common peroneal velocity | 0.275 | 0.15 | 0.315 | 0.103 |
| Right sural latency | −0.102 | 0.607 | 0.232 | 0.235 |
| Left sural latency | −0.326 | 0.091 | 0.375 | 0.05 |
| Right sural amplitude | 0.847 | <0.001 | 0.135 | 0.495 |
| Left sural amplitude | 0.791 | <0.001 | 0. 307 | 0.307 |
Data analysed by Pearson correlation; r-correlation coefficient; p<0.05 is considered statistically significant.
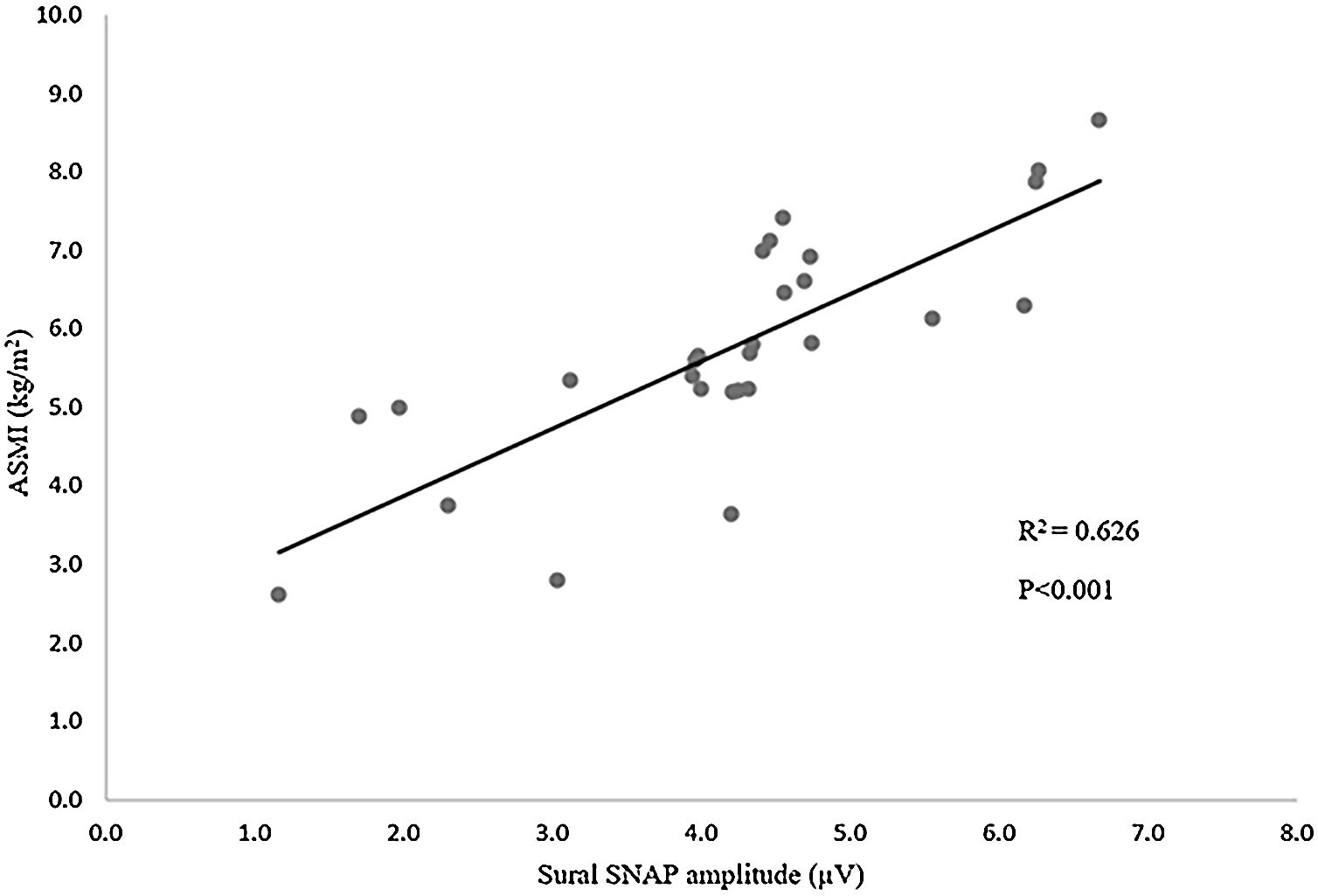
Figure 1 demonstrates positive relation between ASMI and sural SNAP amplitude. (R2=0.626, p<0.001).
Table 5 shows the results of multiple linear regression with ASMI as the dependent variable. The regression model shows that the Sural SNAP amplitude is an independent determinant of ASMI after adjustment for potential confounders like age, duration of diabetes, and HbA1C (B=0.739; p<0.001).
