Disease-related malnutrition (DRM) continues to be a very significant healthcare problem, both in our hospitals and in the community. It is often not properly diagnosed or treated, despite a growing body of evidence highlighting its clinical and economic consequences. The transition between clinical care in the hospital and community services (Primary Care (PC) and Nursing Homes) is a key element in the prevention, detection and treatment of DRM. In October 2020, the Spanish Society of Endocrinology and Nutrition (SEEN) and the main societies of PC physicians in our country (SEMERGEN, SEMFYC and SEMG) met for the first time within the virtual NutriSEEN forum. From that moment on, a joint working group was created for this issue. This document tries to establish joint lines of work between the Clinical Nutrition and Dietetic Units (UNCyD) and the Primary Care teams to improve the detection and treatment of DRM. The clinical consequences and costs associated with DRE, nutritional risk screening, assessment and medical nutritional treatment are considered in a coordinated way between the PC teams and the UNCyD, as well as future proposals to improve the management of DRM.
La desnutrición relacionada con la enfermedad (DRE) sigue siendo un problema asistencial muy significativo, tanto en nuestros hospitales como en la comunidad. A menudo, no se diagnostica ni trata adecuadamente, a pesar de un creciente número de pruebas que ponen en evidencia sus consecuencias clínicas y económicas. La transición entre la atención clínica en el hospital y los servicios comunitarios (Atención Primaria (AP) y Residencias Sociosanitarias) es un elemento clave en la prevención, detección y tratamiento de la DRE. En octubre de 2020, la Sociedad Española de Endocrinología y Nutrición (SEEN) y las principales sociedades de médicos de AP de nuestro país (SEMERGEN, SEMFYC y SEMG) se reunieron por primera vez en el seno del NutriSEEN fórum virtual. A partir de ese momento, se creó un grupo de trabajo conjunto para este tema. El presente documento trata de establecer líneas de trabajo conjuntas entre las Unidades de Nutrición Clínica y Dietética (UNCyD) y los equipos de Atención Primaria para mejorar la detección y el tratamiento de la DRE. Se plantean las consecuencias clínicas y costes asociados a la DRE, el cribado de riesgo nutricional, la valoración y el tratamiento médico nutricional de forma coordinada entre los equipos de Atención Primaria y las UNCyD, así como propuestas de futuro para mejorar el manejo de la DRE.
Disease-related malnutrition (DRM) continues to be a highly significant healthcare problem, both in our hospitals as well as in the community. It is often underdiagnosed and poorly treated, despite a growing body of evidence showing its clinical and economic consequences. In Spain, the leading cause of malnutrition is disease. Many acute and chronic diseases and their treatments can affect nutritional status through various mechanisms involving appetite, nutrient absorption and assimilation, and different metabolic changes. The aetiology and prevalence of DRM have been extensively characterised in several clinical practice guidelines issued by the European Society for Clinical Nutrition and Metabolism (ESPEN).1–3 In addition to purely clinical factors, educational and socioeconomic factors such as poverty, loneliness, or estrangement can also contribute to the onset of DRM.
The transition between hospital clinical care and community services (primary care [PC] and care homes) is key to the prevention, detection and treatment of DRM. Several studies have highlighted the importance of continuity in nutrition therapy and care following hospital discharge, supporting the need to design joint action protocols and establish a coordination channel between clinical nutrition and diet therapy units and primary care that is flexible and adapted to the health and social circumstances.4 Some Spanish Autonomous Communities, such as Castile and Leon, have developed health and social care guidelines that can act as a benchmark.5 At the same time, the Alianza Más Nutridos [More Nourished Alliance] (www.alianzamasnutridos.es) has also established specific protocols.6
In October 2020, the Spanish Society of Endocrinology and Nutrition (SEEN) and Spain's leading societies of primary care doctors (SEMERGEN, SEMFYC and SEMG) met for the first time at the NutriSEEN virtual forum (www.seen.es/portal/actividad-plataforma-formacion/nutriseen-forum-virtual-2020) to discuss the needs of doctors from both specialisms in the approach to DRM. This meeting also saw the creation of a joint DRM working group. This document aims to establish joint working guidelines for clinical nutrition and diet therapy units and PC teams to improve the detection and treatment of DRM.
