To compare the cost-effectiveness of sensor-augmented pump therapy (SAP) [continuous subcutaneous insulin infusion (CSII) plus real-time continuous glucose monitoring (RT-CGM)] with low glucose suspend (MiniMed™ Veo™) and CSII alone in patients with type 1 diabetes mellitus (T1DM) at high risk of hypoglycemia in Spain.
MethodsThe IQVIA CORE Diabetes Model was used to estimate healthcare outcomes as life-years gained (LYGs) and quality-adjusted life years (QALYs), and to project lifetime costs. Information about efficacy, resource utilization, and unit costs (€2016) was taken from published sources and validated by an expert panel. Analyses were performed from both the Spanish National Health System (NHS) perspective and the societal perspective.
ResultsFrom the NHS perspective, SAP with low glucose suspend was associated to a €47,665 increase in direct healthcare costs and to increases of 0.19 LYGs and 1.88 QALYs, both discounted, which resulted in an incremental cost-effectiveness ratio (ICER) of €25,394/QALY. From the societal perspective, SAP with low glucose suspend increased total costs (including direct and indirect healthcare costs) by €41,036, with a resultant ICER of €21,862/QALY. Considering the willingness-to-pay threshold of €30,000/QALY in Spain, SAP with low glucose suspend represents a cost-effective option from both the NHS and societal perspectives. Sensitivity analyses confirmed the robustness of the model.
ConclusionsFrom both the Spanish NHS perspective and the societal perspective, SAP with low glucose suspend is a cost-effective option for the treatment of T1DM patients at high risk of hypoglycemia.
Evaluar la relación coste-utilidad del sistema integrado (MiniMed® Veo®) con suspensión en hipoglucemia frente a la infusión subcutánea continua de insulina (ISCI) en el tratamiento de pacientes con diabetes tipo 1 (DM1) y alto riesgo de hipoglucemias en España.
MétodosSe utilizó el modelo de diabetes IQVIA CORE para estimar los resultados en salud expresados como años de vida ganados (AVG) y años de vida ajustados por calidad (AVAC) y los costes a lo largo de la vida de los pacientes. La información sobre la eficacia, el consumo de recursos y los costes unitarios (2016€) fue obtenida de fuentes publicadas y validadas por un panel de expertos. En el escenario principal se consideró la perspectiva del Sistema Nacional de Salud (SNS) y, en un escenario alternativo, la de la sociedad en general.
ResultadosBajo la perspectiva del SNS el tratamiento con el sistema integrado con suspensión en hipoglucemia se asoció con mayores costes sanitarios directos (47.665€) y un incremento de 0,19 AVG y 1,88 AVAC, resultando en un ratio coste-utilidad incremental (RCUI) de 25.394€/AVAC. Considerando la perspectiva de la sociedad, los costes totales (sanitarios directos e indirectos) se incrementaron en 41.036€, siendo el RCUI resultante de 21.862€/AVAC. Los análisis de sensibilidad confirmaron la robustez de los resultados en todos los escenarios evaluados.
ConclusiónConsiderando el umbral de máxima disposición a pagar para España de 30.000€/AVAC, el sistema integrado con suspensión en hipoglucemia representa una opción eficiente en comparación con la ISCI tanto desde la perspectiva del SNS como de la sociedad en su conjunto.
