Our aim was to characterise a cohort of patients with Cushing's disease (CD) who did not present pituitary adenoma in magnetic resonance imaging (MRI), needing a catheterisation of the inferior petrosal sinus (CIPS), and to study the pathological findings of the pituitary gland in these subjects after transsphenoidal surgery in order to establish the aetiology of CD. Furthermore, we evaluated possible differences in the features of the diagnosis between hyperplasia and adenoma.
Subjects and methodsWe included 16 subjects. 17 CIPS were done. Hormonal parameters were measured using standard methods. A microscopic histochemical study following standard procedures and immunohistochemical analysis was performed. The diagnostic criteria for adenoma and hyperplasia were based on the WHO classification.
ResultsOne patient was excluded for presenting an ACTH-producing bronchial neuroendocrine tumour. The 15 subjects with CD have a positive CIPS test indicating hypophyseal ACTH production. After transsphenoidal surgery, 12 patients showed a microadenoma and three (20%) a corticotroph cell hyperplasia. We found four recurrences after the transsphenoidal surgery (26%), with a mean time for recurrence of 105 months. We found that recurrence was more frequent in subjects with hyperplasia, and in those subjects with lower right/left ACTH ratio.
ConclusionOur study, which was focused on patients with CD with no pituitary adenoma detected by MRI and a positive CRH test after CIPS, has found that 20% showed corticotroph cell hyperplasia as the cause of CD. Right/left ACTH ratio after CIPS was useful to differentiate adenoma from hyperplasia. This finding may have important prognostic and treatment implications. More studies are necessary to confirm our result.
Nuestro objetivo fue caracterizar una cohorte de pacientes con enfermedad de Cushing (EC) que no presentaban adenoma hipofisario en la resonancia magnética (RM), precisando cateterismo del seno petroso inferior (CSPI), y analizar los hallazgos patológicos de la glándula pituitaria en estos sujetos tras la cirugía transesfenoidal con el fin de establecer la etiología de la EC. Además, evaluamos las posibles diferencias en los hallazgos diagnósticos entre hiperplasia y adenoma.
Sujetos y métodosIncluimos a 16 sujetos. Se realizaron 17 CSPI. Los parámetros hormonales se midieron utilizando métodos estándar. Se realizó un estudio histoquímico microscópico siguiendo procedimientos estándar y análisis inmunohistoquímico. Los criterios de diagnóstico de adenoma e hiperplasia se basaron en la clasificación de la OMS.
ResultadosUno de los pacientes fue excluido por presentar un tumor neuroendocrino bronquial productor de ACTH. Los 15 sujetos restantes presentaron una prueba CSPI positiva que indica una producción de ACTH hipofisaria. Después de la cirugía transesfenoidal, 12 pacientes mostraron un microadenoma y 3 (20%) una hiperplasia de células de corticotropina. Hubo 4 recidivas (26%) tras la cirugía (tiempo medio de recidiva 105 meses). La recidiva fue más frecuente en los pacientes con hiperplasia y en aquellos con cociente derecha/izquierda de ACTH más bajo.
ConclusiónEn nuestro estudio, centrado en pacientes con EC sin adenoma hipofisario detectado por RM y una prueba de CRH positiva tras CSPI, el 20% presentaba hiperplasia de células corticotropas como causa de EC. Además, el cociente de ACTH derecha/izquierda tras CSPI fue útil para diferenciar adenoma de hiperplasia. Este hallazgo puede tener importantes implicaciones tanto pronósticas como de tratamiento. Son necesarios más estudios para confirmar nuestros resultados.
