To assess an artificial pancreas system during aerobic (AeE) and anaerobic exercise (AnE).
MethodsA pilot clinical trial on five subjects with type 1 diabetes (four males) aged 37±10.9 years, diabetes diagnosed 21.2±12.2 years before, insulin pump users, and with a mean HbA1c level of 7.8±0.5%. Every subject did three AeE and three AnE sessions. Blood glucose levels were monitored by the artificial pancreas system during exercise and up to 4h later. Before the start of exercise, 23g of carbohydrates were administered orally.
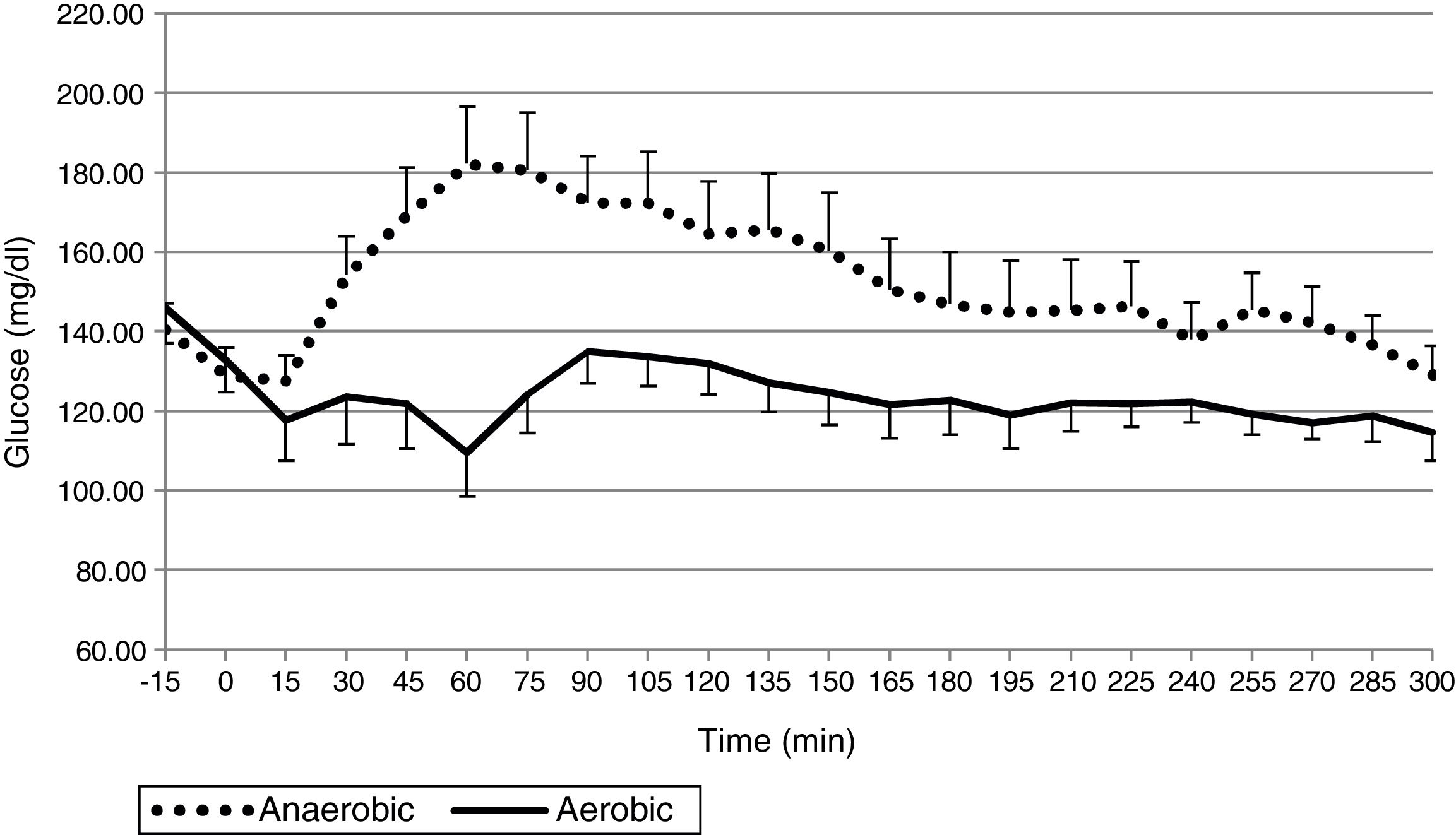
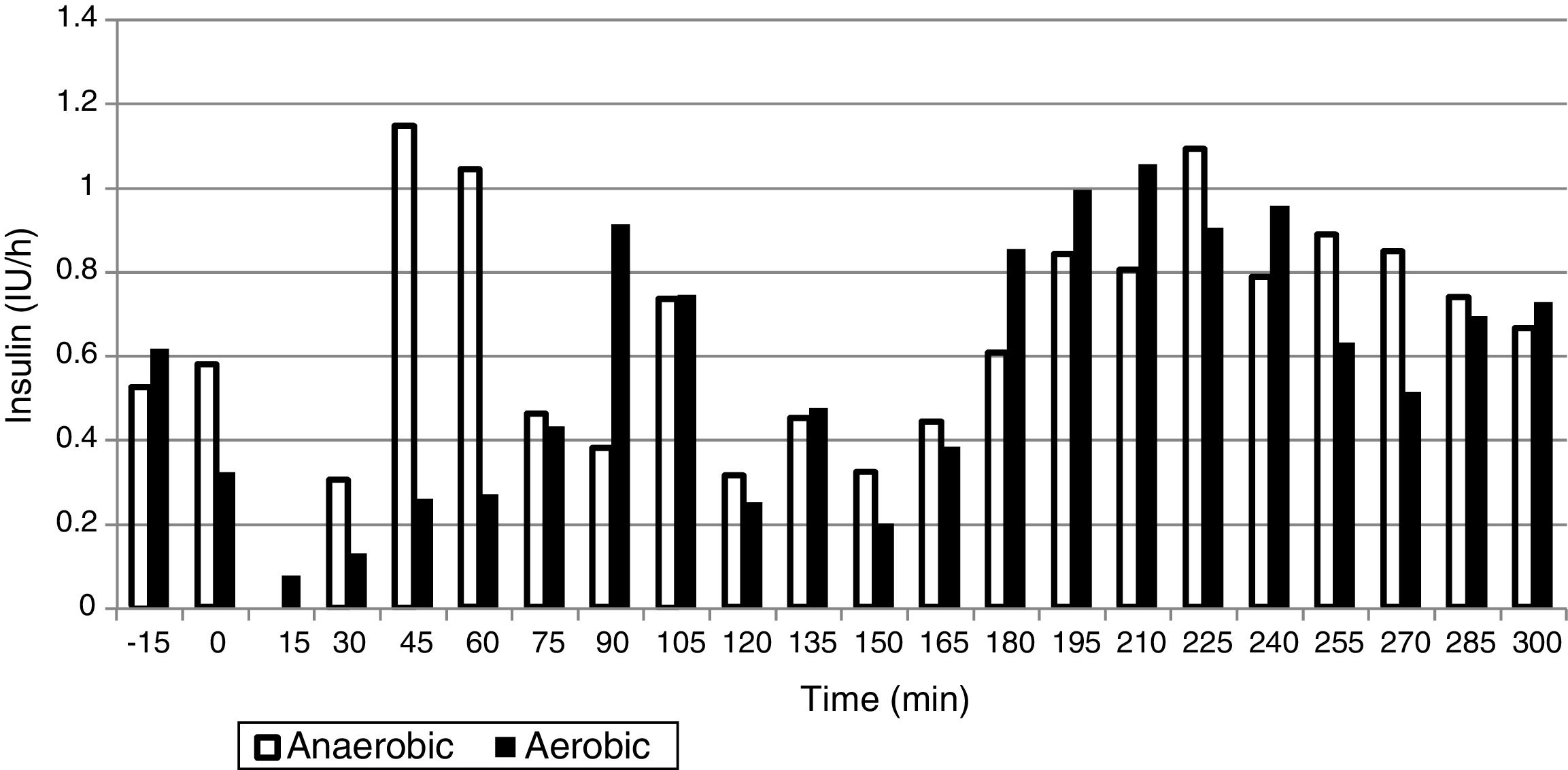
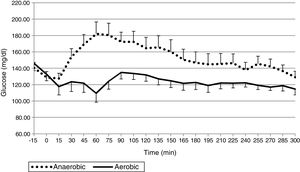
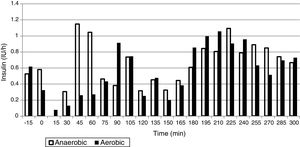
ResultsThe mean glucose level was 124.0±25.1mg/dL in the AeE studies and 152.1±34.1mg/dL in the AnE studies. Percent times in the different glucose ranges of 70–180, >180, and <70mg/dL were 89.8±18.6 and 75.9±27.6%, 7.7±18.4 and 23.2±28.0%, and 2.5±6.3 and 1.0±3.6% during the AeE and AnE sessions, respectively. Only six rescues with carbohydrates (15g) were required during the studies (four in AeE and two in AnE). Total insulin dose during the 5h of the study was 3.1±1.0IU in the AeE studies and 3.5±1.3IU in the AnE studies.