Costs associated with the treatment of diabetes mellitus and its complications constitute a substantial proportion of total healthcare expenses in Spain. Thus, there is an urgent need for measures to achieve complete control of this disease and to prevent progression of the related complications1.1 Optimal glycaemic control is associated with reduced chronic complications and consequently lower diabetes-related costs; however, approximately 75.8% of patients with type 1 diabetes mellitus (T1DM) are not reaching their glucose control target.2
Among patients with chronic poor glycaemic control and patients whose control escalation is limited by hypoglycaemia, continuous subcutaneous insulin infusion (CSII) therapy is usually more effective than multiple daily insulin injections (MDI). A Spanish study conducted over a five-year period revealed that CSII therapy can reduce hypoglycaemic events, and showed long-term maintenance of this clinical benefit without increasing glycosylated haemoglobin (HbA1c) levels.3 From an economic perspective, a budget impact analysis conducted in Spain demonstrates that the increased costs associated with CSII vs. MDI therapy in patients with T1DM and recurrent severe hypoglycaemia are completely counterbalanced by the reduction of severe hypoglycaemic events, with CSII even generating savings for the Spanish National Health System (NHS).4
Intensive insulin treatment needs frequent blood glucose testing to enable adequate adjustment of insulin dosing to achieve near-normoglycaemia in patients with T1DM.5 However, intermittent blood glucose measurements do not provide information regarding the extent of fluctuations or the speed or and direction of the changes over time. Technological advances over the last 15 years have led to the development of devices that provide continuous glucose monitoring (CGM), with information about glycaemic trends and different types of alarms (hyperglycaemia, hypoglycaemia, etc.).6 Moreover, some of these systems can be combined with CSII therapy—enabling sensor-augmented pump therapy (SAP), in which low glucose suspend can be activated when blood glucose levels drop to a pre-set value (hypoglycaemia threshold). Overall, SAP with low glucose suspend therapy together with the treatment of diabetes-associated complications is more expensive than CSII, but is reportedly associated with reduced HbA1c levels and decreases in the number, duration, and severity of hypoglycaemia events compared to CSII.7,8
In the present study, we aimed to estimate the costs and healthcare outcomes of SAP with low glucose suspend vs. CSII alone for the treatment of T1DM patients at a high risk of hypoglycaemia in Spain.
Materials and methodsModel structureThe IQVIA CORE Diabetes Model has been validated and widely used to simulate diabetes development9 enabling estimation of healthcare outcomes in life-years gained (LYG) and quality-adjusted life years (QALY), as well as life-long costs for T1DM patients at a high risk of severe hypoglycaemia. This model comprises 17 Markov sub-models that simulate the evolution of various diabetes-associated complications, such as angina, myocardial infarction, congestive heart failure, stroke, peripheral vascular disease, diabetic retinopathy, macular oedema, cataracts, hypoglycaemia, ketoacidosis, nephropathy, neuropathy, foot ulcers and amputation, pulmonary oedema, depression, and all-cause mortality.
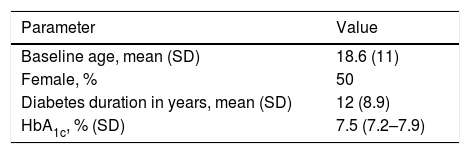
Treatment options and healthcare outcomesBaseline characteristics were based on data from a randomized clinical trial8 that included patients with T1DM at a high risk of hypoglycaemia.10 The mean patient age was 18.6 years, and 50% were females. The mean diabetes duration was 12 years, and the mean HbA1c level was 7.5% (Table 1). The rates of severe hypoglycaemic events after six months of treatment were 0 events/100 patients/month with SAP with low glucose suspend vs. 2.2 events/100 patients/month with CSII alone.
Baseline characteristics.7
| Parameter | Value |
|---|---|
| Baseline age, mean (SD) | 18.6 (11) |
| Female, % | 50 |
| Diabetes duration in years, mean (SD) | 12 (8.9) |
| HbA1c, % (SD) | 7.5 (7.2–7.9) |
SD, standard deviation.