Clinical consequences and costs associated with disease-related malnutritionAs a result of malnutrition, patients have more complications, more and longer hospital stays, more readmissions, greater loss of independence and reduced quality of life.7 This increases costs due to more days in hospital, more complementary tests, the need for artificial nutritional support and/or medicines to treat complications, or more visits to health centres and/or emergency departments.8,9 The PREDyCES multicentre observational study10 found that 23% of patients on admission and 23.4% at discharge suffered from DRM. The mean length of hospital stay of malnourished patients was 11.5 ± 7.5 days vs 8.5 ± 5.8 days (p < 0.001) in the controls, and the cost of care was €8,590 ± €6,127 vs €7,085 ± €5,625 (p < 0.015). Extrapolation of these results to the Spanish National Health System as a whole showed that the estimated cost of hospital malnutrition in Spain was at least, 1,143 million euros per year.11
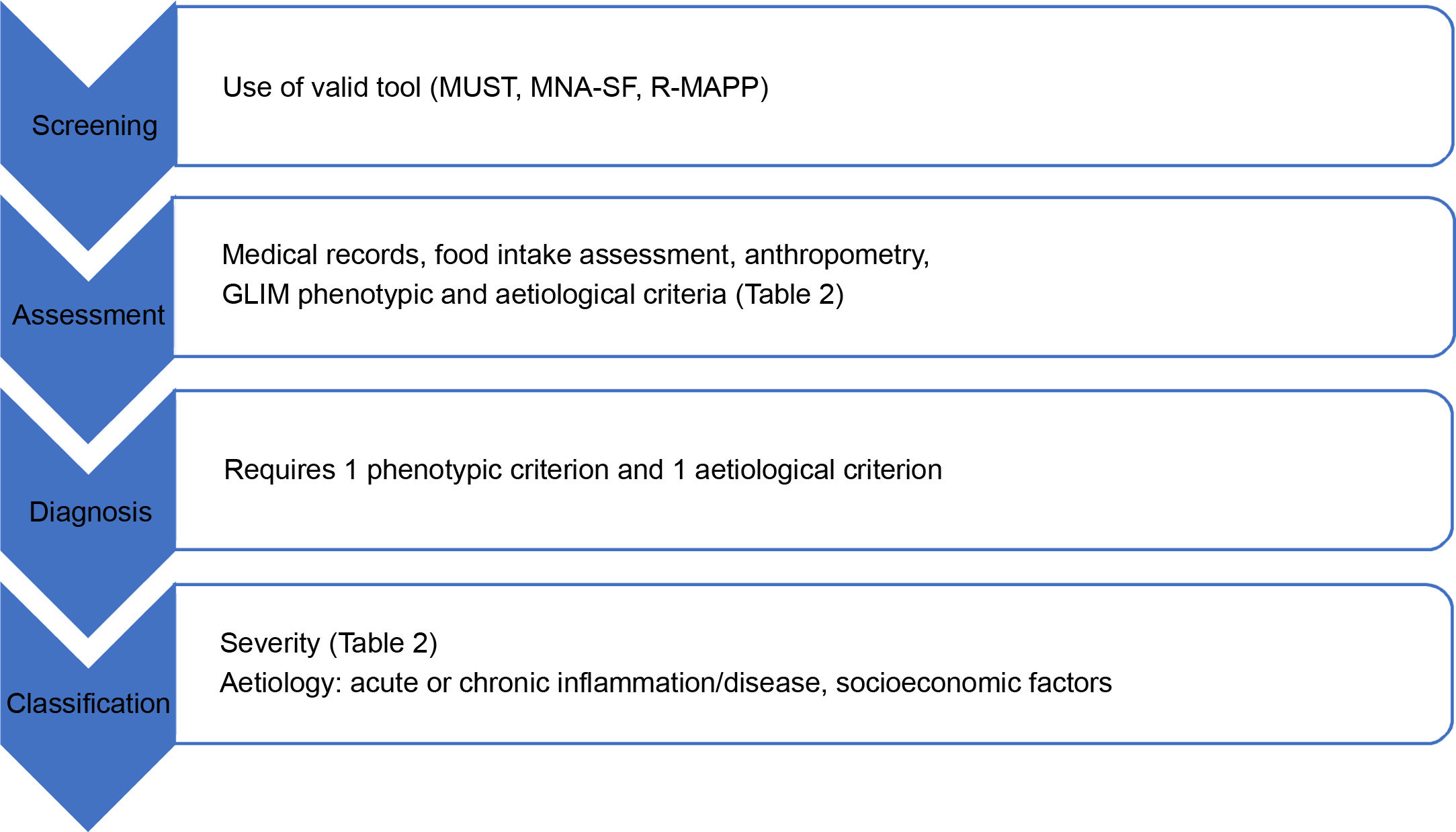
Nutritional risk screening, when and how?DRM is a condition that meets all the screening recommendation requirements: its prevalence is significant (particularly in older patients or people with chronic diseases), there are tools to detect it (and here, the role of nursing staff is critical), and early treatment can be administered to improve patient prognosis.12 Nutritional screening is a standardised procedure that can identify malnourished or at-risk individuals who could benefit from appropriate nutritional therapy. This is particularly relevant in older people, chronically ill patients, patients with multiple diseases, institutionalised patients or people with diseases of special nutritional risk, such as oncological, gastrointestinal, renal, infectious or cardiorespiratory diseases. It is important to remember that DRM is more common in older adults, but it is also underdiagnosed in complex chronic patients due to its multifactorial origin and insidious clinical course. PC can identify certain patient aspects that are vital for its diagnosis, such as the risk factors listed in Table 1.
Malnutrition risk factors (table prepared by the authors).
| Disease-related factors | Acute diseases or exacerbation of chronic diseases, multiple concomitant conditions, polypharmacy, frailty, cognitive impairment, depression, poor dental condition |
| Social factors | Institutionalisation, hospitalisation, isolation, loneliness, being a widow/widower, low cultural or economic status |
| Functional factors | Loss of autonomy in basic activities (eating) and instrumental activities (shopping, cooking), sensory alterations (taste and smell) |
| Dietary factors | Chronic alcoholism, poor diet, poor availability of, or access to, food |
If well thought out, the DRM approach can be extremely beneficial without requiring huge investments in terms of time or material resources. As such, it should be included in the prevention and follow-up programmes of chronic patients with onset of DRM in primary care. A nutritional risk screening method should ideally be included in the electronic medical record in all these high-risk clinical scenarios. Screening methods should be valid, reliable, reproducible, practical and associated with specific action protocols. The nutritional screening guidelines issued by the European Society for Clinical Nutrition and Metabolism (ESPEN)13 stipulate that every nutritional screening tool requires formal and evidence-based validation and should include three elements on nutritional status: current body mass index (BMI), recent unintentional weight loss and information about recent dietary intake. The nutritional screening methods proposed by the ESPEN and the American Society for Parenteral and Enteral Nutrition include Nutritional Risk Screening 2002, Malnutrition Universal Screening Tool (MUST), Mini Nutritional Assessment Short-Form (MNA-SF), Short Nutritional Assessment Questionnaire (SNAQ) and the Malnutrition Screening Tool. The use of a particular method will depend on the study population (adults-elderly) and care level (community-hospital), as well as the preferences and capacity of each site.12,13 ESPEN particularly recommends using the MUST in the community and MNA-SF in older patients,14 so we recommend the preferential use of these methods. They should be administered monthly to institutionalised patients by the PC team or in care homes and at least annually to risk groups and whenever a patient's clinical status changes.