ConclusionsBlood glucose response to AeE and AnE exercise is different. The evaluated artificial pancreas system appeared to achieve effective and safe blood glucose control during exercise and up to 4h later. However, new control strategies that minimize patient intervention should be designed.
Evaluar de forma exploratoria un sistema de páncreas artificial durante la realización de ejercicio aeróbico (EAe) y anaeróbico (EAn).
MétodosEnsayo clínico piloto con 5 sujetos con diabetes tipo 1 (4 hombres) de 37±10,9 años, 21,2±12,2 años de evolución de la diabetes tipo 1, usuarios de infusor de insulina y una HbA1c de 7,8±0,5%. Cada uno de los pacientes realizó 3 estudios de EAe y 3 de EAn. El control de la glucemia se realizó mediante el algoritmo de páncreas artificial durante el ejercicio y las 4h posteriores al mismo. Previo al inicio del ejercicio físico se administraron 23g de hidratos de carbono.
ResultadosLa media de glucosa fue de 124,0±25,1mg/dL en los estudios de EAe y de 152,1±34,1mg/dL en los de EAn. Los porcentajes de tiempo en 70-180, >180 y <70mg/dL fueron: 89,8±18,6% y 75,9±27,6%; 7,7±18,4% y 23,2±28,0%; 2,5±6,3% y 1,0±3,6% durante el EAe y EAn, respectivamente. Únicamente fueron necesarios 6 rescates con 15g de hidratos de carbono en el total de los estudios (4 en EAe y 2 en EAn). La dosis total de insulina durante las 5h de estudio en los estudios de EAe fue de 3,1±1,0UI y de 3,5±1,3UI en los EAn.
ConclusionesLa respuesta glucémica al EAe y al EAn es diferente. El sistema de páncreas artificial evaluado parece controlar de forma eficaz y segura la glucemia durante el ejercicio y las 4h posteriores al mismo, aunque es necesario el diseño de nuevas estrategias de control que minimicen la intervención del paciente.
In recent decades, therapeutic advances in the management of type 1 diabetes mellitus (DM1) have significantly increased patient life expectancy. However, individuals with DM1 have a 4–8-fold increased risk of developing cardiovascular disease compared with individuals without diabetes.
Physical exercise has shown multiple benefits in terms of diminished cardiovascular risk, including an improved lipid profile, reduced body weight and fat, and lower blood pressure. The most recent clinical guidelines therefore recommend regular physical exercise in patients with DM1.1 However, it is also widely known that blood glucose management during and after physical exercise is complex regardless of the treatment modality used, since exogenous insulin administration is unable to mimic the complex physiological system involved in blood glucose regulation.
Multiple factors such as the type of exercise, its duration and intensity, the physical condition of the patient, the time of day of exercise, or the performance of exercise or not during the previous days all condition the glycemic response to exercise in patients with DM1. The implication of so many factors means that predicting blood glucose behavior in response to physical exercise is a highly complex matter. As a result, it is difficult to standardize therapeutic management during and after exercise. Nevertheless, some recent guidelines2 have summarized the information obtained from a range of studies analyzing the physiological response to exercise in patients with DM1. This has provided general advice on the management of blood glucose during the different types of physical exercise, which subsequently needs to be individualized.
Artificial pancreatic systems use the interstitial blood glucose values collected by a continuous monitoring sensor, with an automatic mathematical algorithm-based calculation of the insulin dose to be administered in order to maintain blood glucose values within a target range. These systems have been shown to improve glycemic control in patients with DM1 in home-based studies as compared to conventional systems in which the patients themselves are responsible for taking treatment decisions.3,4 However, unpredictable and rapidly varying changes in insulin requirements associated with physical exercise in patients with DM1 pose a challenge for artificial pancreatic systems. In particular, the initial decrease in glucose levels usually observed after the start of aerobic exercise (AeE) poses a challenge for unihormonal artificial pancreas systems, since their only possible action is to arrest insulin infusion, and if this is done once the drop in glycemia has been detected, the measure usually proves completely ineffective.