Cushing's disease (CD) is a rare disease (1–3 cases per million subjects year) with important consequences in terms of morbidity and mortality. It usually presents an insidious evolution with non-specific symptoms, that makes the early diagnosis difficult.1–3 The optimisation of the screening diagnosis tests has increased early diagnosis4 contributing to changing the traditional aetiologies of CD. Pituitary adenoma is the cause of 80% of ACTH dependent Cushing's syndrome (CS) and 15% is due to ectopic origin.2,5 Nuclear magnetic resonance imaging of the hypothalamus and pituitary gland (MRIHH) with gadolinium is useful to identify pituitary adenomas. This procedure is the most sensitive and specific.2,5 In the case that MRIHH does not localise the pituitary adenoma, a catheterisation of the inferior petrosal sinuses (CIPS) with CRH stimulation is usually performed to confirm excess autonomous ACTH production in the pituitary, before recommending transsphenoidal surgery with a curative intent.6
The rate of remission after surgery is about 80% when the adenoma is localised by MRIHH, with a recurrence of 15%. In contrast, when the adenoma is not detected by MRIHH, the remission rate is only about 60% in long follow up periods.7 Furthermore, previous studies have shown a higher risk of recurrence when MRIHH is negative but there is a positivity in the CIPS test.8–10
Corticotroph cell hyperplasia is an exceptional cause of CD which has been described in some case reports in patients with confirmed pituitary origin of hypercortisolism after removing the pituitary mass showing the histological study hyperplasia of corticotrophin cells.6,11,12 In addition, there is a matter of debate if corticotroph cell hyperplasia is or is not required as the initial step of CD.
Based on previous data, we hypothesise that in patients with confirmed pituitary ACTH production (positive CIPS test) but no detectable pituitary adenoma by NMRHH the possibility of finding corticotroph cell hyperplasia as a cause of CD should be higher than in other ACTH dependent CS cohorts. In this sense, some authors have tried to find specific characteristics which could be useful to differentiate hyperplasia from adenoma because many times the diagnosis of corticotroph hyperplasia is overlooked.13
In this sense, our aims were to characterise a cohort of patients with ACTH-dependent CS without a pituitary adenoma detected by MRIHH and subjected to a CIPS stimulation test with CRH and to study the pathological findings of the pituitary gland in those subjects after transsphenoidal surgery. Furthermore, we evaluated possible differences in the features of the diagnosis between hyperplasia and adenoma.
Subjects and methodsSubjectsWe studied 16 subjects with ACTH dependent CS with no image of adenoma in the MRIHH. Pre-surgery, all of them had a CIPS stimulation test with CRH to demonstrate pituitary origin of ACTH overproduction. The subjects were consecutively recruited from our outpatient clinic.
All the patients studied attended our Endocrinology Unit at the Hospital Clinico Universitario of Valencia [Valencia Clinical University Hospital] in the period from 1992 to 2014 and signed an informed consent approved by the Ethics Committee of our centre. The CIPS tests were carried out in the Interventional Radiology Unit form our centre by the same group of radiologists. All of them were performed from 1992 to 2013. The same neurosurgical team (two skilled neurosurgeons) performed the interventions from 1992 to 2014.
One subject was excluded from the study, because the CIPS tests showed that it was not a CD and additional studies indicated an ectopic production of ACTH (bronchial carcinoid). After tumour removal, the patient maintained remission criteria for a four-year follow-up period.
In 15 subjects the CIPS test confirmed that the cause of CD was of hypophyseal origin. All of them were treated with transsphenoidal surgery. When the neurosurgeon detected a microscopic lesion, enucleation was performed. In the cases where no microlesions were found a hemihypophysectomy was done, guided by the result of the right/left plasma ACTH gradient after maximum stimulation with CRH. After surgery, the removed tissue was analysed in the Pathological Anatomy Service of our Centre for cell characterisation and immunohistochemistry was performed (see below).
Inclusion criteriaThe inclusion criteria were: man or woman, aged from 18 to 70 years, ACTH-dependent CS and negative MRIHH for adenoma, CIPS: baseline plasma ACTH central to peripheral gradient≥2 or after maximum stimulation plasma ACTH central to peripheral gradient≥3, transsphenoidal surgery and histopathological characterisation after the surgery.
ACTH-dependent CS was diagnosed if the patient fulfilled the four following criteria: (1) Cortisoluria more than three times the upper normal value (in our laboratory>150μg/24h); (2) Two days suppression test with 0.5mg of dexamethasone every 6h: 9.00am cortisol value ≥2μg/dl; (3) Overnight 1mg dexamethasone suppression test at 23h: 9.00am cortisol value≥2μg/dl; and (4) 9.00am ACTH value≥20pg/ml.
Exclusion criteriaThe exclusion criteria were: ACTH-independent Cushing Syndrome (9.00am ACTH value≤10pg/ml), ACTH dependent Cushing's syndrome with MRIHH that showed pituitary adenoma, no surgery and/or histological studies.