The MUST15,16 was developed by the British Association of Parenteral and Enteral Nutrition, initially for community screening. However, it can also be used for institutionalised patients or in the hospital setting. In the community, it predicts the admission rate and primary care attendance. It includes BMI, weight loss over 3-6 months and the effect of the acute disease on dietary intake over the last five days. A score of 0 is considered a low risk of malnutrition, 1 moderate risk and >2 high risk. It is associated with action protocols and has been translated into Spanish, available at: https://www.bapen.org.uk/images/pdfs/must/spanish/must-toolkit.pdf
The MNA-SF17 was validated as an independent screening tool based on the MNA long form. It has a sensitivity of 85%, a specificity of 84% and a very good correlation with the long form. It consists of five clinical questions about reduced food intake, unintentional weight loss, degree of mobility, presence of stress or acute disease and neuropsychological problems. It adds the last section consisting of BMI assessment or calf circumference when BMI cannot be measured. It classifies patients as malnourished (0–7 points), at risk of malnourishment (8–11 points) or with normal nutritional status (12–14 points). Its results are associated with morbidity and mortality indicators and social function, as well as with the frequency of doctor's visits. The Spanish version can be downloaded from: https://www.mna-elderly.com/forms/mini/mna_mini_spanish.pdf
The COVID-19 pandemic has driven the further expansion of telemedicine, resulting in the creation of a remote nutritional screening tool called R-MAPP18 (https://www.rmappnutrition.com/es), which combines the use of the MUST as a screening method with a questionnaire on sarcopenia risk, SARC-F, to assess loss of muscle mass. Other digital tools like MioApp (https://mioapp.es/) also facilitate nutritional screening and assessment.
Nutritional assessment in primary careThe positive screening of a patient gives rise to the need to conduct a nutritional assessment to determine their nutritional status and establish medical nutrition therapy should the malnutrition diagnosis be confirmed. The nutritional assessment performed by the PC team should include at least the following19–21:
- -
Clinical and medication history. Of special interest are those points that could influence nutritional status or reduced food intake, such as gastrointestinal symptoms, socioeconomic status and intercurrent conditions.
- -
Food intake assessment. This can be performed using visual scales, food intake quartiles, 24-h reminder or prospective dietary record.
- -
Anthropometric evaluation. Height, current weight, usual weight and percentage of unintentional weight loss over the last six months must be recorded. Calf circumference can be a very useful measurement for estimating muscle mass, once oedema has been ruled out.22
- -
Lab tests. A general blood test including albumin and C-reactive protein.
By integrating all the above data, a tentative diagnosis of malnutrition can be made using a tool such as the subjective global assessment23 or MNA.24 The Global Leadership Initiative on Malnutrition recently established criteria for the diagnosis of malnutrition25, which requires at least one phenotypic criterion and one aetiological criterion for confirmation (Table 2). Once confirmed, it is classified according to severity by the phenotypic criterion and according to aetiology26 (Table 2), as can be seen by the process depicted in Fig. 1.
GLIM criteria.25
| Moderate malnutrition | Severe malnutrition | ||
|---|---|---|---|
| Phenotypic criteria | Unintentional weight loss (%) | 5–10% within past 6 months or 10–20% beyond 6 months | >10% within past 6 months or >20% beyond 6 months |
| Low BMI (kg/m2) | <20 if <70 years or <22 if ≥70 years | <18.5 if <70 years or < 20 if ≥70 years | |
| Reduced muscle mass | Mild-moderate deficit | Severe deficit | |
| With validated body composition measuring technique | |||
| Aetiological criteria | Reduced food intake or reduced absorption | ≤50% of energy requirements >1 week | |
| Any food intake reduction >2 weeks | |||
| Any chronic gastrointestinal condition that adversely impacts nutrient assimilation or absorption | |||
| Inflammation | Acute disease or surgery | ||
| Associated with chronic disease | |||
Diagnosis of malnutrition: one phenotypic criterion + one aetiological criterion.
Screening process and nutritional assessment in PC (prepared by the authors).
Adapted from Cederholm et al.25.