The primary analysis was conducted from the perspective of the Spanish National Healthcare System (NHS), and thus considered direct healthcare costs. In an alternative analysis, we also assessed the societal perspective by including the indirect costs associated with T1DM. We employed a lifetime horizon, and applied an annual 3% reduction rate to both costs and healthcare outcomes.10
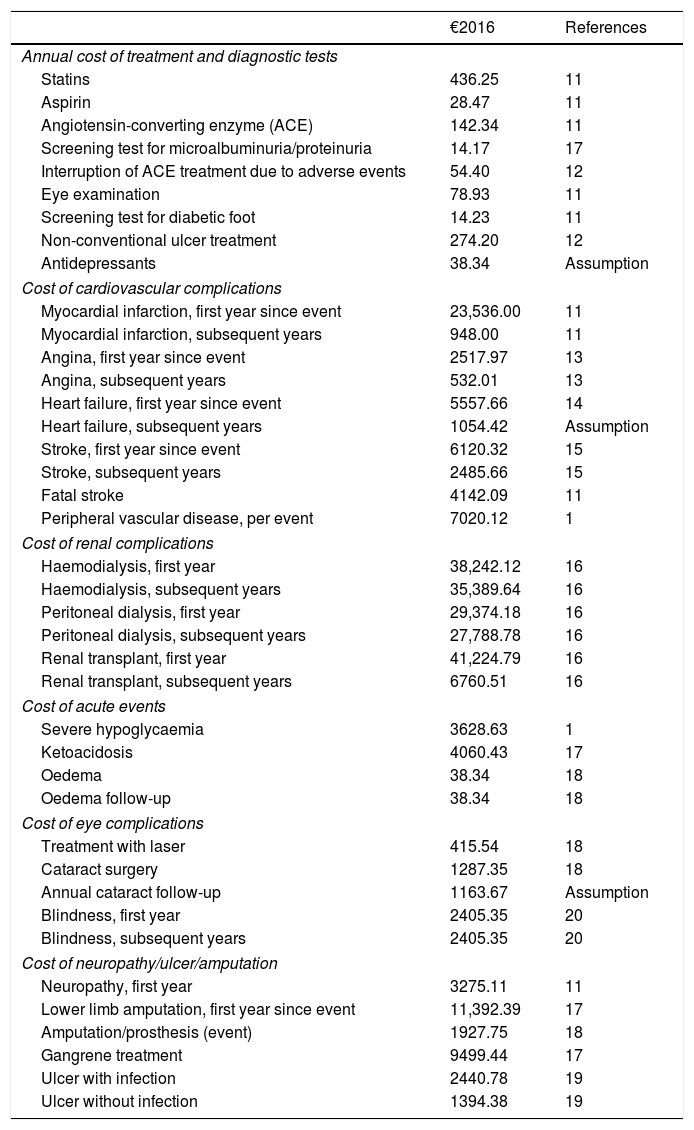
Resources used and cost per unitThe data regarding the utilized resources and unitary costs were obtained from various published sources,11–20 and validated by a panel of experts. For intervention costs only, the incremental cost of SAP with low glucose suspend therapy relative to CSII therapy was applied. Costs were updated to €2016 using the relevant variation of the consumer price index (Table 2). In our analysis, we considered 70% sensor adherence, i.e. 43 sensors per year, each worn over a 6-day period.21 For analysis from the societal perspective, we considered the average annual salary in Spain, the average ages of integration into working life and retirement, and the days of sick leave due to diabetes-associated complications.22
Costs of the management of diabetes and associated complications.
| €2016 | References | |
|---|---|---|
| Annual cost of treatment and diagnostic tests | ||
| Statins | 436.25 | 11 |
| Aspirin | 28.47 | 11 |
| Angiotensin-converting enzyme (ACE) | 142.34 | 11 |
| Screening test for microalbuminuria/proteinuria | 14.17 | 17 |
| Interruption of ACE treatment due to adverse events | 54.40 | 12 |
| Eye examination | 78.93 | 11 |
| Screening test for diabetic foot | 14.23 | 11 |
| Non-conventional ulcer treatment | 274.20 | 12 |
| Antidepressants | 38.34 | Assumption |
| Cost of cardiovascular complications | ||
| Myocardial infarction, first year since event | 23,536.00 | 11 |
| Myocardial infarction, subsequent years | 948.00 | 11 |
| Angina, first year since event | 2517.97 | 13 |
| Angina, subsequent years | 532.01 | 13 |
| Heart failure, first year since event | 5557.66 | 14 |
| Heart failure, subsequent years | 1054.42 | Assumption |
| Stroke, first year since event | 6120.32 | 15 |
| Stroke, subsequent years | 2485.66 | 15 |
| Fatal stroke | 4142.09 | 11 |
| Peripheral vascular disease, per event | 7020.12 | 1 |
| Cost of renal complications | ||
| Haemodialysis, first year | 38,242.12 | 16 |
| Haemodialysis, subsequent years | 35,389.64 | 16 |
| Peritoneal dialysis, first year | 29,374.18 | 16 |
| Peritoneal dialysis, subsequent years | 27,788.78 | 16 |
| Renal transplant, first year | 41,224.79 | 16 |
| Renal transplant, subsequent years | 6760.51 | 16 |
| Cost of acute events | ||
| Severe hypoglycaemia | 3628.63 | 1 |
| Ketoacidosis | 4060.43 | 17 |
| Oedema | 38.34 | 18 |
| Oedema follow-up | 38.34 | 18 |
| Cost of eye complications | ||
| Treatment with laser | 415.54 | 18 |
| Cataract surgery | 1287.35 | 18 |
| Annual cataract follow-up | 1163.67 | Assumption |
