Development of cystic fibrosis-related diabetes (CFRD) is associated with worsening of nutritional status and lung function, as well as increased mortality. The relevance of diagnosing the «pre-diabetic» status in these patients has not been addressed and the utility of HbA1c measurement in these patients is under discussion.
AimTo study and characterise the different categories of carbohydrate metabolism impairment in paediatric patients with cystic fibrosis.
Patients and methodsA transversal study for characterisation of carbohydrate metabolism impairment according to clinical and anthropometric status and genetic background in 50 paediatric patients with cystic fibrosis (CF) was undertaken. Oral glucose tolerance tests (OGTT) for determination of glucose and insulin levels measurement and continuous subcutaneous glucose monitoring (CSGM) were performed.
Results6% of patients presented with CFRD, 26% impaired glucose tolerance, 10% an indeterminate glucose alteration and 2% impaired fasting glucose. The severity of glycaemic impairment correlated positively with age and negatively with standardised height (p < 0.05) with intergroup differences in HbA1c levels (p < 0.01), with the latter correlating with the duration of hyperglycaemia throughout CSGM. No intergroup differences in mutation prevalence, pulmonary function test, nutritional status or disease exacerbations in the previous year were found. The daily enzyme replacement dose correlated with the glucose area under the curve (AUC, p < 0.05) but not with insulin-AUC.
ConclusionsAn older age and greater enzyme replacement need are correlated with more severe carbohydrate metabolism impairment and lower standardized height in paediatric CF patients, with HbA1c correlating with the duration of hyperglycaemia. The study of the full glucose/insulin AUCs throughout the OGTT affords no additional information compared to glucose determination at 120 min in these patients.
El desarrollo de diabetes mellitus en pacientes afectos de fibrosis quística (DRFQ) se relaciona con peor estado nutricional y función pulmonar y aumento de mortalidad. La relevancia del diagnóstico de los estados «prediabéticos» es desconocida, cuestionándose la utilidad clínica de la HbA1c en estos pacientes.
ObjetivoEstudiar y caracterizar las alteraciones del metabolismo hidrocarbonado en pacientes pediátricos afectos de FQ.
Pacientes y métodosCaracterización antropométrica, clínica y genética y estudio de prevalencia de las alteraciones del metabolismo hidrocarbonado en 50 pacientes pediátricos afectos de FQ, mediante determinación de glucosa e insulina en test de tolerancia oral (TTOG) y monitorización continua de glucosa subcutánea (MCGS).
ResultadosUn 6% de pacientes presentaron DRFQ, 26% intolerancia a los hidratos de carbono, 10% alteración indeterminada de la glucosa y 2% alteración de glucemia en ayunas. La gravedad de la alteración glucémica se correlacionó positivamente con la edad y, negativamente, con la talla de los pacientes (p < 0,05), observándose diferencias intergrupos en HbA1c (p < 0,01), correlacionándose esta con el tiempo de hiperglucemia en MCGS, pero no en las mutaciones subyacentes, parámetros de función respiratoria, estado nutricional ni número de exacerbaciones en el año previo. Existía una correlación de las necesidades enzimáticas con el área bajo la curva (AUC) de glucemia (p < 0,05), pero no con AUC-insulinemia.
ConclusionesUna mayor edad y necesidad de aportes enzimáticos se relacionan con mayor afectación del metabolismo hidrocarbonado y menor talla estandarizada en pacientes afectos de FQ, siendo la HbA1c útil en la estimación del tiempo de hiperglucemia. El estudio del AUC de glucemia/insulinemia no aporta información adicional frente a la determinación de glucemia a los 120 min.
Cystic fibrosis (CF) is the most common autosomal recessive disease in the Caucasian population; however, its reported prevalence in Spain (1/2810–1/3743 newborns)1 appears to be overestimated, as newborn screening has shown ethnicity and country of origin to have an impact on this prevalence. CF is due to mutations in the CFTR gene (ΔF508 being the predominant mutation), which encodes the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Dysfunction of this protein causes an abnormality in chloride and sodium transport in epithelial secretion cells, leading to multisystemic clinical signs, especially in the respiratory tract (progressive lung impairment) and gastrointestinal system (exocrine pancreatic insufficiency and liver disease).2
Disease progression gradually induces pancreatic fibrosis, which initially causes exocrine insufficiency. Endocrine insufficiency usually develops later on and causes carbohydrate metabolism impairment, which may culminate in the onset of a particular type of diabetes mellitus (DM) called cystic fibrosis-related diabetes (CFRD). CFRD usually develops in the second decade of life.3 It affects up to 20% of adolescents with CF and around 50% of adults with CF;4 hence, CFRD research and treatment has gained importance as these patients’ survival has increased.