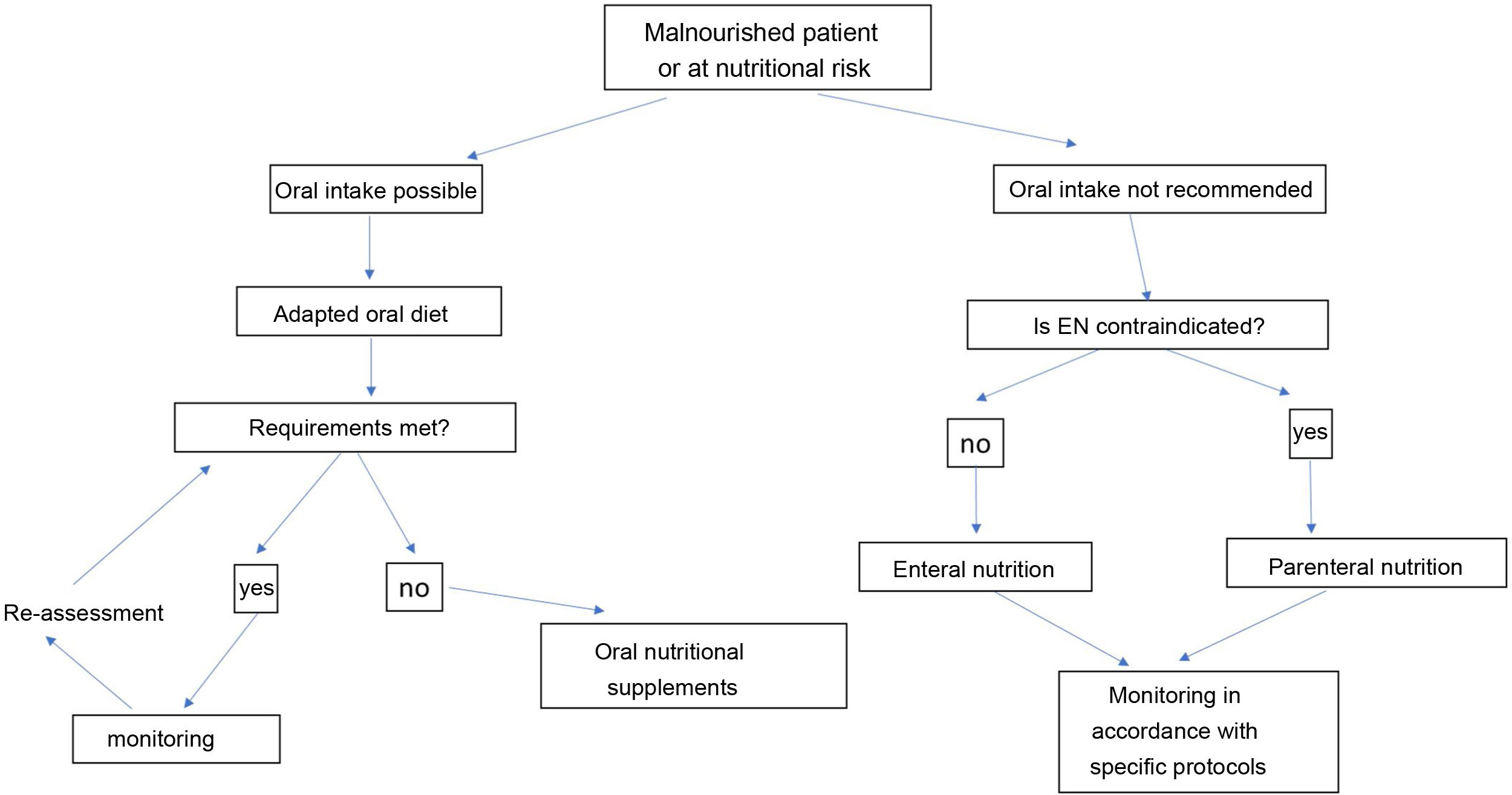
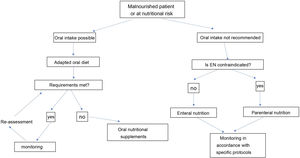
The nutritional therapy of malnourished or at-risk patients includes an increasingly complex strategy of therapeutic measures, which must adapt to the general and specific objectives and the clinical circumstances of each patient (Fig. 2). This algorithm begins with treatment, which includes recommendations based on oral intake. The prescription of diet therapy should be tailored to each individual patient and adapted to the patient’s clinical and personal characteristics, as well as to cultural and socioeconomic factors and patients’ personal preferences. The aims of diet therapy are:
- •
To prevent disease-related malnutrition.
- •
To treat malnutrition.
- •
To help control symptoms.
- •
To have a beneficial effect on the course of the disease.
Most clinical nutrition and diet therapy units boast dietary recommendations adapted to the most relevant diseases. However, accessibility to this content is not always equal across the different care levels. Moreover, people often seek information about their disease and diet therapy from reliable sources such as scientific societies, patient associations or official bodies or indiscriminately online. Table 3 details the main recommendations for correct diet therapy prescribing. Additional information can be found on the SEEN website (www.seen.es).
Dietary prescription: main general and specific recommendations (table prepared by the authors).
| General recommendations for a dietary prescription |
| Conduct a detailed assessment of the patient’s nutritional status and food intake |
| Identify the diet therapy, adapting the general and specific recommendations to the characteristics and underlying disease of each patient |
| Assess the patient's understanding and acceptance of the proposed dietary measures |
| Establish a plan to monitor diet therapy compliance and effectiveness |
| Dietary enhancement |
| Use more fatty cooking methods. Add oil, cream, mayonnaise, melted or grated cheese |
| Add grains or instant mash potato powder |
| Include pulse purées, fruit purées, dairy desserts |
| Use unfunded nutritional modules and supplements |
| Increase protein intake: powdered milk, egg white, ground almonds |
| Tips to adapt diet to symptoms and other clinical conditions |
| Dysphagia |
| Avoid double textures (e.g. solid and liquid together, such as soups), fibrous, hard foods (bread, whole nuts), sticky foods (sliced bread, croquettes), and foods that disintegrate or crumble (rice, biscuits) |
| Use thickeners in water and liquid foods |
| Nausea and vomiting |
| Avoid fatty foods, caffeine, very cold or very hot foods |
| Spread your daily intake over several small portions, include rest |
| Diarrhoea |
| Ensure adequate hydration |
| Avoid fatty foods, vegetables, whole pulses, fruit with skin, milk containing lactose |
| Constipation |
| Ensure adequate hydration |
| Increase intake of fibre-rich foods |
For a patient with DRM, the type and characteristics of nutritional support will depend on:
- •
Prior malnutrition and its degree.
- •
Clinical status and comorbidities.
- •
The estimated time to recover oral intake.
- •
Whether hospitalised or in community care.
When patients cannot meet their nutritional goals by oral intake alone, we recommend prescribing medical nutrition therapy following the algorithm proposed in Fig. 2. This begins by deciding whether the patient can swallow safely and effectively, which will not be possible in severe oropharyngeal dysphagia, mucositis or anatomical or functional pharyngoesophageal abnormality. Several formulas of different nutritional compositions are adapted to different clinical situations for oral and tube feeding or ostomy.
Oral nutritional supplementsOral nutritional supplements (ONS) (unfortunately and quite incorrectly called "shakes") are a medical treatment proven to reduce mortality, length of hospital stay, readmission rate, and process costs27,28 in patients with DRM. Their effectiveness is currently supported by several clinical trials with a high level of evidence and high strength of recommendation in the clinical practice guidelines.29–31 Improved functionality in activities of daily living, quality of life and muscle strength support the use of ONS beyond the costs to fund it. However, profound inequalities in nutritional therapy prescription and funding models persist in Spain, deriving from a series of regulations governing the prescription of dietary products across the country (Table 4).