Remission and recurrence criteriaWe considered remission criteria to be when 9.00am cortisol value (last taking prednisone as replacement therapy at 9.00am on the day before)<5μg/dl at 14 days after the intervention or 9.00am cortisol value<1.8μg/dl at three months after surgery.14,15
The recurrence criteria were absence of remission criteria and abnormal overnight 1mg dexamethasone suppression test: 9.00am cortisol value≥2μg/dl.
All the subjects were followed up after surgery at 14 days, 3, 6 and 12 months. Subsequently an annual follow up with measurement at 9.00am of cortisol, cortisoluria and the performance of an overnight 1mg dexamethasone suppression test was performed, if the patient maintained remission criteria.
MethodsClinicalClinical parameters were collected by clinical interview: age, gender and personal history (hyperglycaemia, hypertension, obesity, depression and osteoporosis; and non-related to CS, such as surgical background and other disease).
Anthropometric parameters as height, weight, waist circumference and body mass index were measured by standardised methods.
Hormonal valuesPlasma cortisol was measured by chemiluminescent competitive immunoassay with the Analyzer Cobas 8000 (Roche Diagnostics, Rotkreuz, Switzerland), considering normal as 5–25μg/dl at 9.00am. Plasma ACTH was measured using ELISA with the Analyzer Cobas 8000 (Roche Diagnostics, Rotkreuz, Switzerland), considering normal as 5–60pg/ml. Urinary cortisol was determined using the competitive chemiluminescent Analyzer (Siemens) Architect immunoassay technique.
Two days suppression test with 0.5mg of dexamethasone consisted in the administration of 0.5mg of dexamethasone orally every 6h for 2 days and measurement of cortisol at 9.00am the next day.
The overnight 1mg dexamethasone suppression test consisted in the administration of 1mg of dexamethasone at 11.00pm and measurement of cortisol at 9.00am the next day.
Two days suppression test with 2mg of dexamethasone consisted in the administration of 2mg of dexamethasone every 6h orally for two days and measurement of cortisol at 9.00am the next day.
Magnetic resonance imaging (MRI)The MRIHH was performed with the administration of gadolinium in the Radiology Service of our centre. High-resolution, contrast-enhanced and thin-section MRI scans were done. Cuts of less than a millimetre were done to detect microadenomas. This test was interpreted by two expert radiologists dedicated to endocrine image analysis in our centre.
Catheterisation of inferior petrosal sinuses (CIPS)Catheterisation was performed by two experienced radiologists at our centre after 8 hours’ fasting. We used local anaesthesia in order to perceive presentation of problems like otalgia during the procedure.