Despite certain similarities to type 1 DM (T1DM), CFRD constitutes a singular clinical entity. Pancreatic beta cells start declining in numbers at an early age,5,6 and its pathophysiology involves multiple mechanisms of synergistic action, such as direct cell damage, underlying genetic alteration and hyperglycaemia itself, which induces pancreatic islet cell damage and contributes to peripheral insulin resistance in a process called glucotoxicity.5
The importance of CFRD, as well as the carbohydrate metabolism impairment that may precede it (called “stages of prediabetes”), lies in the impact thereof on the overall clinical course of a patient with CF.7 Blood glucose levels exceeding 144 mg/dl (8 mmol/l) have been seen to increase airway glucose excretion,8 thus promoting growth of micro-organisms and, consequently, numbers of respiratory infections, which could later lead to worsening of lung function. Insulin therapy has been seen to improve nutritional status,9 and thus aid in reducing mortality in patients with CFRD.10,11 This means that early detection thereof is clinically significant.
An initial diagnosis of CFRD is generally made in periods of increased insulin resistance (lung infections or use of systemic corticosteroids)11 and is commonly preceded by weight loss, decreased growth (in infancy/childhood) and a decline in lung function. Classic symptoms of DM (polydipsia and polyuria) and ketoacidosis are rare in CFRD,5,6 insulin deficiency develops more gradually in CFRD than in T1DM.
Oral glucose tolerance testing (OGTT) is considered the standard test for its diagnosis,6,12 since up to two thirds of patients with CFRD do not present fasting hyperglycaemia,10 suggesting that the presence of hyperglycaemia at intermediate points in the test could be useful as a clinical predictor.10,13,14
Continuous subcutaneous glucose monitoring (CSGM) enables detection of intermittent postprandial periods of hyperglycaemia in patients whose fasting blood glucose and OGTT yield normal results. As a result, some authors believe that it is more sensitive for early detection of these abnormalities preceding CFRD onset.15,16 However, the clinical significance of these periods of hyperglycaemia is not well established,13,17 and CSGM is not currently recommended as a screening method.18
HbA1c is an unreliable marker for the diagnosis of CFRD, in general and in children in particular; the argument is that HbA1c levels can be found to be falsely low, for two reasons.19 Namely, 1) periods of intermittent postprandial hyperglycaemia do not appear to significantly affect glycosylation of haemoglobin20 and 2) a drop in mean red blood cell lifespan, which is characteristic in patients with cystic fibrosis, would lead to lower HbA1c results for a given blood glucose level.21 Different authors have found that the sensitivity of HbA1c for the diagnosis of CFRD is just 50%, compared to OGTT, suggesting that HbA1c levels are not correlated to plasma blood glucose levels.22
The American Diabetes Association (ADA) and the International Society for Pediatric and Adolescent Diabetes (ISPAD) recommend annual screening for CFRD using OGTT, starting at age 10 in all patients with CF.4,6 However, blood glucose abnormalities develop gradually, with documented rates of carbohydrate metabolism impairment of 42%–78% in children under this empirical limit established for screening.23,24
Neither the ADA nor the ISPAD recommendations cite the possible importance of determining blood insulin levels over the course of OGTT, even though this could be useful for evaluating insulin reserve capacity in stages prior to CFRD onset.25
The objectives of this study were: 1) to investigate the prevalence of carbohydrate metabolism impairment in paediatric patients with CF in relation to anthropometric measurements, clinical stage and CFTR variants; 2) to evaluate the possible usefulness of HbA1c as an indirect indicator of time in hyperglycaemia in these patients; and 3) to explore whether examining the AUCs of glucose and insulin over the course of OGTT provides information in addition to isolated determination of blood glucose levels 120 min after glucose intake.
Patients and methodsThe study included 50 patients (23 girls [46%] and 27 boys [54%]), with a mean age of 12.27 (±3.72 years) with CF, with no other known concomitant or underlying diseases and in a stable disease stage, with neither intercurrent conditions nor treatment with systemic corticosteroids in the past eight weeks, and not on treatment with CFTR potentiators. Of the 50 patients, 16 were prepubescent, 24 were going through puberty and 10 had completed puberty.
