Resistance to thyroid hormones (RTH) was first described in 1967 by Refetoff et al. as a genetic syndrome characterized by diminished tissue sensitivity to thyroid hormones.1,2 Most cases of RTH are due to mutations of the thyroid hormone receptor beta (THRβ) gene, which encodes for one of the two types of T3 nuclear receptor, called thyroid receptor β.3
Although the contribution of RTH to the development of differentiated thyroid carcinoma (DTC) is not known, the available evidence suggests that the presence of mutations of the receptor β gene may play a tumorigenic role.4
A 35-year-old woman with a history of migraine was referred to the endocrinology outpatient clinic due to thyroid hormone alterations detected at the Department of Gynecology, which she had visited in her wish to become pregnant. The laboratory tests showed free T4 2.1ng/dl (normal range 0.8–1.8) and TSH 5.85μU/ml (normal range 0.55–4.78). The physical examination revealed grade 2 diffuse goiter, a body weight of 60kg, a body mass index (BMI) within normal limits (23kg/m2), and a heart rate of 94bpm. The patient was asymptomatic. Laboratory testing was repeated, confirming the mentioned thyroid alterations: free T4 1.90ng/dl, free T3 5.02pg/ml (normal range 2.3–4.2) and TSH 4.84μU/ml.
Because of the suspicion of secondary hyperthyroidism versus RTH, pituitary magnetic resonance imaging and thyrotropin-releasing hormone (TRH) testing were requested, with normal findings in both cases. The TSH subunit α/TSH molar ratio was found to be under 1. The above findings led us to suspect that the patient had RTH. A genetic study was requested, with confirmation of the mentioned diagnosis. The patient presented the mutation in heterozygosis p.Arg438his in exon 10 of the THRα gene. In view of these findings, the patient was again questioned about her family history, and laboratory and genetic tests were recommended for her first degree relatives (who do not live in Spain).
In addition, due to the presence of diffuse goiter, thyroid ultrasound was performed, revealing an enlarged thyroid gland containing a solid nodule in the lower third of the right thyroid lobe with punctate calcifications measuring 12mm in maximum diameter. Because of the suspicious ultrasound characteristics, a cytological study was made, the results being consistent with papillary thyroid carcinoma.
The patient underwent a total thyroidectomy (initial lobectomy was proposed but rejected), with a histopathological diagnosis of classical well-differentiated thyroid papillary carcinoma (pT1b), with disease-free surgical margins. The rest of the gland exhibited a chronic nodular, hyperplastic T lymphocytic pattern.
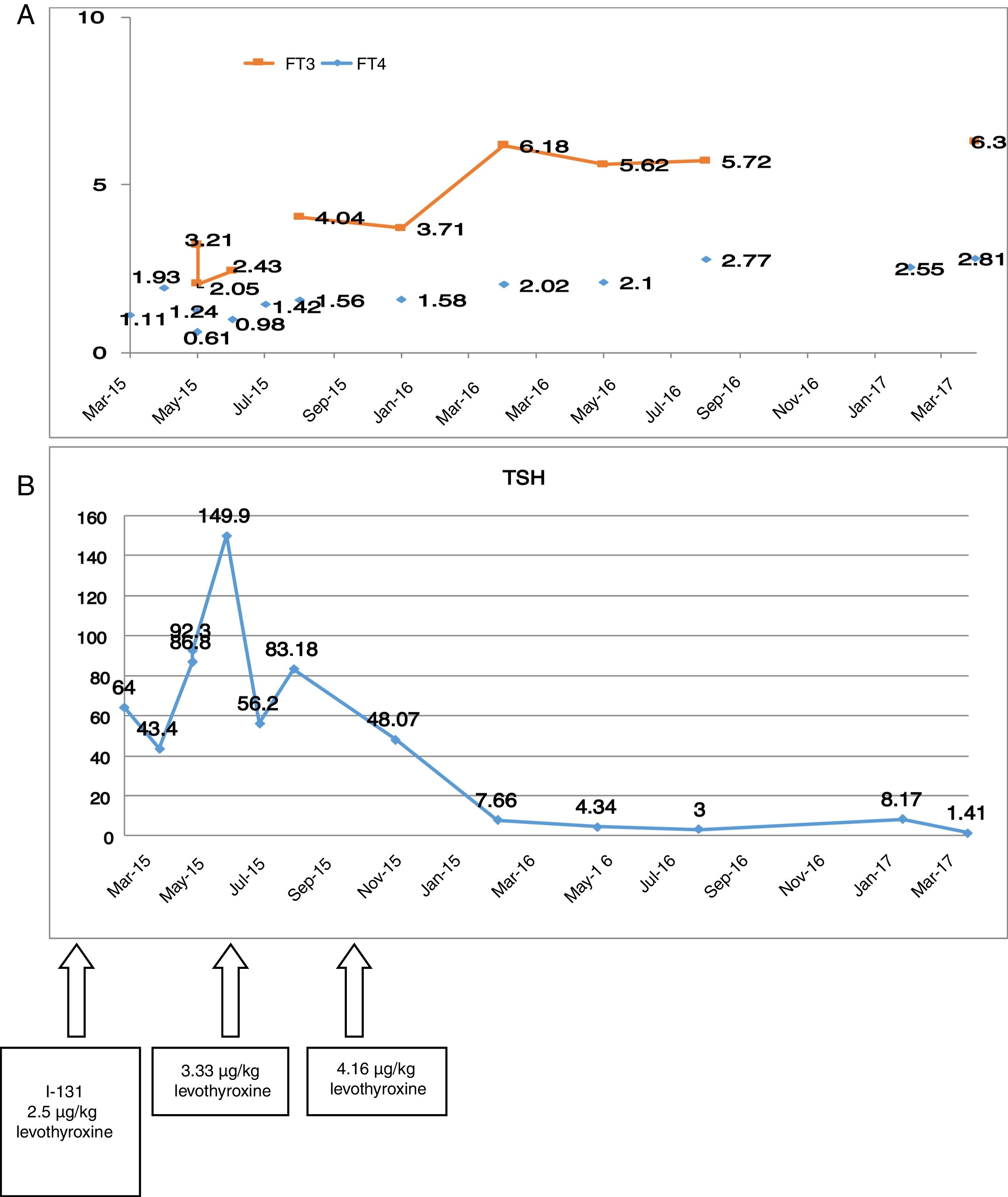
Preoperative thyroid function tests showed the following: TSH 3.4μU/ml, free T4 1.86ng/dl and free T3 7.15pg/ml. After surgery, the patient was discharged with 100μg of levothyroxine (1.6μg/kg). Although this was a low risk papillary carcinoma, we decided to administer an ablative dose of I-131 two months after surgery (50mCi), following the suspension of levothyroxine. After admission, the levothyroxine dose was increased to 2.5μg/kg. Fig. 1A and B shows the difficulty of controlling the TSH levels after surgery and, the evolution of the free T3 and free T4 levels.
At the last control visit, the DTC showed an excellent response to treatment. In relation to RTH, the patient remained clinically asymptomatic, with the administration of 4.16μg/kg body weight of levothyroxine daily.