A peripheral vein was catheterised in one arm to collect samples of peripheral blood. Catheterisation of the femoral veins with two 5F introducers was done. For each introducer, we passed a 5F catheter to reach inferior petrosal sinus. The procedure was performed under fluoroscopic control. To check the correct position of the catheter, a small amount of iodinated contrast was inserted. To obtain detailed information about the procedure see reference.16
After correct catheterisation, CRH was administered (1μg/kg). ACTH was measured at – 5, baseline (0) and 2, 3, 5, 10min after the administration of CRH.
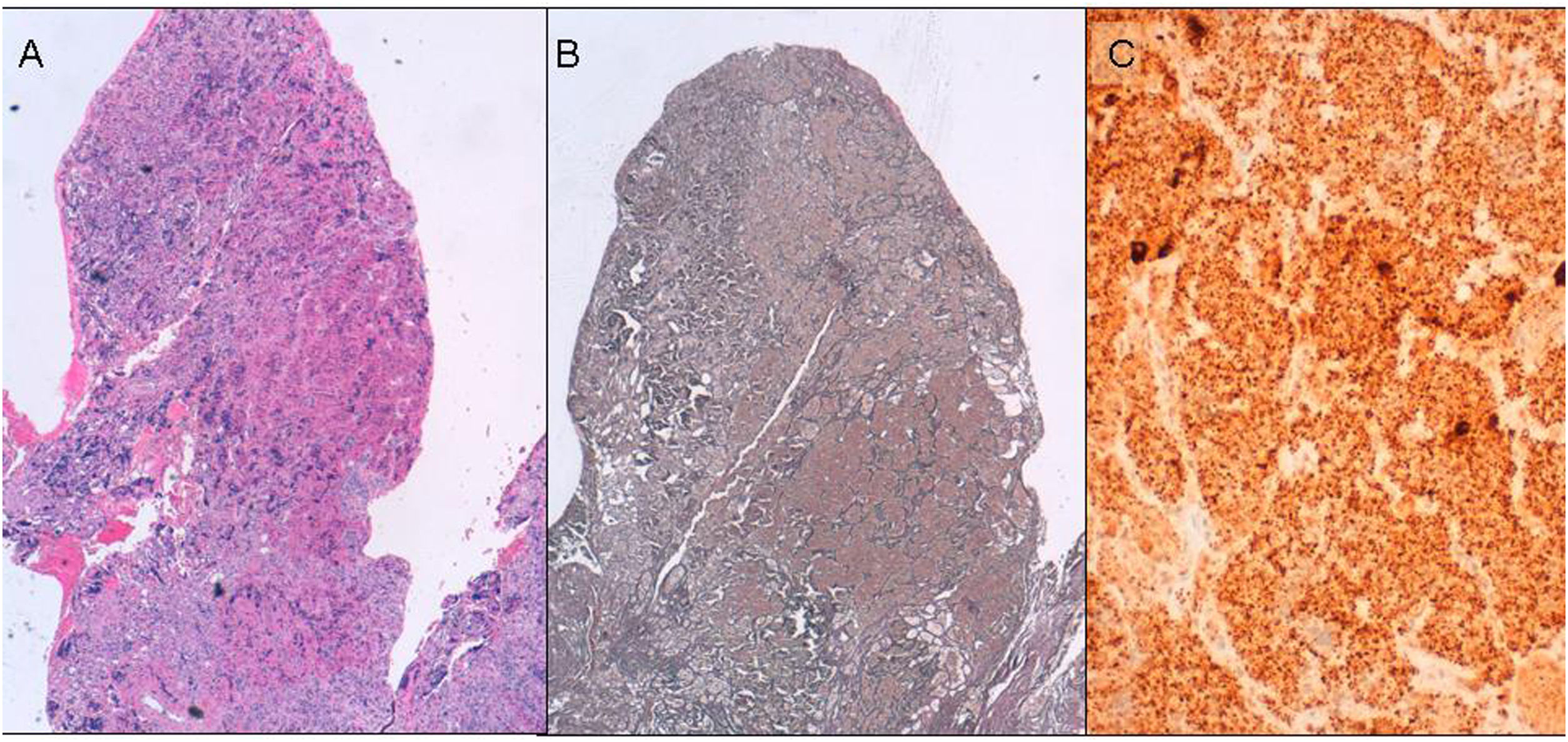
Histopathological studyWe performed a microscopic study from formalin-fixed, paraffin-embedded specimens. In each case a routine H.E. staining, reticulin histochemical study following standard procedures and immunohistochemical analysis were performed17 (Fig. 1).
Samples were sectioned for immunostaining sections, processed by Envision Flex (Dako) and stained with antibodies to ACTH (Dako; 1/200), prolactin, (Novocastra; 1/400), TSH (Dako; prediluted), HGH (Dako; 1/1000), FSH (Dako; 1/50), LH (Dako1/100) and Ki67 (Dako; prediluted). Antigenic retrieval was performed with PT-Link, and low pH buffer. We detected antibody binding with DAB as the chromogen.
The diagnostic criteria for adenoma and hyperplasia were based on the WHO classification.18 In summary: the architectural pattern in acini for normal tissue, expanded acini for hyperplasia and nodular pattern for adenoma. The reticulin network is essential in differential diagnosis. It is surrounding the acini in normal tissue, expanded in hyperplasia and lost in adenoma. Finally, cells are monomorphous in both pathological cases and heteromorphous in normal tissue.
Statistical methodsData were analysed using the Statistical Package for the Social Sciences (SPSS 12.1.3 for Windows; SPSS Chicago, IL, USA). Quantitative variables are expressed as mean and standard deviation. The qualitative variables are expressed in total number or percentage.
The comparative analysis of the variables was made using the Mann–Whitney test. Qualitative variables were analysed using the Chi square test. We considered a p value below 0.05 to be statistically significant.
ResultsWe studied 16 individuals. One of them was excluded because CS was due to a bronchial carcinoid tumour producing ACTH. Finally, we included 15 individuals (11 women and 4 men) in the study. All of them presented CD with no adenoma localised by high-resolution, contrast-enhanced and thin-section MRIHH. In the 15 subjects a CIPS stimulation test with CRH was performed prior to surgery. In all of them, the test showed a pituitary origin of CD. We show the clinical and biological characteristics of the studied patients in Tables 1 and 2, including data about lateralisation (except for patient 8 for whom it was not possible to evaluate both sides).