The following data were recorded for the study patients: demographic and anthropometric data (age, sex, weight and standardised height), nutritional status and growth parameters (through calculation of Waterlow’s classification and standardised growth rate in the past year), as well as lung function status, by means of forced spirometry, through forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), expressed in terms of z-score of the percentage predictive value for sex and height, according to the standard equations of the Global Lung Initiative.26
Severity of exocrine pancreatic insufficiency was indirectly estimated using daily pancreatic enzyme requirements (adjusted per kilogram of body weight) at the time of the study, and clinical course in the year prior to the study was analysed (number of admissions and disease exacerbations, defined as any respiratory infection that would have required a change in the patient’s usual treatment). Micro-organisms isolated in sputum samples collected in the year prior to the study were recorded as well.
All patients underwent OGTT after 12 h of fasting with determination of blood glucose and blood insulin levels (1.75 g/kg; maximum 75 g). Based on the results, patients were divided into the following groups: normal glucose tolerance (NGT), impaired fasting glycaemia (IFG), carbohydrate intolerance (CI) and CFRD, based on blood glucose levels at baseline and after 120 min, according to the ADA-established criteria.4 In addition, a diagnosis of indeterminate glycaemia (INDET) was made in patients in whom, in the absence of another diagnostic category, had exhibited blood glucose levels in OGTT greater than or equal to 200 mg/dl after 30 or 60 min.4
The inclusion of a new, recently proposed group consisting of patients with CF with blood glucose abnormalities, characterised by blood glucose spikes in OGTT greater than 140 mg/dl, but less than 200 mg/dl, called abnormal glucose tolerance 140 (AGT140), was also considered.27
The area under the curve (AUC) of blood glucose and blood insulin levels in OGTT were calculated according to this formula: AUC = 0.25× (baseline value) + 0.5× (value after 30 min) + 0.75× (value after 60 min) + 0.5× (value after 120 min).
The blood sample taken in the baseline (fasting) blood draw was tested for HbA1c levels, as well as other metabolic parameters (fat-soluble vitamins, markers of inflammation, lipid panel, total protein and albumin).
Patients who presented any abnormalities in OGTT were offered the option of undergoing CSGM (Medtronic iPro2®) for seven days. Sixteen patients agreed to undergo CSGM; of them, 11 (68.75%) had been previously classified as having CI, two (12.5%) as having INDET, one (6.25%) as having IFG and two (12.5%) as having CFRD. In them, mean percentage time spent with blood glucose levels exceeding 144 mg/dl8 and total numbers of daily episodes of blood glucose levels greater than 200 mg/dl were assessed.
The study protocol was approved by the Independent Ethics Committee of Hospital Infantil Universitario Niño Jesús [Niño Jesús University Children’s Hospital] in Madrid and conducted in accordance with the “Ethical Principles for Medical Research Involving Human Subjects” adopted in the Declaration of Helsinki by the World Medical Association (WMA) (64th WMA General Assembly, Fortaleza, Brazil, October 2013) and the Spanish personal data protection law (Organic Law on personal data protection of 5 December 2018). The parents or legal guardians of all patients granted their written informed consent, and patients over 12 years of age granted their informed assent, after the study and the procedures included therein had been fully explained to them.
Statistical analysis was performed on the data using the software program SPSS for Windows, version 23 (IBM Corp., Armonk, NY, United States). The normality of the distribution of the quantitative variables studied was examined using the Kolmogorov–Smirnov test, and comparisons were made between the different diagnostic categories established. When these comparisons dealt with variables with a normal distribution, differences were analysed using Student’s t test when comparing two groups and analysis of variance (ANOVA) when comparing more than two groups. When these comparisons dealt with variables without a normal distribution, differences were analysed using the Mann–Whitney U test when comparing two groups and the Kruskal–Wallis test when comparing more than two groups. Correlation studies were conducted using Pearson's r for variables with a normal distribution and Spearman’s rho for variables without a normal distribution.
ResultsOf the patients, 56% (n = 28) showed no glycaemic abnormalities (NGT). CFRD was diagnosed in 6% (n = 3). Among them, 26% (n = 13) presented CI, 10% (n = 5) presented INDET and a single patient presented IFG (this patient was excluded from intergroup comparisons). Within the group of 28 patients with NGT, 23 of them (82%) showed peak blood glucose levels in OGTT greater than 140 but less than 200 mg/dl before 120 min, rendering them eligible for inclusion in the AGT140 group. Thus, if AGT140 were considered a condition of initial carbohydrate metabolism impairment, just five of the 50 patients in the study cohort (10%) would not fall under any of the proposed disease categories.