| Blindness, first year | 2405.35 | 20 |
| Blindness, subsequent years | 2405.35 | 20 |
| Cost of neuropathy/ulcer/amputation | ||
| Neuropathy, first year | 3275.11 | 11 |
| Lower limb amputation, first year since event | 11,392.39 | 17 |
| Amputation/prosthesis (event) | 1927.75 | 18 |
| Gangrene treatment | 9499.44 | 17 |
| Ulcer with infection | 2440.78 | 19 |
| Ulcer without infection | 1394.38 | 19 |
Health-related quality of life is incorporated into economic evaluations via estimation of QALY, based on the correction of survival in LYG, with a utility value representing the patient's subjective preference for certain health conditions. Here we considered utility values based on the published literature,23–25 with a lower utility value assigned when a patient simultaneously presented multiple complications. Since the studied population had a high risk of hypoglycaemic events, we also considered the potential impact on quality of life due to reduced fear of hypoglycaemia in patients treated with SAP with low glucose suspend.24,25
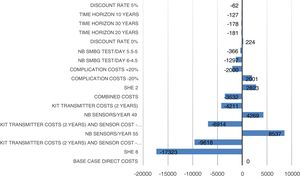
Sensitivity analysisWe performed univariate and bivariate deterministic sensitivity analyses (SAs), along with a probabilistic SA to evaluate the impacts of certain parameters on the results. Univariate deterministic SAs were conducted to evaluate the impact associated with variations in the sensor adherence rate (80% and 90%), rate of severe hypoglycaemic events (2 events/100 patients/month with CSII vs 0 events/100 patients/month with SAP with low glucose suspend; 8 episodes/100 patients/month with CSII vs 0 with SAP with low glucose suspend), reduction rate (0% and 5%), cost of complications (±20%), time-frame of the analysis (10, 20, and 30 years), number of self-monitoring blood glucose tests, and average transmitter life (2 years). Bivariate deterministic SAs were performed to account for variations in both transmitter cost (10% and 20% reduction) and transmitter life (2 years). Probabilistic SA comprised 1000 Monte Carlo simulations, each including 1000 patients, with simultaneous modification of the values of the parameters according to the normal and gamma distributions.
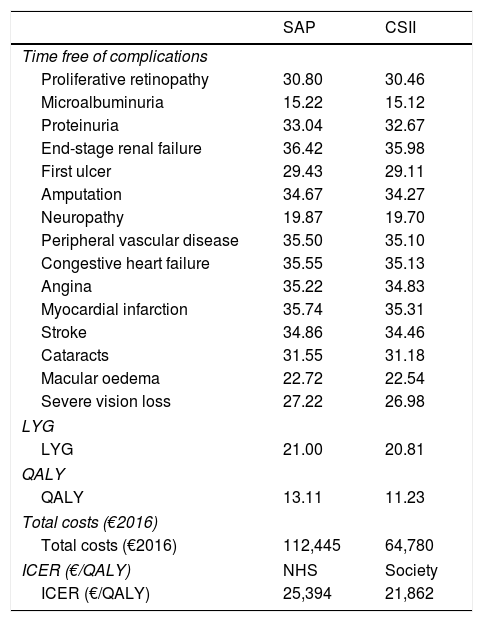
ResultsIn terms of disease burden, treatment with SAP with low glucose suspend was associated with increases of 0.19 in LYG and 1.88 in QALY, both discounted, compared to CSII alone. The use of SAP with low glucose suspend was also associated with a reduced cumulative incidence and delay to onset of diabetes-related complications. The most pronounced delay was for end stage renal disease, myocardial infarction and congestive heart failure. The mean onset of all complications was delayed by 0.33 years with SAP with low glucose suspend compared with CSII alone. Table 3 presents the time free of complications, LYG, QALY, and total costs associated with both therapies and with the management of complications.