Clinical characteristics and hormonal values of patients with ACTH dependent Cushing syndrome with no adenoma detected by MRIHH and studied with CIPS.
| n=15 | Average±standard deviation |
|---|---|
| Age (years) | 38.1±7.9 |
| BMI (kg/m2) | 27.5±3.6 |
| Cortisoluria (mg/24h) | 653.1±509.4 |
| 9.00am Cortisol (μg/dl) | 31.9±12.6 |
| 9.00am ACTH (pg/dl) | 75.3±57.7 |
| Cortisol 9.00am after two days suppression test with 0.5mg of dexamethasone every 6h (μg/dl) | 21.2±9.8 |
| Cortisol 9.00am after two days suppression test with 2mg of dexamethasone every 6h (μg/dl) | 13.5±10.4 |
| Catheterisation follow up (years) | 10.1±6.5 |
| Central ACTH (pg/dl) | 506.1±646.4 |
| Peripheral ACTH (pg/dl) | 71.7±67.6 |
| Central/peripheral baseline ACTH ratio | 9.4±10.7 |
| Peripheral ACTH after CRH stimulation (pg/dl) | 110.7±122.0 |
| Central ACTH after CRH stimulation (pg/dl) | 1874.6±1493.1 |
| Central/peripheral ACTH ratio after CRH | 43.3±37.1 |
| ACTH ratio after CRH (lateralisation) | 7.3±6.1 |
| Follow up after surgery (years) | 9.7±6.3 |
BMI: body mass index; MRIHH: magnetic resonance imaging of the hypothalamus and hypophysis; CIPS: catheterisation of inferior petrosal sinus.
Characteristics of the patients with ACTH dependent Cushing syndrome.
| Case no. | Age (years) | Gender | Right ACTH (pg/dl) | Left ACTH (pg/dl) | Right/left ACTH ratio | Remission | Histological diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | 48 | Male | 1369 | 1794 | 1.3 | Yes | Left adenoma |
| 2 | 41 | Male | 648 | 61 | 10.6 | No | Right adenoma |
| 3 | 33 | Female | 1908 | 1307 | 1.4 | No | Hyperplasia |
| 4 | 39 | Female | 221 | 1797 | 8.1 | Yes | Left adenoma |
| 5 | 23 | Male | 1237 | 59.8 | 20.6 | Yes | Right adenoma |
| 6 | 41 | Male | 246 | 5143 | 20.9 | Yes | Left adenoma |
| 7 | 45 | Female | 770 | 55 | 14 | Yes | Right adenoma |
| 8 | 46 | Female | NA | NA | NA | No | Hyperplasia (right adenoma after second surgery) |
| 9 | 46 | Female | 689 | 4425 | 6.4 | Yes | Left adenoma |
| 10 | 28 | Female | 2172 | 33 | 65.8 | Yes | Right adenoma |
| 11 | 38 | Female | 18.2 | 4.4 | 4.55 | Yes | Right adenoma |
| 12 | 40 | Female | 436 | 1816 | 4.1 | Yes | Left adenoma |
| 13 | 23 | Female | 2351 | 642 | 3.6 | Yes | Right adenoma |
| 14 | 41 | Female | 1973 | 183 | 10.7 | Yes | Right adenoma |
| 15 | 40 | Female | 1227 | 2001 | 1.63 | No | Hyperplasia |
Right/Left ratio was calculated dividing the highest value by the lowest.
NA: not available.
After transsphenoidal surgery, 12 patients (80%) showed a microadenoma and three (20%) a corticotroph cell hyperplasia (Table 2). During the follow-up period (1992–2015) four recurrences out of 15 patients (26%) were observed. The mean time for recurrence was 105 months (more than eight years). In Table 3 we show the clinical and hormonal parameters classifying the studied subjects as still being in remission or not. We found that recurrence was more frequent in subjects with hyperplasia, and in those subjects with lower right/left ACTH ratio.