None of the patients with CFRD required insulin therapy at diagnosis. Significant differences were found in the overall prevalence of carbohydrate metabolism impairment in relation to the colonising micro-organism, in cases in which it was known (χ2 46.94; p < 0.05), these being present in 100% of patients colonised by methicillin-resistant Staphylococcus aureus (MRSA).
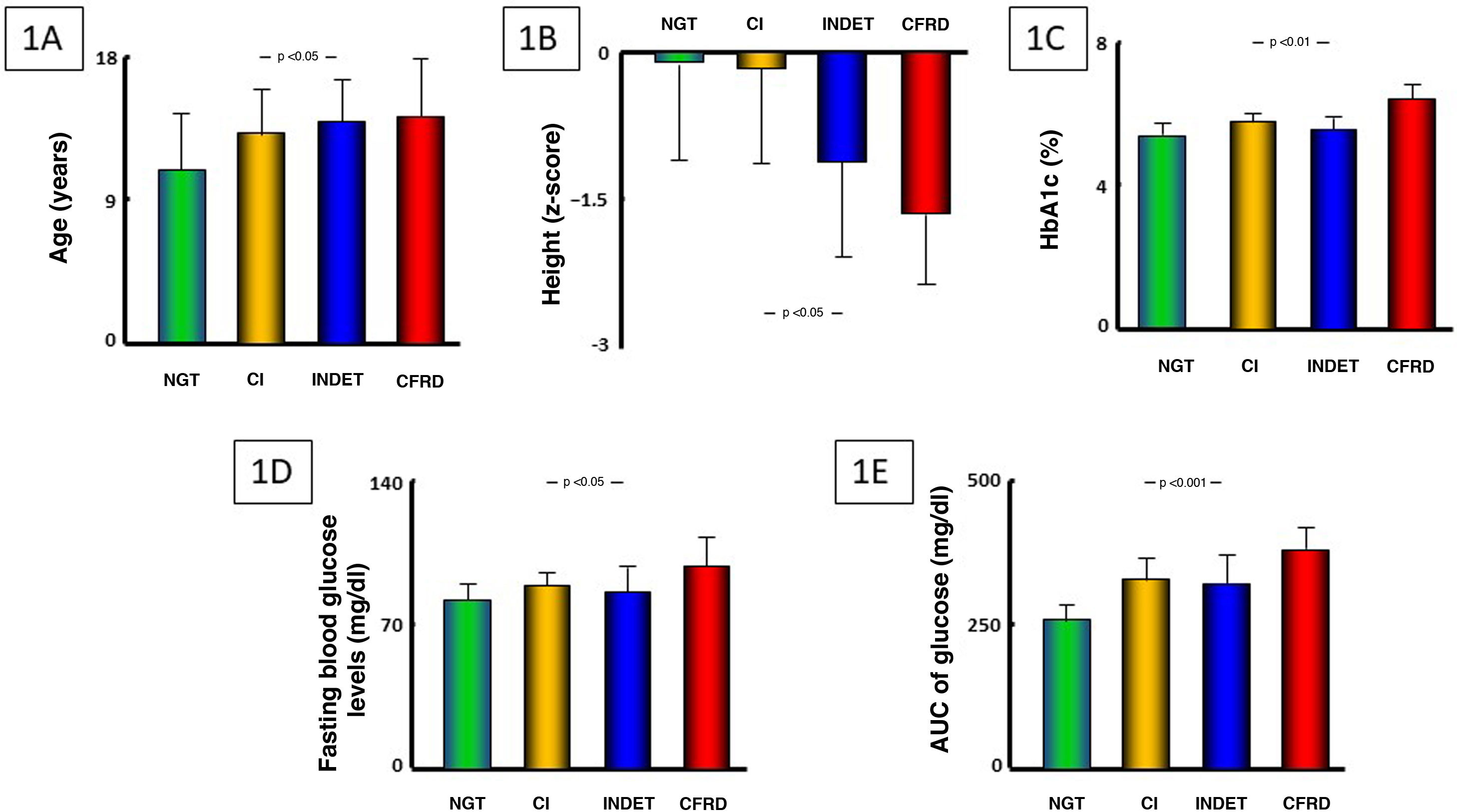
A homozygous ΔF508 mutation was seen in 30% of the patients, a heterozygous ΔF508 mutation in 46% and other combinations of mutations in 24%. No statistically significant differences were found in the prevalence of underlying CFTR mutations between the patient groups established, based on glycaemic abnormalities (NGT/INDET/CI/INDET). Furthermore, no between-group differences were found in terms of FEV1 z-score, FVC z-score (Table 1), numbers of admissions or numbers of exacerbations in the past year.