Basic regulations governing the prescribing of food for special medical purposes (FSMP) in the Spanish National Health System (table prepared by the authors).
| Spanish Royal Decree 1030/2006 of 15 September, establishing the Spanish National Health System’s Portfolio of special services |
| Spanish Royal Decree 1205/2010 of 24 September, establishing the foundations for the inclusion of food for special medical purposes in the portfolio of the Spanish National Health System and the establishment of its maximum funding amounts |
| Spanish Royal Decree-Law 16/2012 of 20 April, on urgent measures to guarantee the sustainability of the Spanish National Health System and improve the quality and safety of its portfolio |
| Order SSI/1640/2012 of 18 July, modifying Annex vi of Spanish Royal Decree 1030/2006, establishing the Spanish National Health System’s Portfolio of special services and Updating procedure |
In principle, these regulations only cover the provision of complete formulas, including special complete formulas suitable for metabolic disorders and the requirements of a specific disease, formulas for allergies or intolerance, nutritional modules for metabolic disorders and the preparation of modular home enteral nutrition (HEN) diets and dietary products for congenital metabolic disorders. This prescription must be authorised (endorsed) by the pharmacy inspection of the health area where the patient resides. This requirement must be met before withdrawing the product from the pharmacy, apart from two autonomouscommunities of Spain (Catalonia and Galicia), where dispensing in the outpatient setting is controlled by the hospital pharmacy departments.
Both models are frequently marred by problems of inequity in dispensing authorisation when faced with the same clinical situation in two different health areas or two differentautonomous communities. This issue is exacerbated by the almost complete failure to update the lists of diseases for which the Spanish National Health System funds this nutritional therapy and the different interpretations of pharmacy inspectors when facing clinical scenarios not accounted for by clearly outdated regulations. This situation has given rise to certain flexibility, including through meetings and consensus statements between regional scientific societies and pharmacy inspectors, with adaptation to daily clinical practice in some autonomouscommunities but not in others. In light of the recommendations by scientific societies,1–3,32,33 in most cases based on high levels of scientific evidence, the lack of assessment, diagnosis and treatment plan for DRM as a fundamental pillar to guide nutritional therapy seems paradoxical.25
Enteral nutritionEnteral nutrition is the administration of certain nutrients through the digestive tract. This technique is more physiological, simpler, cheaper and carries a lower risk of complications than parenteral nutrition. It also preserves the integrity of the intestinal mucosa due to its trophic effect, preventing atrophy of the intestinal villi and maintaining the barrier effect of the immune system.34 Enteral nutrition requires the digestive system to be functioning to such a degree as to be able to receive, digest and absorb nutrients. It may be non-invasive (through nasogastric or nasoenteric, duodenal or jejunal tubes) or invasive by ostomy (gastrostomy or jejunostomy). Deciding whether a patient requires long-term ostomy will depend on their clinical status and underlying disease, as well as estimating that they will require this support for at least 4-6 weeks. Home enteral nutrition is possible, and patients and carers must be adequately informed and educated. In this regard, resources from the SEEN virtual classroom for patients and carers (https://www.seen.es/portal/aula-virtual/nutricion-enteral-domiciliaria-ned) may be helpful.
Parenteral nutritionWhen oral nutrition is not possible or is insufficient, enteral nutrition should first be considered. However, this is contraindicated in cases of complete obstruction of the small or large intestine, paralytic ileus, gastrointestinal perforation with peritonitis, severe malabsorption, severe acute gastrointestinal bleeding or nonsurgical gastrointestinal ischaemia. In these cases, parenteral nutrition should be administered. Long-term home parenteral nutrition can be considered in patients with chronic intestinal failure (short-bowel syndrome, unsolvable intestinal obstruction or pseudo-obstruction) with a stable clinical status and who have completed an advanced training programme at a clinical nutrition and diet therapy unit. For this form of nutritional therapy, the resources of the SEEN virtual classroom (https://www.seen.es/portal/aula-virtual/nutricion-parenteral-domiciliaria-npd) and websites such as https://fallointestinal.com/ may also be useful to patients and carers.
Assessment criteria at the nutrition consultationPatients with a positive nutritional risk screening outcome who could benefit from medical nutrition therapy should be referred to a clinical nutrition and diet therapy unit (Table 5). Once the appropriate treatment with dietary counselling has been prescribed, patients should be reassessed after four weeks to confirm treatment efficacy by means of clinical parameters, such as weight or calf circumference, or blood tests. Assessment by a clinical nutrition and diet therapy unit is recommended if there is no improvement.19
Situations that require a clinical nutrition consultation (table prepared by the authors)
| Malnourished patients who do not meet nutritional requirements with dietary recommendations and require medical nutrition therapy |
| Malnourished patients with symptoms not conducive to dietary therapy (vomiting, diarrhoea, abdominal pain, etc.) |
| Patient's with impaired oral intake (e.g. dysphagia) |
| Malnourished patients with severe or complex chronic diseases requiring a specific nutritional approach (e.g. kidney disease, cirrhosis, multiple concomitant conditions, etc.) |
| Patients requiring multidisciplinary clinical management and follow-up (e.g. anorexia nervosa, neoplastic disease, etc.) |
| Patients with severe malnutrition or at risk of refeeding syndrome |
| Patients with dietary deficiency refractory to oral and intramuscular treatment |
When patients with DRM are referred from primary care to a clinical nutrition and diet therapy unit for assessment and follow-up, they should provide sufficient clinical information to facilitate clinical care and avoid duplicating tests.
- •
Ideally, medical records and complementary tests (blood tests, imaging tests) should be accessible to different healthcare settings.
- •
To facilitate coordinated care, clinical nutrition consultation referral protocols should be established and adapted to the modalities of each centre (e-consultation, synchronous consultation, etc.).
- •
Patients and their families should have the necessary information to understand the disease and care process and correctly cooperate throughout follow-up.
- •
Essential clinical data that are also necessary for screening should be provided, such as weight, height, BMI and the percentage of unintentional weight loss, as well as data on suspected dysphagia.