Clinical and hormonal values of patients with ACTH dependent Cushing syndrome classified depending on recurrence.
| Dependent ACTH CS in remission (n=11) | Dependent ACTH CS with recurrence (n=4) | p | |
|---|---|---|---|
| Age (years) | 38.4±8.8 | 37.5±6.3 | 0.512 |
| BMI (kg/m2) | 26.5±2.98 | 29.3±4.5 | 0.920 |
| Cortisoluria (μg/24h) | 759.3±597.8 | 849.2±334.2 | 0.712 |
| Baseline Cortisol (μg/dl) | 32.1±13.11 | 29.9±8.43 | 0.599 |
| Baseline ACTH (pg/dl) | 79.2±70.19 | 70.3±35.22 | 0.174 |
| Cortisol after two days suppression test with 0.5mg of dexamethasone every 6h (μg/dl) | 21.9±7.1 | 18.6±9.3 | 0.143 |
| CIPS (years) | 10.3±4.8 | 14.8±6.2 | 0.619 |
| Central ACTH at baseline (pg/dl) | 415.8±579.4 | 350.3±470.7 | 0.229 |
| Peripheral ACTH at baseline (pg/dl) | 78.5±80.45 | 53.0±43.5 | 0.146 |
| Central ACTH after maximal stimulus with CRH (pg/dl) | 1556.7±1490.6 | 1914.2±2274.0 | 0.512 |
| Central/peripheral baseline ACTH ratio | 8.6±12.2 | 7.3±9.0 | 0.954 |
| Central/peripheral ACTH ratio after CRH stimulation | 40.6±39.2 | 31.8±29.6 | 0.508 |
| Right/left ACTH ratio after CRH stimulation | 8.8±6.1 | 2.21±1.3* | 0.012 |
| Time from surgery (years) | 10.0±4.9 | 14.0±6.1 | 0.669 |
| Time free of recurrence (months) | – | 105.0±100.6 | – |
| Adenoma/hyperplasia | 11/0 | 1/3* | 0.011 |
BMI: body mass index; CIPS: catheterisation of inferior petrosal sinus; CS: Cushing syndrome.
The patients who presented recurrence remain currently in remission after a minimum follow-up period of 18 months (radiotherapy or a new surgery was needed). Unfortunately, 12 patients had panhypopituitarism and three partial hypopituitarism (three subjects hypogonadism and one hypothyroidism).
Finally, we also evaluated the characteristics of subjects with adenoma and hyperplasia (Table 4). We found that those patients with diagnosis of adenoma showed higher baseline ACTH levels and higher right/left ACTH ratio during CIPS.
Clinical and hormonal values of patients with ACTH dependent Cushing syndrome classified depending on histological diagnosis after surgery.
| Patients with corticotroph adenoma (n=12) | Patients with corticotroph hyperplasia (n=3) | p | |
|---|---|---|---|
| Age (years) | 39.2±8.2 | 33.7±6.0 | 0.168 |
| BMI (kg/m2) | 27.8±3.6 | 26.4±2.7 | 0.306 |
| Cortisoluria (μg/24h) | 682.5±555.1 | 357.5±234.9 | 0.484 |
| Baseline Cortisol (μg/dl) | 32.7±14.2 | 28.9±0.9 | 0.664 |
| Baseline ACTH (pg/dl) | 87.2±60.0 | 31.7±3.9* | 0.016 |
| Cortisol after two days suppression test with 0.5mg of dexamethasone every 6h (μg/dl) | 23.8±9.8 | 13.3±5.1 | 0.052 |
| CIPS (years) | 10.7±6.4 | 7.7±7.6 | 0.633 |
| Central ACTH at baseline (pg/dl) | 560.1±717.2 | 307.8±262.2 | 0.938 |
| Peripheral ACTH at baseline (pg/dl) | 82.9±72.2 | 30.4±20.5 | 0.186 |
| Central ACTH after maximal stimulus with CRH (pg/dl) | 2041.2±1603.1 | 1319.3±1100.2 | 0.800 |
| Central/peripheral baseline ACTH ratio | 9.2±11.3 | 10.3±8.9 | 0.697 |
| Central/peripheral ACTH ratio after CRH stimulation | 41.4±33.5 | 49.7±55.6 | 0.842 |
| Right/left ACTH ratio after CRH stimulation | 8.9±5.9 | 1.5±0.9* | 0.036 |
| Time from surgery (years) | 10.5±6.3 | 6.7±6.6 | 0.383 |
| Time free of recurrence (months) | 69.5±81.6 | 64±70.3 | 0.891 |
BMI: body mass index; CIPS: catheterisation of inferior petrosal sinus.