Resistance to thyroid hormones involves an altered tissue response to thyroid hormones. It is observed in one out of every 40,000 births, affecting both sexes equally, and exhibits an autosomal dominant hereditary pattern.5 Eighty-five percent of all cases are due to mutations of the THRβgene, while less than 1% are attributable to mutations of the THRαgene.6 The main features that should cause us to suspect the syndrome are elevated T4 and usually also elevated T3 concentrations, with normal or moderately elevated TSH levels, accompanied by goiter (affecting 2 out of every 3 patients). The TSH response to TRH is normal or incremented, and the final diagnosis is confirmed by THRβ gene sequencing.
Several cases of RTH in patients with DTC have been reported in recent years. Although the contribution of RTH to the development of thyroid carcinoma is not known, the presence of diffuse goiter and elevated TSH levels may contribute to nodule growth and the development of DTC. Mutations of the THRβ gene cause an increase in TSH, which in turn elevates adenylate cyclase activity, incrementing cAMP production and promoting cell growth. Point somatic mutations have been reported in different areas of the receptor β gene in patients with liver, kidney, breast and colon carcinoma.4 A study published by Puzianowska et al. identified the presence of mutations of the THRβ gene in 93.8% of patients with papillary thyroid carcinoma, and of THRα gene mutations in 62.5%. The mutation was not found in healthy individuals, but was present in 11–22% of the patients with adenomas. The authors concluded that these findings suggest that mutations of the thyroid hormone receptor gene could contribute to the development of papillary thyroid carcinoma.7
With regard to the management of RTH syndrome, in most patients with receptor β defects high circulating thyroid hormone levels compensate for the receptor defects and the patient is clinically under euthyroid conditions. No treatment is therefore required. Triiodothyroacetic acid (TRIAC), a thyroid hormone analog, has been used in some cases to suppress TSH without increasing the thyromimetic effect in peripheral tissues due to its two properties: greater binding to beta than to alpha receptors (less action upon the cardiovascular system), and greater degradation.8,9
However, the aforementioned compensatory mechanism fails if the thyroid reserves are depleted, as in our patient who underwent total thyroidectomy. In these cases, treatment with supraphysiological levothyroxine doses ranging from 500 to 1000μg/day is advocated. If tachycardia develops, it can be treated with beta-blockers.
It has been reported that the combination of levothyroxine and TRIAC may be effective in patients undergoing total thyroidectomy and presenting thyroid cancer.10
Lastly, it should be noted that in patients seeking to become pregnant, genotyping of the fetus with fetal DNA from the chorionic villi or amniocentesis is recommended in order to assess mutation of the thyroid hormone receptor gene. If the fetus is found to be affected, no treatment during pregnancy is required. By contrast, if the fetus is not affected, it is advisable for the mother not to present free T4 levels more than 20% above the upper limit of normal in order to prevent fetal loss and hyperthyroidism during pregnancy.11
Please cite this article as: Guijarro de Armas MG, Pérez Blanco C, Carrasco Lara P, Merino Viveros M, Pavón de Paz I. Coexistencia de síndrome de resistencia a hormonas tiroideas y cáncer diferenciado de tiroides. Endocrinol Diabetes Nutr. 2018;65:474–476.