It is essential to be clear about the aim of nutritional therapy and if dietary recommendations or changes have already been provided as the first step of nutritional therapy, as well as if there is any related action protocol and if follow-up will be shared (Table 6).
Considerations for referral to a clinical nutrition consultation (checklist for referral) (table prepared by the authors)
| Is nutritional screening positive? (MUST, MNA-SF, R-MAPP) |
| Are height, current weight and usual weight known? |
| Does the patient cough when swallowing liquids? Is dysphagia suspected? |
| Suspicion based on medical history or prior episodes |
| EAT-10 screening35 |
| Is there a specific nutritional therapy goal? |
| Has the patient received basic dietary recommendations? |
| Is there a related action protocol? |
| Pharmacological treatment review and optimisation: Rule out that symptoms are caused by adverse effects |
| Reason for referral |
| Need for re-assessment |
| Inadequate treatment |
| Lack of protocol |
The use of e-consultations, defined as an asynchronous non-face-to-face written interconsultation between professionals from different healthcare fields with an integrated medical records system, improves accessibility and facilitates administrative procedures. Many patients could initially be assessed by e-consultation if available (Table 7, modified by Gorgojo et al.36) as long as essential clinical data are provided, which are also required for referral to a face-to-face appointment (Table 6).
Patient referrals to nutrition consultations (table prepared by the authors).
| e-consultation | Face-to-face |
|---|---|
| If patient has: | If patient has: |
| Lab test abnormalities | Been rejected for remote consultation |
| Stable chronic disease | Sensory perception limitations |
| Difficulty coming to the medical centre | Complexity requiring physical examination |
| Recent hospital discharge | Instability with clinical deterioration |
| If patient requires: | If patient requires: |
| Extension of treatment | Treatment in consultation or day hospital |
| Consultations about test results | Functional or body composition assessment |
| Multidisciplinary consultations (e.g. ALS) | |
| Diagnostic tests (e.g. dysphagia test) | |
| Instrumental techniques (e.g. tube/catheter placement) |
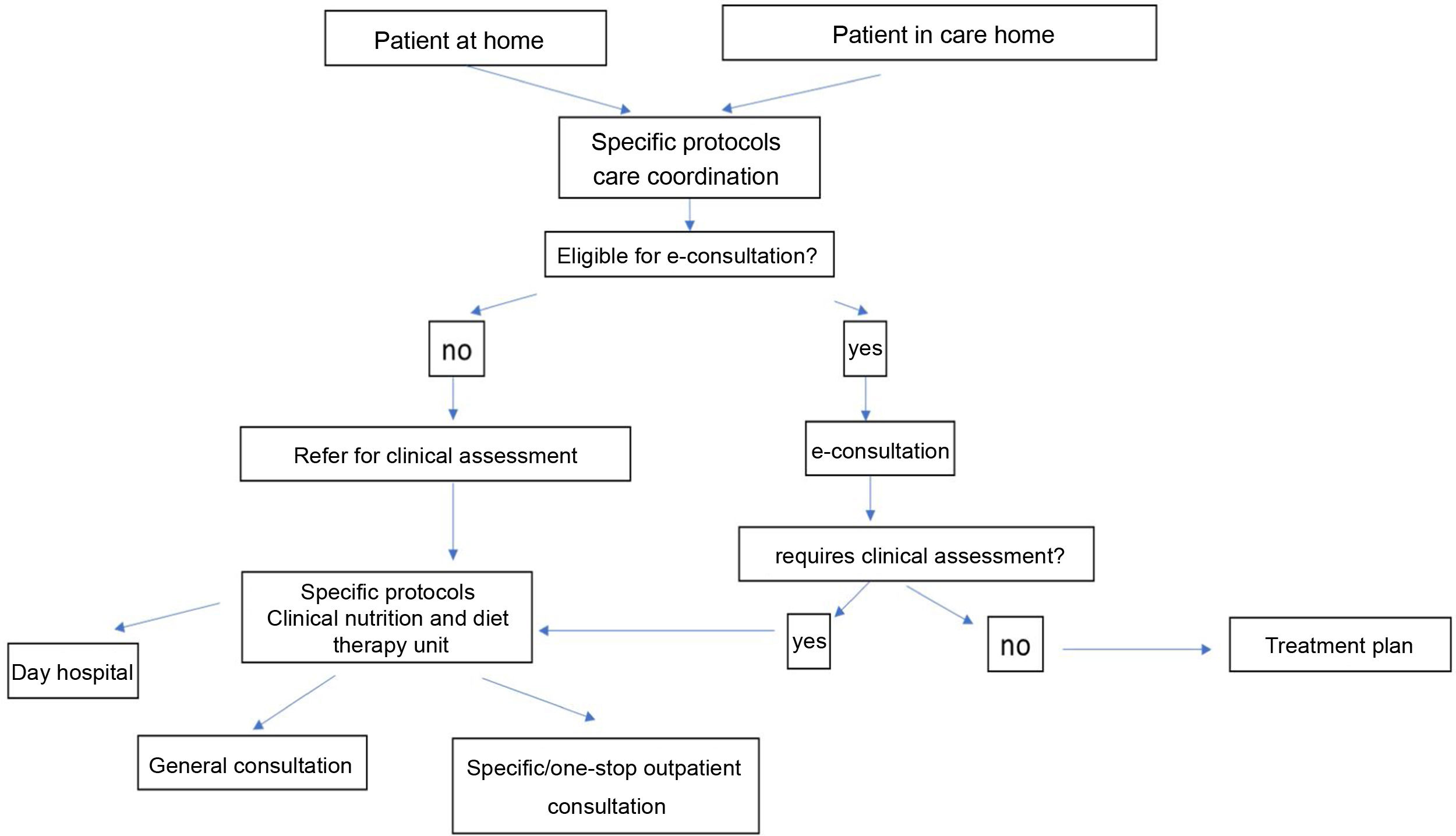
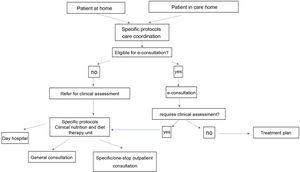
Fig. 3 shows an algorithm for coordinated care between PC, care homes and clinical nutrition and diet therapy units. Patient referral should, whenever possible, follow specific care protocols to promote communication between different healthcare levels. To facilitate care and reduce response time, this algorithm proposes using e-consultation in the first instance. E-consultation will enable those patients requiring a face-to-face clinical assessment to be identified in accordance with specific protocols or, in some instances, will allow the treatment plan to be directly modified or updated. Patients referred for clinical assessment could be evaluated, following specific care protocols, in a specialist consultation (ALS, eating disorders, etc.), in a general clinical nutrition consultation, or be seen at a day hospital (e.g., problems with the gastrostomy).