Comparison of variables studied (mean ± standard deviation) between groups, based on patients’ carbohydrate metabolism status.
| NGT | CI | INDET | CFRD | p | |
|---|---|---|---|---|---|
| Age | 10.9 ± 3.7 | 13.3 ± 3.0 | 14.6 ± 2.6 | 14.9 ± 3.6 | 0.034* |
| Height (SDS) | –0.11 ± 1.00 | –0.16 ± 1.00 | –1.10 ± 1.00 | –1.63 ± 0.70 | 0.047* |
| Growth rate (SDS) | –0.87 ± 2.50 | 1.53 ± 2.64 | 1.77 ± 2.10 | –1.71 ± 2.70 | 0.067 |
| Waterlow height (%) | 98.7 ± 4.9 | 99.1 ± 4.1 | 96.0 ± 3.7 | 92.0 ± 2.7 | 0.079 |
| FEV1 (z-score) | –1.98 ± 1.49 | –1.65 ± 1.02 | –2.05 ± 2.00 | –1.08 ± 1.37 | 0.566 |
| FVC (z-score) | –1.51 ± 1.45 | –0.84 ± 0.97 | –1.02 ± 0.78 | –0.79 ± 0.76 | 0.457 |
| Exacerbation in past year | 3.7 ± 2.7 | 2.7 ± 2.1 | 2.8 ± 2.1 | 2.3 ± 0.9 | 0.930 |
| Pancreatic enzyme dose (103 IU/kg/day) | 3.83 ± 2.69 | 4.16 ± 2.28 | 3.61 ± 2.04 | 4.94 ± 2.79 | 0.369 |
| Ferritin (ng/ml) | 29.1 ± 10.9 | 28.1 ± 11.8 | 43.2 ± 19.0 | 34.0 ± 12.0 | 0.343 |
| CRP (mg/dl) | 0.44 ± 0.30 | 0.40 ± 0.10 | 1.52 ± 2.00 | 0.33 ± 0.23 | 0.673 |
| HbA1c (%) | 5.52 ± 0.30 | 5.80 ± 0.25 | 5.58 ± 0.32 | 6.43 ± 0.37 | 0.002* |
| Baseline blood glucose levels (mg/dl) | 82.15 ± 7.60 | 89.15 ± 5.80 | 85.80 ± 11.60 | 98.67 ± 13.90 | 0.016* |
| Baseline blood insulin levels (µU/ml) | 5.45 ± 2.40 | 8.94 ± 5.60 | 6.60 ± 2.90 | 9.03 ± 1.40 | 0.075 |
| AUC of blood glucose levels | 260.2 ± 31.6 | 328.4 ± 37.9 | 322.5 ± 47.3 | 381.5 ± 35.8 | 0.000* |
| AUC of blood insulin levels | 60.3 ± 28.7 | 100.5 ± 63.6 | 71.1 ± 43.2 | 87.5 ± 26.9 | 0.148 |
AUC: area under the curve; CFRD: cystic fibrosis-related diabetes; CI: carbohydrate intolerance; CRP: C-reactive protein; FEV1: forced expiratory volume in one second expressed in terms of z-score; FVC: forced vital capacity (expressed in terms of z-score); HbA1c: glycosylated haemoglobin; INDET: indeterminate glycaemia; NGT: normal glucose tolerance; p: level of statistical significance; SDS: standard deviation score (z-score); Waterlow height: Waterlow’s classification for height.
An older age and a greater impact on height depending on severity of carbohydrate metabolism impairment were seen (both p < 0.05) (Table 1, Fig. 1A and B); growth rate in the past year approached but did not achieve statistical significance (p = 0.07). No differences were found in terms of sex or growth rate based on stage of pubertal development.
Comparison between groups established, based on carbohydrate metabolism impairment presented by patients (excluding abnormalities in fasting blood glucose levels [n = 1]).
AUC: area under the curve; CFRD: cystic fibrosis-related diabetes; CI: carbohydrate intolerance; INDET: indeterminate glycaemia; NGT: normal glucose tolerance.