Our group has recently evaluated an artificial pancreas system (the saphenous auxiliary feedback [SAFE] system) during the postprandial period, with favorable results.5 Since the postprandial period is also characterized by rapid and poorly predictable changes in glucose levels, the present study was designed so that a controlled exploratory assessment of the behavior of this artificial pancreas system during AeE and anaerobic exercise (AnE) could be conducted.
Material and methodsAn exploratory clinical trial was carried out on five patients with DM1. The study protocol was approved by the Ethics Committee of Hospital Clínic de Barcelona (Barcelona, Spain), and patient's informed consent was obtained before the start of participation in the study. The inclusion criteria were as follows: patients between 18 and 60 years of age with DM1 for at least 1 year, and treated with an insulin pump for at least 6 months, with glycosylated hemoglobin (HbA1c) concentration 6–8.5%. The Clarke test was used to rule out unnoticed hypoglycemia in all patients.6
Each subject completed six exercise studies in the hospital setting, spaced at least 1 week apart. In three of the studies, the patient performed AeE, with resistance exercise in the remaining three studies. The sequence in which each patient performed the different types of exercises was randomly assigned by software.
The patients were instructed to use a real-time continuous monitoring system on the initial study visit and subsequently wore the system for 1 week before the start of the controlled exercise trials. The information obtained from this first week of monitoring was used to calculate the following parameters: the insulin/carbohydrate ratio, the sensitivity factor, basal insulin requirements, and basal insulin on board. These parameters allowed both adjustment of the regular insulin pump treatment of the patient and individualized adjustment of the artificial pancreas system.5,7,8
After the completion of each study, the patient assessed the intensity of physical exercise by completing the Borg Rating of Perceived Exertion scale.9 The patient likewise completed this scale after each exercise session at home during the full study participation period.
Study devicesContinuous subcutaneous insulin infusion (CSII) was performed using Paradigm Veo® devices, and continuous glucose monitoring (CGM) was carried out using Enlite2® sensors, both from Medtronic Minimed (Northridge, CA, USA). Two CGM sensors were inserted 24h before the study. Although the glucose controller only used information from one of the two sensors (that chosen as the primary sensor prior to initiating the controller after MARD [mean absolute relative difference] analysis of both in the previous hours), the purpose of the second sensor was to inform the glucose controller in the case of the persistent failure of the primary sensor (defined as an absolute relative difference between sensor glucose and venous glucose >40% in one reading or >30% in two consecutive reading periods). Calibration of the CGM systems was performed using Contour Next Link® glucometers (Ascensia Diabetes Care Holdings AG, Basel, Switzerland). During the study, the venous glucose levels were also measured every 15min using the YSI 2300 Stat Plus Glucose Analyzer reference method (YSI 2300; YSI Incorporated Life Sciences, Yellow Springs, OH, USA).
The characteristics of the artificial pancreas system have been described in detail elsewhere.5 The system comprises a PID (proportional integral derivative) primary controller designed to take the glucose levels to a defined target value. The particularity of the system is the addition of an SMRC (sliding mode reference conditioning)-type external controller. This controller estimates insulin on board in each period using a pharmacokinetic model and compares it to an upper insulin limit calculated for each patient on a case-to-case basis using data from the week prior to patient monitoring and the settings of the parameters of the regular insulin pump treatment of the patient. When the estimated insulin on board exceeds the upper limit set for each patient, the SMRC system modifies the glucose target value of the primary controller in order to limit the administered insulin and thus avoid hypoglycemia secondary to excessively high active insulin levels.
In relation to exercise, the controller has two main actions: (a) the target glucose level increases from 100 to 150mg/dL from the start of exercise during a 45min period and then gradually falls over 2h until 100mg/dL are again reached; and (b) during the same time period, the reference baseline of the controller from which deviations are computed to calculate the insulin dose to be administered at each time point switches from that of the patient during regular CSII therapy to 0, thereby reducing the insulin dose to be administered.