Analysis results.
| SAP | CSII | |
|---|---|---|
| Time free of complications | ||
| Proliferative retinopathy | 30.80 | 30.46 |
| Microalbuminuria | 15.22 | 15.12 |
| Proteinuria | 33.04 | 32.67 |
| End-stage renal failure | 36.42 | 35.98 |
| First ulcer | 29.43 | 29.11 |
| Amputation | 34.67 | 34.27 |
| Neuropathy | 19.87 | 19.70 |
| Peripheral vascular disease | 35.50 | 35.10 |
| Congestive heart failure | 35.55 | 35.13 |
| Angina | 35.22 | 34.83 |
| Myocardial infarction | 35.74 | 35.31 |
| Stroke | 34.86 | 34.46 |
| Cataracts | 31.55 | 31.18 |
| Macular oedema | 22.72 | 22.54 |
| Severe vision loss | 27.22 | 26.98 |
| LYG | ||
| LYG | 21.00 | 20.81 |
| QALY | ||
| QALY | 13.11 | 11.23 |
| Total costs (€2016) | ||
| Total costs (€2016) | 112,445 | 64,780 |
| ICER (€/QALY) | NHS | Society |
| ICER (€/QALY) | 25,394 | 21,862 |
QALY, quality-adjusted life years; LYG, life-years gained; CSII, continuous subcutaneous insulin infusion; ICER, incremental cost-effectiveness ratio; NHS, National Health System; SAP, sensor augmented pump.
From the Spanish NHS perspective, direct healthcare costs were €47,665 higher for treatment with SAP with low glucose suspend compared to treatment with CSII alone (€112,445 vs. €64,780). All cost items associated with both therapy and management of various complications were higher in the case of SAP with low glucose suspend, with therapy costs having the greatest impact on total cost. The costs associated with complications were higher in the case of SAP with low glucose suspend treatment due to a “survival paradox” in which patients accrue direct costs and experience complications over a longer period of time owing to higher life expectancy. The incremental cost-effectiveness ratio (ICER) estimated from the Spanish NHS perspective was €25,394 per QALY gained.
From the societal perspective, total costs (direct and indirect) were increased by €41,036 with SAP with low glucose suspend compared to CSII therapy (€217,272 vs. €176,236), yielding an ICER of €21,862 per QALY gained. Therefore, SAP with low glucose suspend was associated with greater clinical benefits despite the higher costs relative to therapy with CSII alone. The generally accepted willingness-to-pay threshold in Spain is €30,000 per QALY26.26 Thus, these results indicate that SAP with low glucose suspend is a cost-effective option for T1DM patients at a high risk of hypoglycaemia, from both the Spanish NHS perspective and the societal perspective.