Differences were found between groups in HbA1c levels (p < 0.01), fasting blood glucose levels (p < 0.05) and glucose AUC (p < 0.001), but not in baseline blood insulin levels and not in insulin AUC (Table 1 and Fig. 1C–E). In addition, there were direct correlations between HbA1c levels and the AUC of blood glucose levels (r = +0.49; p < 0.001) and blood glucose levels after 120 min in OGTT (r = +0.54; p < 0.001). By contrast, no correlation was seen between, on the one hand, HbA1c levels and, on the other hand, fasting blood glucose or blood insulin levels or the AUC of insulin. In patients with a record of CSGM, mean interstitial glucose (MIG) was 101.1 ± 11.6 mg/dl and estimated HbA1c 5.1% ± 0.4%, with no significant correlation seen between this and capillary HbA1c. However, a direct correlation was indeed seen between mean percentage of time spent with blood glucose levels exceeding 144 mg/dl (which was 4.87% ± 4.36%) and HbA1c levels (r = +0.57; p < 0.05), whereas daily mean numbers of episodes of blood glucose levels >200 mg/dl were extremely low (0.63 ± 0.95).
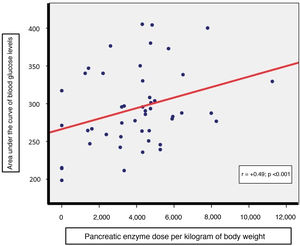
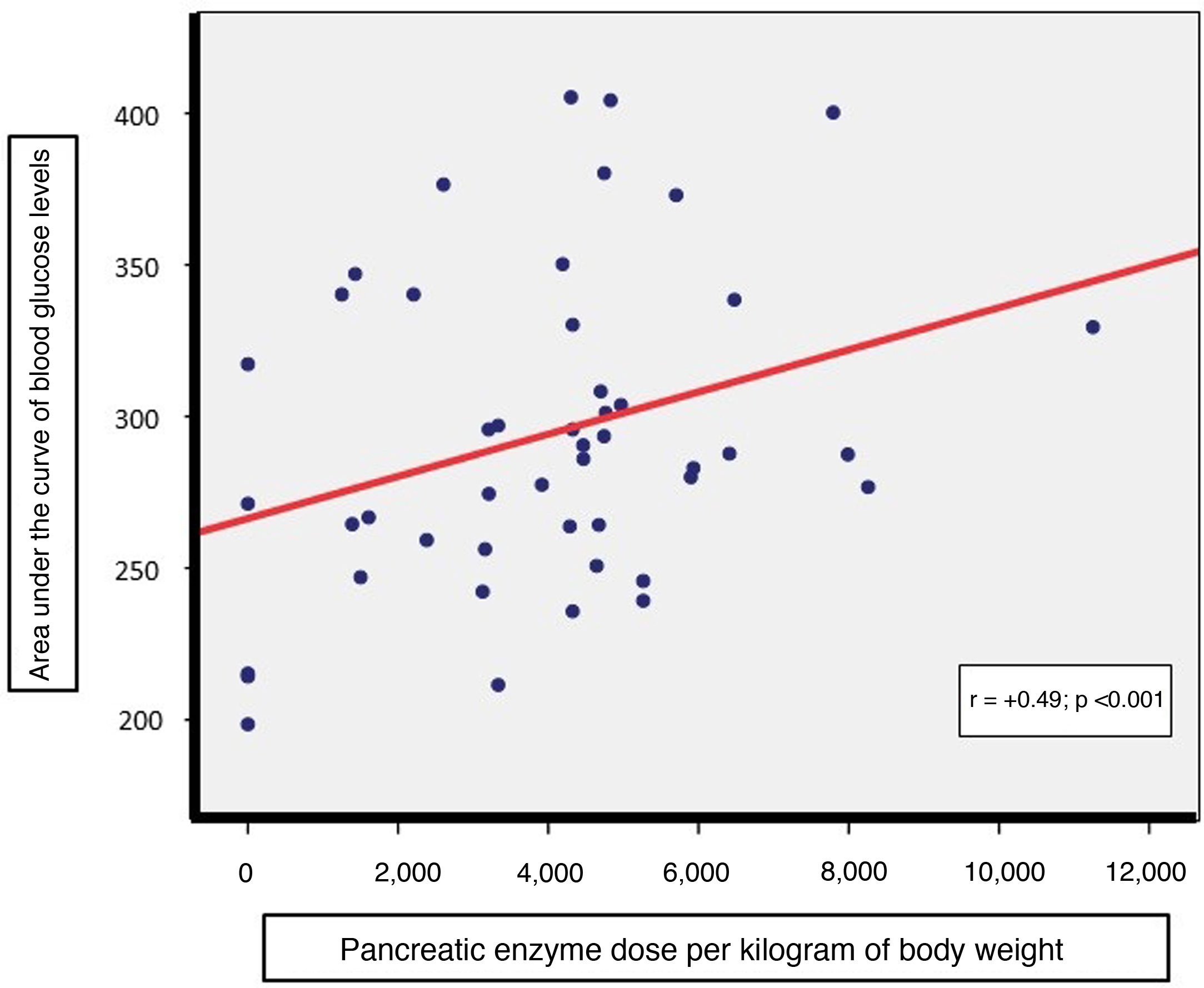
Intergroup comparisons of parameters referring to nutritional status (Waterlow’s classification for weight, fat-soluble vitamins, albumin and lipid panel), markers of inflammation (ferritin and CRP) and total pancreatic enzyme requirements (per kilogram of body weight) showed no significant differences (Table 1). However, a direct correlation was indeed detected between daily pancreatic enzyme dose (per kilogram of body weight) required by patients and the AUC of blood glucose levels (r = +0.49, p < 0.001, Fig. 2); this was not the case with the AUC of blood insulin levels.
DiscussionIn this study, approximately half of paediatric patients with CF (or up to 90% considering the abnormalities defined as AGT14027) had some sort of blood glucose abnormality in OGTT that put blood glucose levels over the limit that causes bronchial secretion, which could promote bacterial colonisation and have an impact on the overall clinical course of the disease, including somatic growth. In addition, an older age and need for enzyme replacement therapy were linked to more severe carbohydrate metabolism impairment. HbA1c proved useful in estimating time in hyperglycaemia, while tests to determine baseline blood glucose and blood insulin levels (as well as their AUCs in OGTT) provided no additional information versus determination of blood glucose levels after 120 min.