Physical exercise studiesThe patients arrived at the clinical research unit at 8 a.m. having previously had breakfast at home along with the administration of the bolus suggested by the insulin pump, based on the usual insulin/carbohydrate ratio. Each patient had the same breakfast on each of the 6 days of the study. Upon arrival, two venous lines were placed: one for arterialized venous blood sampling10 and the other for glucose/insulin infusion. To ensure comparable metabolic conditions between studies, patients could receive intravenous glucose/insulin in order to secure a plasma glucose level of about 150mg/dL before artificial pancreas blood glucose control was started. At 10:45 a.m., automatic blood glucose control was started, and every 15min the system was informed of the glycemia value of the sensor and decided on the insulin to be administered during the next 15min through the variation of the baseline of the pump during that period. The automatic glucose control was maintained until 4 p.m. Physical exercise started at 11 a.m., after the patient had consumed 23g of carbohydrate (CH) in gel form (Diabalance® rapid-acting glucose gel; Esteve, Barcelona, Spain) and an isotonic drink (Aquarius®, The Coca Cola Company, Atlanta, GA, USA).
The aerobic exercise studies comprised three series of 15min of stationary bicycle at 60% of maximum heart rate, with 5min of rest between them. In the resistance exercise studies, the patient performed five series of eight repetitions of four weight exercises involving different muscle groups, with a weight equivalent to 70% of maximum capacity, with periods of 90s of rest between repetitions.
When plasma glucose dropped to <70mg/dL in two consecutive measurements, 15g of oral CH was administered until recovery from hypoglycemia.
Statistical analysisSince this was an exploratory study, no comparisons were made between the two types of exercise. Calculations were limited to mean and standard deviations of plasma and interstitial blood glucose, percentage time within range in hypo- and hyperglycemia, and the insulin doses administered.
ResultsThe study comprised five subjects with DM1, with the following characteristics: four males, with a mean age of 37±10.9 years, 21.2±12.2 years since DM1 onset, a body mass index (BMI) 24.9±1kg/m2, and HbA1c 7.8±0.5%.
The mean plasma glucose level during exercise and the succeeding 4h was 124.0±25.1mg/dL in the AeE studies and 152.1±34.1mg/dL in the AnE studies. As can be seen in Figs. 1 and 2, resistance exercise induced an initial hyperglycemic peak that was subsequently compensated for by an increase in insulin administration, while AeE induced a drop in blood glucose levels from the first minutes of exercise, despite the fact that the artificial pancreas system stopped administering insulin from the start of exercise. The results were similar on analyzing the glycemia data obtained from the continuous monitoring sensor (AeE: 129.0±27.7; AnE: 152.1±34.1).
The percentage time between 70 and 180mg/dL was 89.8±18.6% in the AeE studies and 75.9±27.6% in the AnE studies. Likewise, the percentage hypoglycemia time was 2.5.±6.3% in the AeE studies and 1±3.6% in the AnE studies.
The insulin doses administered during the 5h of study were 3.1±1.0IU in the AeE studies and 3.5±1.3IU in the AnE studies. The distribution of insulin administration is shown in Fig. 2.
With regard to the perception of exercise intensity, both types of exercise were perceived as being of similar intensity, with a Borg score in the AeE studies of 11.5±1.8 versus 10.9±2.6 in the AnE studies. These data were similar to the results obtained in the exercise sessions at home during the duration of the clinical trial (10.6±2.1).
DiscussionThe results of this exploratory study confirm that subjects with DM1 under automated glycemic control using an artificial pancreas differ significantly with regard to the glycemic response to AeE and resistance exercise. While AeE induces a fast and greater drop in glucose levels, resistance exercise tends to increase blood glucose initially, with a less pronounced fall afterwards.
Previous studies by Yardley et al.11,12 in patients treated with both multiple doses of insulin and CSII showed AnE to induce a lower initial blood glucose decrease, thereby facilitating the prevention of hypoglycemia associated with exercise, which constitutes one of the main barriers against physical activity in patients with DM1. In addition, AnE facilitated glycemic control during the hours after exercise, with more stable glucose levels than after AeE. These data were confirmed by a subsequent meta-analysis13 documenting the glycemic fluctuations after different types of exercise in various studies.
The physiopathological basis of these findings has not been fully established. However, in both the aforementioned studies11,12 and in other later publications14 in which different blood markers were measured, it has been suggested that the greater increases in cortisol, catecholamine, and lactate levels during resistance exercise appear to be the main factors underlying this difference in initial glycemic response to the two types of exercise.