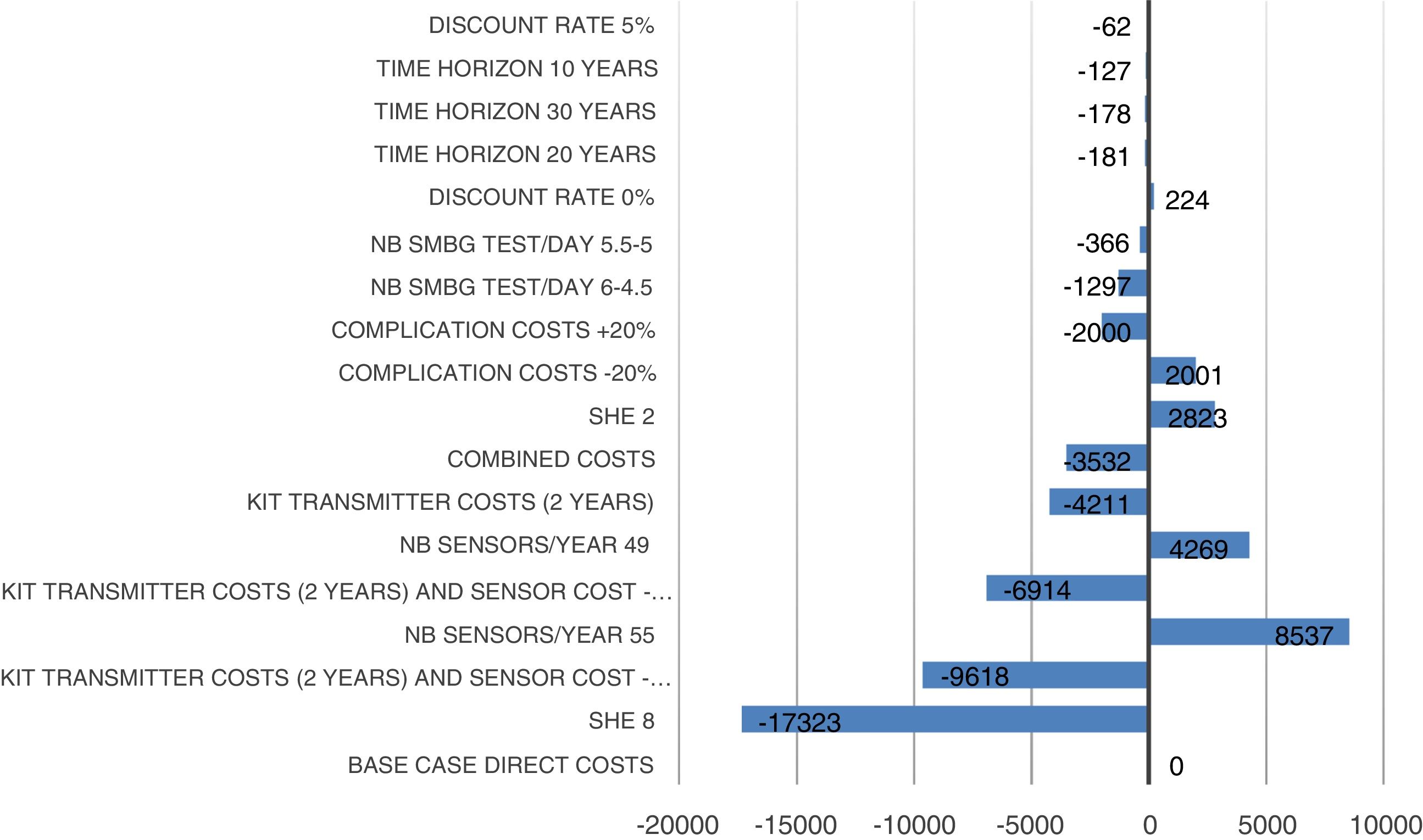
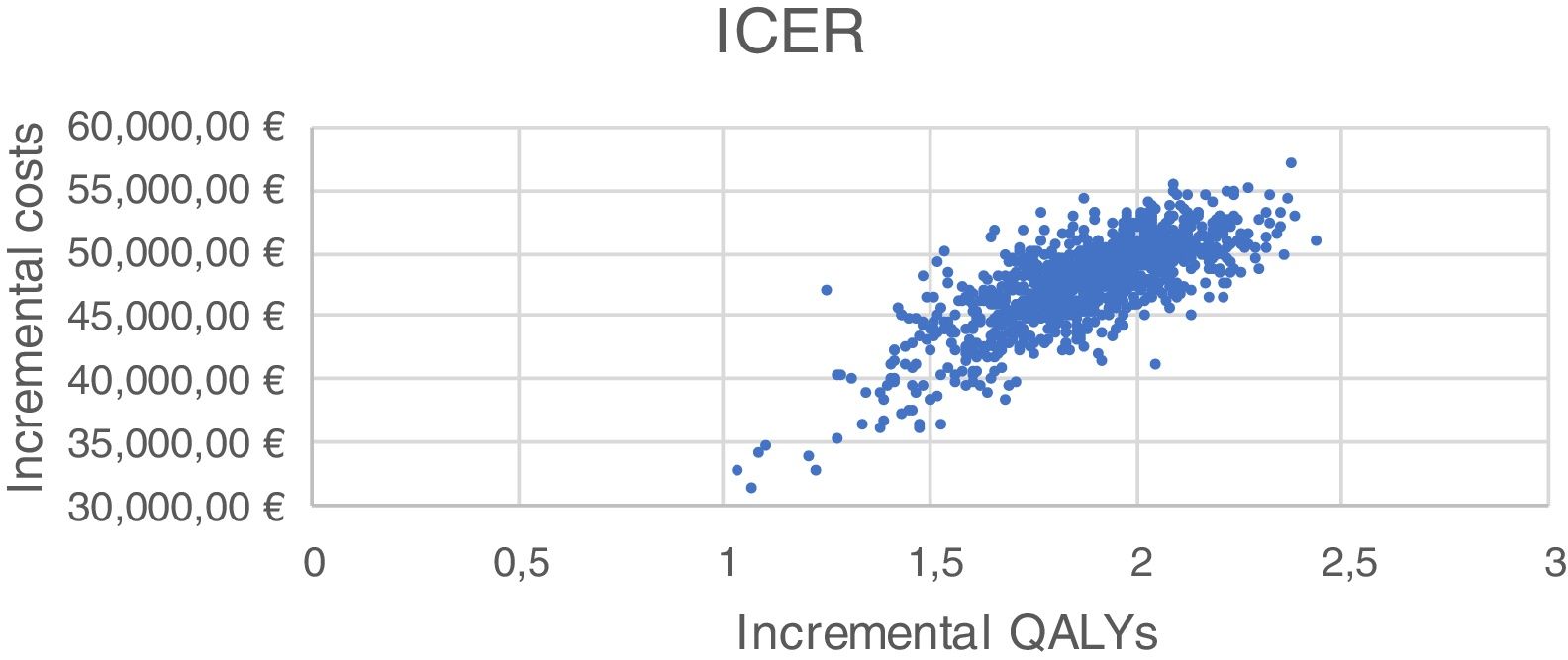
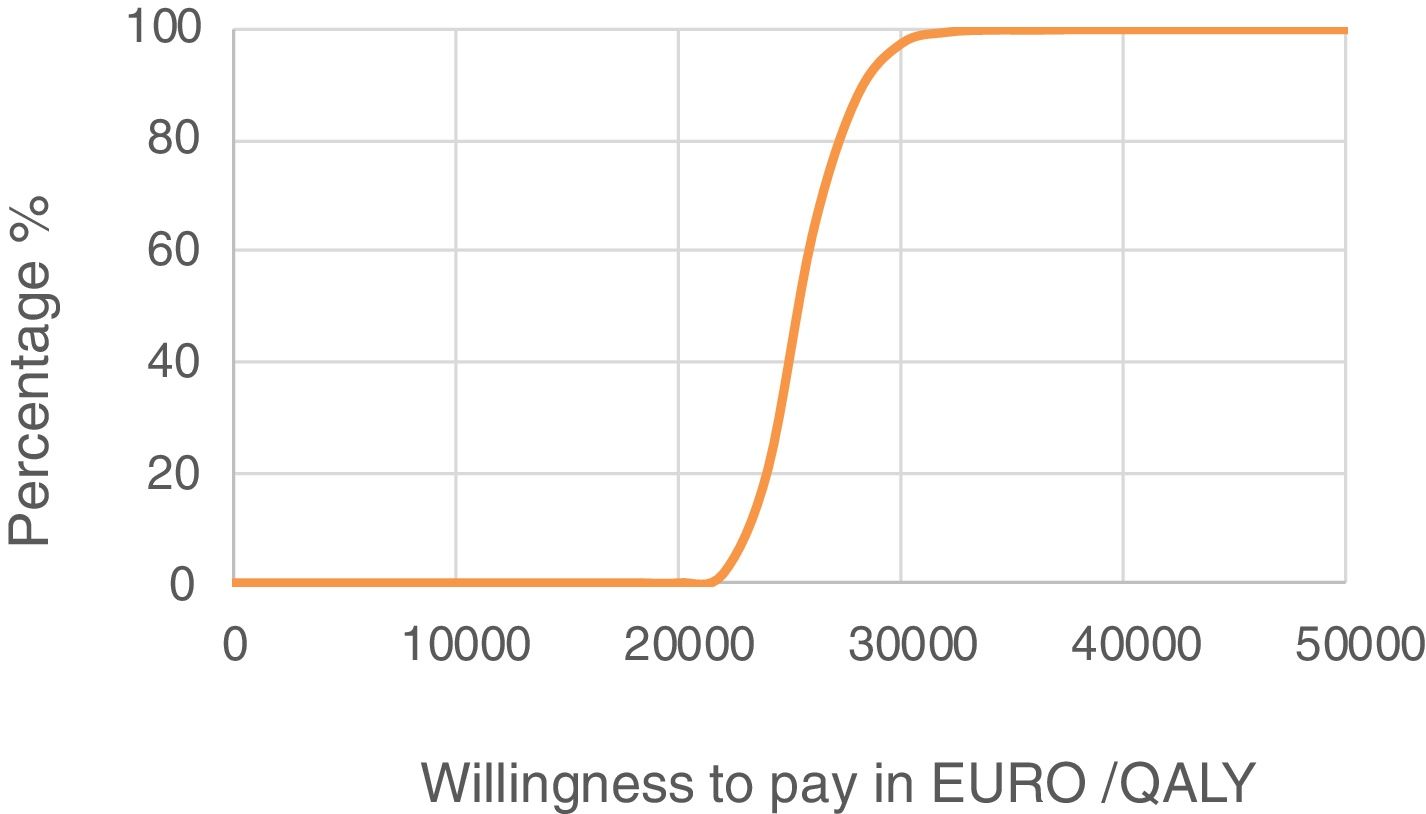
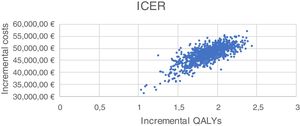
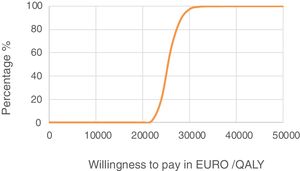
Sensitivity analysis resultsThe results of the various conducted SAs confirmed the robustness of the model. The following parameters modified in the deterministic SA showed the greatest impact on the base case results: change in the number of severe hypoglycaemia events, treatment adherence, and the simultaneous variation in the transmitter cost plus the transmitter lasting longer. Fig. 1 shows the results of the sensitivity analysis. The probabilistic SA was represented via the cost-effectiveness plane, with each point representing the ICER value of every 1000 Monte Carlo simulations conducted (Fig. 2). Of these 1000 simulations, 97.5% were below the €30,000 per QALY threshold26 (Fig. 3).