Our study confirmed previously reported data concerning the prevalence of blood glucose abnormalities in patients with CF, carbohydrate intolerance being the most commonly found such abnormality, as well as relationships between age, height impairment and severity of carbohydrate metabolism impairment.23,24,28 In our sample, the youngest patient with CFRD was 12.5 years old, but we found patients under 10 years of age with CI (the youngest was 6.58 years old). Consequently, even though both the ADA and the ISPAD recommend starting screening for CFRD at age 10 in patients with CF, these younger children with CI could constitute a risk group for early-onset diabetes, as previously suggested.23,24 A recent meta-analysis found that these stages of prediabetes are not uncommon under 10 years of age, representing a predictive factor for CFRD onset and contributing to a worse prognosis in these patients.29
In addition, if AGT140 were to be considered initial carbohydrate metabolism impairment in CF, though the recency of the proposal of this category has precluded endorsement thereof by associations for the study of diabetes to date, then the percentage of patients with no carbohydrate metabolism impairment would be very limited, even under age 10. However, in our study, the limited numbers of patients in the latter subgroup (five out of 28) barred determination of significant differences between these five patients and the 23 patients with AGT140, and thus also barred speculation as to the potential clinical significance of AGT140.
The direct correlation found between pancreatic enzyme dose per kilogram of body weight and the AUC of blood glucose levels during OGTT backed the notion that there is a relationship between a greater decline in exocrine pancreatic secretion and the onset of endocrine abnormalities.3 Thus, in these patients, evaluation of changes in daily enzyme needs could be useful for suspecting possible worsening of pre-existing blood glucose abnormalities.
The absence of a relationship between severity of blood glucose abnormalities and impairment of respiratory function, determined by FEV1 z-score and FVC z-score values, corroborated the results of prior studies,23,24,28 though the existing data in the literature on this association were not conclusive, as other groups have postulated the existence of this link.14,30 This may be influenced, at least in part, by the limited prevalence of CFRD in our cohort and its lack of need for insulin therapy (initial stage of CFRD with limited metabolism impairment). Similarly, the relationship between the presence of CI or CFRD and the prevalence of underlying CFTR mutation reported by other authors30,31 were not reproduced in this study, though the sample size did limit the potential for determining this association.