Algorithm for care coordination between PC, care homes and clinical nutrition and diet therapy units (as per the criteria listed in Table 9) (prepared by the authors).
Clinical nutrition consultations allow more complex patients to be assessed and treated. Coordination of this clinical activity with that performed in primary care will facilitate shared clinical management. Coordination between the PC team, endocrinology and nutrition specialists and other healthcare professionals involved (nursing and dieticians-nutritionists, to name a few, as listed in the SEEN portfolio of services37) is required to resolve patients' nutritional problems.
Home enteral nutritionTreatment with HEN usually begins in the hospital setting, whether during admission, at a day hospital or during a nutrition consultation. Patients receiving home enteral nutrition usually undergo follow-up in primary care for their underlying disease and other conditions. The follow-up of HEN patients by a clinical nutrition specialist will depend on their clinical characteristics, their need to be treated by a specialist consultation (e.g. ALS, oncology, etc.) or the presence of complications.38 Generally speaking, chronic HEN patients require at least one annual check-up by a clinical nutrition specialist. Some clinical scenarios in which HEN patients followed up in PC should be referred to a clinical nutrition specialist for assessment, following the algorithm shown in Fig. 3, are described below.
- •
Access route-related problems:
- ○
The need for post-pyloric access due to the risk of aspiration.
- ○
Planning long-term access (gastrostomy).
- ○
Gastrostomy problems, replacement required.
- ○
- •
Gastrointestinal complications in enteral nutrition, poor tolerance of enteral nutrition.
- •
Nausea, diarrhoea, abdominal distension.
- •
- •
Metabolic complications or other concomitant diseases.
- •
Diabetes/stress-induced hyperglycaemia, renal failure, hyponatraemia.
- •
- •
Other clinical scenarios:
- •
Dietary deficiency refractory to oral treatment.
- •
Treatment with home parenteral nutrition (HPN) usually begins in the hospital setting, whether in the hospital ward or the day hospital. Given the complexity of this treatment, all patients receiving HPN require follow-up by a clinical nutrition specialist, with more frequent check-ups than for HEN. Coordination between PC and the clinical nutrition and diet therapy unit facilitates better clinical follow-up in these patients. Some clinical scenarios in which patients should be referred from PC to a clinical nutrition and diet therapy unit are described below.
- •
Catheter-related problems that do not require emergency care.
- •
Change in clinical status that requires the nutritional therapy to be modified:
- ◦
Reduced food/fluid intake.
- ◦
Gastrointestinal symptoms, vomiting, diarrhoea, increased ostomy output.
- ◦
Onset or worsening of other diseases, blood test abnormalities:
- ◦
- •
Anaemia.
- •
Hypokalaemia, hyponatraemia, hypomagnesaemia.
- •
Liver function test abnormalities.
- •
Diabetes.
- •
Renal failure.
The discharge information (from both doctors and nurses) of all hospitalised patients with DRM should include (Table 8): diagnosis and severity of malnutrition; whether or not the patient has dysphagia; nutritional therapy administered during hospital stay; progression of patient's nutritional status during hospital stay (weight at discharge, functional status); treatment goals; dietary recommendations at discharge; medical nutrition therapy after discharge; nutritional supplements; enteral nutrition by tube or ostomy; parenteral nutrition; tube/ostomy or catheter care plan; other treatments (micronutrients, antidiarrhoeals, including unfunded medications); the need for clinical check-ups by a clinical nutrition specialist; proper identification of the clinical team responsible for the patient during admission; coordination between hospital care and primary care; and contact details of the hospital contact person.
Considerations at discharge of patients with DRM (checklist for continuity at discharge) (table prepared by the authors).
| Yes | No | |
|---|---|---|
| Does the patient have disease-related malnutrition? | ||
| Does the patient have dysphagia? | ||
| Has the patient received nutritional therapy during admission? | ||
| Modified oral diet | ||
| Oral nutritional supplements | ||
| Enteral nutrition by tube/ostomy | ||
| Parenteral nutrition | ||
| Nutritional status at discharge | ||
| Weight, BMI | ||
| Anthropometry, dynamometry | ||
| Does the patient require nutritional therapy after discharge? | ||
| Modified oral diet | ||
| Oral nutritional supplements | ||
| Enteral nutrition by tube/ostomy | ||
| Parenteral nutrition | ||
| Does the patient require follow-up by a nutrition specialist after discharge? |
The clinical assessment in PC of all patients following hospital discharge must include aspects pertaining to nutritional status. By its very nature, hospitalisation itself gives rise to nutritional risk. The need for nutritional assessment and follow-up at discharge by PC will depend on the clinical status and nutritional risk of each individual patient. Below is a patient categorisation proposal based on nutritional risk and the need for treatment after hospitalisation (Table 9).