We found four recurrences from 15 CD studied (26%), after transsphenoidal surgery. A similar recurrence rate was found in other studies. In addition, in patients with no adenoma detected by MRIHH, treated with hemihypophysectomy, the remission rate is about 50%.19 Moreover, when the follow up period is longer (more than 10 years) the rate of recurrences increased to 26% in subjects with detectable adenoma by MRIHH.7,17
In our cohort, three patients showed corticotroph cell hyperplasia as the pathological finding, which represents 20% of the total number of studied cases. This result confirmed our hypothesis. It is remarkable that all the patients with hyperplasia had recurrence. However, only one out of 12 (8.3%) with diagnosis of adenoma presented recurrence (Table 3). When we analysed the factors which could be related to recurrence, we found that those patients with diagnosis of adenoma showed higher baseline ACTH levels and higher right/left ACTH ratio during the CIPS test (Table 4). In fact, subjects with hyperplasia presented weak lateralisation or it was even absent. Although more studies are necessary, a CIPS test could be useful to anticipate the diagnosis of hyperplasia. Furthermore, the CIPS test was able to guide the neurosurgical procedure. The neurosurgeon performed an exploration of the pituitary gland, mainly guided by the presence of lateralisation after the administration of CRH. In those cases, in which an adenoma was observed, it was enucleated. In cases in which no type of lesion was evident, hemihypophysectomy was performed based on previous CIPS test lateralisation. The concordance between the CIPS test and the location of the lesion was very high (Table 2).
There are few data about the parameters associated to recurrence. Ciric et al.10 showed that peripheral to central ACTH gradient at baseline or after maximum stimulation with CRH was associated with a high incidence of hormone remission in the immediate postoperative period. However, in this study all CD patients presented adenoma in the MRIHH.10 Therefore, the results observed by Ciric et al. are not comparable with those found in the present study, in which only CD patients with no adenoma in MRIHH were included.
Catalino et al. also evaluated diagnostic features, surgical management, and clinical outcomes to compare corticotroph hyperplasia and histopathologically proven adenomas in patients with Cushing disease. However, no specific findings were obtained.13
In other studies, corticotrophin cell hyperplasia has been described as responsible for ACTH-dependent CS in some case reports.6,11,12 In addition, hyperplasia with transformation to adenoma has been described as a rare cause of CD20,21 and hyperplasia and adenomas have been found in the sample of the same patient with CD.22 It is note that one of our four patients who experienced recurrence showed a corticotroph cell hyperplasia but in the second surgery a microadenoma as the pathological finding was established (Table 2; patient 8).
However, not all the authors agree with the definition of corticotroph cell hyperplasia. We have used diagnostic criteria based on the WHO recommendations.18 In addition, the three patients with corticotroph cell hyperplasia showed remission criteria after surgery suggesting that this pathological finding was the cause of CD. None of them presented persistent disease after surgery.
Our study has some limitations. The sample size is small, although it is representative. In fact, Nishioka et al.9 studied a cohort of 124 patients with ACTH-dependent CS. Only 18 had a negative MRIHH. In addition, the diagnostic criteria of CS have experienced some changes during the time of the study. Even this, our study is original. We have included only patients with no adenoma detected by MRIHH with a median follow-up period of 8 years. In addition, the CIPS test, MRIHH interpretation and pathological studies have been made by the same group of researchers.
In conclusion, the present cohort focused on patients with ACTH-dependent CS with no pituitary adenoma detected by MRIHH and a positive CIPS test after CRH, and 20% of the patients showed a corticotroph cell hyperplasia as the cause of CD. Furthermore, the CIPS test guided surgery in an efficient way, and could be useful to differentiate adenoma and hyperplasia. Nevertheless, multi-centre studies involving a greater number of subjects with ACTH-dependent CS with no adenoma detected by MRIHH are necessary to know the real prevalence of corticotroph cell hyperplasia as the cause of CD. In addition, these studies could identify biological and/or hormonal predictors of hyperplasia diagnosis and recurrence in this subgroup of patients where the rate of recurrences is very high.
Conflict of interestThe authors declare that they have no conflict of interest.
We thank the patients for their cooperation. This work has been developed within a final degree project at the University of Valencia.