Regarding somatic growth, co-occurrence of greater impairment thereof and an older age based on severity of carbohydrate metabolism impairment allows for speculation that disease progression could affect the pubertal growth spurt, leading to worse height z-scores as patients go through puberty. Nevertheless, for this claim to be made, the study would have to have larger groups of patients for a longer period of time.
Blood glucose testing after 30 min and after 60 min during OGTT and calculation of the AUC of blood glucose levels in this study showed no additional advantages over determining blood glucose levels after 120 min, apart from identifying patients with INDET. The (non-significant) trend towards patients with INDET showing more elevated CRP and ferritin levels might suggest that these transient episodes of hyperglycaemia could be influenced by occasional release of insulin due to inflammation or other factors, without there being any real underlying abnormalities in blood glucose levels. Determination of blood insulin levels, both at baseline and during OGTT, and calculation of the AUC of insulin also did not provide additional information. This was consistent with prior findings31 and suggested that these tests should not be advised in regular clinical practice. In addition, determination of fasting blood glucose levels proved uninformative in our cohort; these levels had no correlation with the parameters studied and IFG was rare. This means that they are of doubtful clinical significance, as suggested in other paediatric populations.32
The results of our study suggested that, in patients with CI, CSGM could provide information on the frequency and chronology of episodes of hyperglycaemia. However, the percentage of time with blood glucose levels exceeding 144 mg/dl in these patients was very limited and not correlated with worse respiratory function or a worse clinical stage; hence, it contributed no additional information. Furthermore, no correlation was seen between MIG and HbA1c estimated with real HbA1c, probably influenced by an insufficient duration (seven days) of recording of continuous glucose monitoring and limited numbers of patients who agreed to have the monitor implanted. This was among the study’s limitations. On this basis, although CSGM has been proposed as a more sensitive method than OGTT for detecting risk of progression to CFRD in patients with CI,4 the results of this study precluded speculation as to the potential usefulness thereof as a screening method.
Determination of HbA1c is not considered a sensitive parameter for diagnosing CFRD.4 However, in our study there were significant differences in HbA1c between the different categories of blood glucose abnormalities, as well as direct correlations with the AUC of blood glucose levels, blood glucose levels 120 min after OGTT and the percentage of time spent with blood glucose levels exceeding 144 mg/dl in CSGM. Consequently, HbA1c in patients with CF, even considering the limitations cited in this and in other paediatric diseases with potential carbohydrate metabolism impairment,32 could provide information on the duration and severity of periods of hyperglycaemia over the course of follow-up, as well as point to the possible presence of underlying blood glucose abnormalities, even though it may not be the most sensitive diagnostic parameter of CFRD.
In conclusion, the results of this study suggested that an older age and need for enzyme replacement therapy are linked to greater impairment of glucose metabolism and height in children with CF, with HbA1c being useful in estimating time in hyperglycaemia. Our results did not support testing for blood insulin levels in these patients, as such testing provides no additional information. Blood glucose levels after 120 min in OGTT represent the most efficient determination for screening for glucose metabolism impairment associated with cystic fibrosis, and fasting blood glucose levels are uninformative.
Authors’ contributionsAll of the authors made substantial contributions to the preparation of this manuscript. GAMM and JA developed the study concept and design; JEG, GAMM and AUP were responsible for patient care and data collection; and JEG, AMR, VSS, JA and GAMM performed data analysis and interpretation and drafted the article. All of the authors took part in the critical review of the intellectual content and granted their definitive approval of the version of the study submitted.
FundingThis study did not receive any type of funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank the nursing and support staff of the Pulmonology Unit and Endocrinology Department, especially María José Vergara, for their invaluable assistance with the patients’ diagnostic procedures.






![Comparison between groups established, based on carbohydrate metabolism impairment presented by patients (excluding abnormalities in fasting blood glucose levels [n = 1]). AUC: area under the curve; CFRD: cystic fibrosis-related diabetes; CI: carbohydrate intolerance; INDET: indeterminate glycaemia; NGT: normal glucose tolerance. Comparison between groups established, based on carbohydrate metabolism impairment presented by patients (excluding abnormalities in fasting blood glucose levels [n = 1]). AUC: area under the curve; CFRD: cystic fibrosis-related diabetes; CI: carbohydrate intolerance; INDET: indeterminate glycaemia; NGT: normal glucose tolerance.](https://static.elsevier.es/multimedia/25300180/0000006900000008/v3_202301250732/S2530018022001500/v3_202301250732/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)