Multiple linear regression analysis with ASMI as the dependant variable.
| Variable | Unstandardised B | Standardised co-efficient beta | p-value | 95% CI |
|---|---|---|---|---|
| Sural nerve SNAP amplitude | 0.739 | 0.680 | <0.001 | 0.423–1.054 |
| Age | −0.027 | −0.128 | 0.356 | −0.085–0.032 |
| Duration of diabetes | 0.043 | 0.199 | 0.232 | −0.029–0.115 |
| HbA1C | −0.176 | −0.338 | 0.050 | −0.353–0.175 |
Abbreviations: B, regression coefficient; CI, Confidence Interval; ASMI, Appendicular Skeletal Muscle Index; SNAP, Sensory Nerve Action Potential; HbA1C, glycated haemoglobin.
Adjusted for conventional risk factors like age, duration and HbA1C.
This study investigated the relationship between DPN and skeletal muscle mass and function in T2DM patients. The present study suggested that DPN was associated with a decline in muscle mass, which was found only in men. A previous study with 230 participants conducted in a tertiary care hospital where DPN was diagnosed based on the Michigan Neuropathy Screening Instrument Questionnaire (MNSI-Q) and Physical Examination (MNSI-PE) showed that DPN was associated with decline in muscle function which was observed only in men.13 Zhang et al. had demonstrated a reduction in muscle function, which was correlated with DPN in men.18 Our study results are in concordance with these previous studies. The rationale for gender differences observed may be due to various methods used for assessing sarcopenia and diverse study population.
In the diabetic population, the prevalence of DPN varies from 13% to 68% which is associated with remarkable loss of muscle mass and strength in comparison to those without DPN.19,20 Our study demonstrated a positive correlation between ASMI and common peroneal CMAP amplitude and sural SNAP amplitude with statistical significance. Furthermore, in multiple linear regression analyses, we found a positive relation between ASMI and sural SNAP amplitude after adjustment for confounders like age, duration of diabetes, and HbA1C. This further proves that neuropathy could be an independent determinant for the decline in muscle mass. Various previous studies had correlated muscle function with DPN using subjective tools like different questionnaire scores and clinical examination for evaluation of DPN.13 But in our study, we performed a nerve conduction study of common peroneal and sural nerves to evaluate DPN. Therefore, the clinical implication of our findings may be superior as compared to other studies.
Various pathophysiological mechanisms have been proposed to explain the relationship between peripheral neuropathy and sarcopenia in T2DM patients. First axonal loss is a major factor leading to skeletal muscle deficits in DPN.21 Secondly, alteration in structural muscle protein and contractile properties in skeletal muscle may contribute to muscle loss in DPN patients.22 Third, as skeletal muscle is a major organ for glucose disposal, poor glycaemic control maybe a major factor contributing to DPN and muscle loss. The proposed reason for the development of sarcopenia in T2DM is attributed to loss of insulin sensitivity in diabetes leading to loss of anabolic action of insulin on skeletal muscle.23 Advanced glycation end-product (AGE) receptors are present in both neurons and skeletal muscle. Raised AGEs due to hyperglycaemia may contribute to microvascular injury leading to impaired nerve and muscle function.23 Moreover, inflammation and oxidative stress also negatively affect nerves and muscles.24
In our study, loss of muscle mass in DPN patients was found in males. In addition, there was a significant correlation between ASMI and nerve conduction parameters found in men, but in women, no significance was found between nerve conduction parameters and ASMI. Various factors may contribute to this finding. Few previous studies have demonstrated that diabetes is linked to reduced sensory and motor conduction parameters in both genders, but the alterations have been more in men.25 This kind of sexual dimorphism may be due to the oestrogen-dependent protective effect of human heat shock protein-27 on the peripheral nerves.26 Such an oestrogen-induced protective effect may overcome the neurodegradatory effects that can cause sarcopenia.
This is the first study showing the association of peripheral neuropathy with skeletal muscle mass and function in T2DM patients in Eastern India. The confounding factors affecting the outcome were eliminated during subject selection. The predictive equation for ASMI used in our study was the most cost-effective and accessible method validated with the standard techniques like Bioelectrical Impedance Analysis (BIA) and Dual-energy X-ray Absorptiometry (DEXA) which are highly sensitive. We performed nerve conduction studies for the diagnosis of peripheral neuropathy as compared to other studies which have used questionnaire scores and clinical examination for the diagnosis of DPN.
There was some limitation that warrants discussion. First, the sample size of our study is relatively small. Second, daily physical activity and nutritional status, especially protein intake, which are possible modulators of the relationship between diabetes and skeletal muscle mass were not considered in the present study. Third, as patients are included from single tertiary care hospitals, the results of our study may not be generalised. Sarcopenia is an important factor leading to frailty which in turn decreases the quality of life. Despite affecting a significant number of patients with diabetes, sarcopenia had not gained much attention among physicians and the general population. So further multicentric studies with a larger population and diverse ethnicity are essential for prevention and early intervention of sarcopenia in T2DM patients.
ConclusionOur study demonstrated an association between DPN and skeletal muscle mass. As sarcopenia affects a significant proportion of diabetic patients, periodic assessment of skeletal muscle mass and function is warranted to initiate early lifestyle interventions in these patients which in turn will improve the quality of life.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone declared.
We express our sincere gratitude to the technical staff for extending their support throughout the study duration.