Patient categorisation based on nutritional risk and need for treatment after hospitalisation (table prepared by the authors).
| Description | PC follow-up recommendation | Need for joint follow-up with clinical nutrition and diet therapy unit | |
|---|---|---|---|
| Risk 0 | No nutritional risk | General protocol for assessment and follow-up after hospital discharge | No |
| Patient did not require medical nutrition therapy during admission | |||
| Risk 1 | With nutritional risk or malnutrition | Periodic nutritional assessment as per clinical course | At the discretion of the clinical nutrition and diet therapy unit as per clinical characteristics |
| Patient required medical nutrition therapy during admission and/or requires modified oral diet and nutritional supplements at discharge | |||
| Risk 2 | Patient with nutritional risk or malnutrition and requires home EN or PN | Periodic nutritional assessment as per clinical course. Specific HEN/HPN protocols | Necessary |
In a health system where chronic diseases are the leading health problem, the patient should be an active part of the process.39 Aware of the enormous possibilities patient training offers and exploiting the benefits and widespread use of the Internet, the Spanish Society of Endocrinology and Nutrition (SEEN) has included on its website the Aula Virtual [Virtual Classroom], (https://www.seen.es/portal/aula-virtual) to support the training of patients and carers alike. The SEEN website also boasts a nutritional information section designed specifically for patients: https://www.seen.es/portal/nutricion-enfermedades. As other medical societies have done, the Spanish Society of Primary Care Physicians (SEMERGEN) has a medical website (www.pacientessemergen.es) accredited by the Spanish Medical Professional Accreditation Council. On this platform, doctors offer guidance and answer patients' and carers' health-related questions. The Alianza Más Nutridos [More Nourished Alliance] also offers information (www.alianzamasnutridos.es).
Because of its accessible, comprehensive and longitudinal nature, the clinical primary care team is usually the first port of call for patients regarding nutrition and the information or information overload they are exposed to in the mass media or social networks. Continuing medical education is required to answer queries, detect malnutrition and prescribe nutritional supplements judiciously.40Table 10 lists some resources of interest for patients and carers.
Links of interest for patients and carers (table prepared by the authors).
| Scientific societies |
| Spanish Society of Endocrinology and Nutrition (Sociedad Española de Endocrinología y Nutrición). SEEN.https://www.seen.es/portal |
| Virtual classroom |
| Dietary recommendations |
| Spanish Society of Primary Care Physicians (Sociedad Española de Médicos de Atención Primaria). SEMERGENhttps://www.semergen.es |
| Spanish Society of Family and Community Medicine (Sociedad Española de Medicina de Familia y Comunitaria). semFYC.https://www.semfyc.es/medicos/ |
| Spanish Society of General and Family Physicians (Sociedad Española de Medicos Generales y de Familia). SEMG.https://www.semg.es |
| Association of Nutrition and Dietician Nurses (Asociación de Enfermeras de Nutrición y Dietética)https://www.adenyd.es/ |
| More Nourished Alliance (Alianza mas nutridos).https://www.alianzamasnutridos.es |
| Patient associations |
| Spanish Federation of Associations for the Fight against Kidney Disease (Federación española de asociaciones para la lucha contra las enfermedades renales):https://alcer.org/fines/ |
| Spanish Association of Amyotrophic Lateral Sclerosis (Asociación española de esclerosis lateral amiotrófica); adELA:https://adelaweb.org |
| Spanish Association against cancer (Asociación española contra el cáncer):https://www.aecc.es/es |
| Crohn's Disease and Ulcerative Colitis ACCU Confederation (Confederación ACCU Crohn y colitis ulcerosa):https://accuesp.com |
| We are NUPA (Somos NUPA):https://somosnupa.org/ |
| Administration: |
| Network of Health Schools for Citizens (Red de Escuelas de Salud para la Ciudadanía)https://www.redescuelassalud.es/ |
| Other initiatives |
| Alicia Foundation (Fundación Alicia):https://www.alicia.cat/es/ |
| Angels:https://es.angels-initiative.com |
The current progressive ageing of the population, coupled with a rise in chronic conditions, suggests that clinical situations entailing a risk of DRM are likely to become more commonplace and that medical nutrition therapy will be increasingly prescribed, making coordination between primary care and clinical nutrition and diet therapy units vital. This consensus statement aims to promote such coordination, and we would like to highlight some current/future challenges that must be addressed in healthcare provision and training, research, quality and humanisation.
- •
It must be ensured that the current height and weight of all patients at risk of malnutrition are recorded in their medical records. Nursing staff must also perform initial nutritional screening with periodic check-ups based on nutritional risk.
- •
Promoting proper digital transformation and implementing a system that facilitates access to information and fluid communication between healthcare professionals at different levels of care throughout Spain should be a priority.
- •
Implementing a protocol or clinical pathway for the DRM care process that optimises care at every stage should be promoted. The protocols must establish criteria for using the different clinical care and care coordination modalities, including e-consultation, teleconsultation and telemonitoring.
- •
For the latter two points, coordination with care homes must also be addressed, establishing joint protocols for the diagnosis and treatment of DRM.
- •
It must be ensured that patients with DRM who are referred, both from PC to a clinical nutrition and diet therapy unit as well as from a clinical nutrition and diet therapy unit to PC, are given a report or assessment of their nutritional status and the needs or approaches to be implemented during follow-up.
- •
By SEEN's portfolio of services,37 it is recommended for clinical nutrition and diet therapy units to employ graduates in nutrition and dietetics. Their role could include collaborating with PC to improve DRM screening, diet therapy and treatment compliance.
- •
We consider it essential to improve training in clinical nutrition among family and community medicine and nursing specialists, promoting specific rotations of suitable duration at clinical nutrition and diet therapy units in their training programme.
- •
Continuing education tools and programmes in clinical nutrition aimed at doctors and nurses must be proposed in primary care and care home settings. These activities should be developed by PC together with the reference endocrinology and nutrition departments, with a specific theoretical-practical programme. Their format should facilitate the identification of training needs, participation, evaluation of their effectiveness and transfer to clinical practice.
- •
Developing joint research lines and projects that contribute to a better understanding of and approach to DRM should be encouraged.
- •
Care quality indicators that include health outcomes, aspects concerning the level of satisfaction and the experience of patients and their families with the care process must be defined and applied.
- •
Include patients or their representatives (carers or associations) in the development of clinical processes at all care levels.