Given these differences, the approach adopted should vary depending on the type of exercise carried out by the individual. Since exercise performed by patients is often not only either aerobic or anaerobic, and considering that many other factors are also implicated in glycemic response (intensity, duration, physical activity over the previous days, etc.), establishing general recommendations for glycemic management during exercise is a very complicated matter. In this respect, a series of factors should be taken into account by patients when deciding which behavior is required. An online survey of over 500 patients with DM115 subjected to different treatment modalities showed the management of blood glucose levels during exercise to be highly variable among patients, and many of them reported important difficulties in controlling blood glucose during exercise.
The main objective of artificial pancreas systems is to secure adequate glycemic control, freeing the patient from the constant decision making currently associated with the management of DM1. Growing evidence that these systems are able to improve glycemic control as compared to current therapies has been obtained from uncontrolled studies of relatively long duration.3,4 However, the management of certain situations such as blood glucose control in the postprandial period or during exercise remains a challenge for these systems.
The main difficulty facing artificial pancreatic systems in glycemic control during exercise lies in the delay associated with interstitial fluid glucose monitoring and insulin administration in the subcutaneous tissue, the action profile being much slower than in the case of endogenous insulin. Physiologically, in people without DM1, the start of exercise causes a drop in blood insulin.16 Given the kinetics of subcutaneous insulin analog injection, it is not possible to mimic this behavior in artificial pancreatic systems, even if exercise has been preset, thereby allowing for pre-dosing actions. As a result, a number of studies using different strategies for glycemic control during exercise have been published in recent years.14,17–19
One of the most widely used strategies is the administration of CH before and/or during exercise. Patel et al.20 used this approach with a proportional integral derivative (PID) artificial pancreas system, avoiding hypoglycemia in sessions of intense AeE, though at the expense of relatively high blood glucose values and an intake of 30–45g of CH per exercise session.
Another strategy has involved the presetting of exercise to the artificial pancreas system before the start of exercise, allowing the algorithm to modify certain parameters to afford less aggressive insulin administration, thereby reducing the risk of hypoglycemia. This approach was used in the study carried out by Jayawardene et al.,14 involving CH intake before exercise, based on the previous blood glucose levels. However, the announcement of exercise took place 120min before the start of exercise, and this approach appears to be impractical in real life, outside the controlled clinical trial setting.
Other groups have attempted to add monitors of heart rate and other signals to the artificial pancreas system in order both to detect the performance of exercise17,21 and to discriminate between types of exercise.22 These systems have been shown to adequately detect the performance of exercise and even discriminate between AeE and AnE, though as commented above, introducing changes in the artificial pancreas system once exercise has started appears insufficient to prevent the drop in glucose levels associated with AeE.
On the other hand, bihormonal artificial pancreas systems a priori should offer advantages over unihormonal systems in the context of physical exercise, for in addition to stopping insulin infusion, they can administer glucagon to mitigate the tendency toward hypoglycemia. The only published study comparing a unihormonal versus a bihormonal system18 reported a decrease in the number of hypoglycemic episodes, though with a non-negligible percentage of exercise sessions in which a hypoglycemic episode occurred (11.8 and 6.25% of the AeE sessions and intervals, respectively, using the bihormonal system).
Lastly, the use of ultra-fast insulin analogs that have shown a faster action peak, improving postprandial glycemia control in patients on CSII therapy,23,24 theoretically should offer benefits in terms of glycemia control with artificial pancreatic systems, particularly in situations where (as during exercise) the glucose levels vary rapidly. However, to date no studies have evaluated these new drugs in artificial pancreatic systems during exercise.
In our pilot study, we evaluated an artificial pancreatic system specifically designed for glycemic control during the postprandial period in the context of AeE and AnE. The protocol included the previous intake of CH, with globally satisfactory glycemia control during exercise and over the following 3h being obtained. We believe that presetting physical exercise may be a very efficient strategy for avoiding hypoglycemia, though very early presetting is probably not feasible in the context of everyday life. On the other hand, the ingestion of CH before exercise is also an effective safety strategy, though ideally artificial pancreatic systems should be able to avoid obligatory intake before physical exercise in patients with DM1. It is therefore necessary to design new and more effective control strategies in relation to physical exercise, capable of minimizing patient intervention.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Quirós C, Bertachi A, Giménez M, Biagi L, Viaplana J, Viñals C, et al. Control de la glucemia durante el ejercicio físico aeróbico y anaeróbico mediante un nuevo sistema de páncreas artificial. Endocrinol Diabetes Nutr. 2018;65:342–347.