In this study, we performed analyses among adults in Spain with T1DM, who had a past history and a high risk of hypoglycaemia. This was the first study in this setting to evaluate the costs and healthcare outcomes of intensive treatment using SAP with low glucose suspend versus CSII therapy alone, which are two insulin therapies currently employed in clinical practice in Spain. Our analysis revealed that SAP with low glucose suspend was a cost-effective option for treatment of these patients relative to treatment with CSII alone, from both the NHS perspective and the societal perspective (even more cost-effective from the second one). This finding is in line with the results of previous economic evaluation studies conducted in different countries, including the United Kingdom,27 Sweden.28 and France,29 which also suggest that SAP with low glucose suspend can be considered a cost-effective treatment option compared to CSII alone, from both the societal perspective and the health system perspective in each country.
Compared to other European countries, in Spain CSII therapy is less commonly used—reportedly, in less than 5% of T1DM patients.2 This is mainly, although not exclusively, due to economic reasons based on the device purchase price. In this setting, the use of CGM is even more restricted. The Spanish Diabetes Association Working Group on Diabetes and Technology recently published a consensus document based on scientific evidence, presenting the main indications for CGM use and analysing specific cases that warrant consideration of public funding for real-time CGM plus CSII.6 These cases include patients with a high risk of recurrent severe, non-severe, or nocturnal hypoglycaemia, and patients with hypoglycaemia unawareness resulting in a disabling situation, since hypoglycaemia contributes to poor glycaemic control and commonly recurring hypoglycaemia may cause depression, fatigue, and anxiety, as well as fear of hypoglycaemia, which has a negative impact on the patient's quality of life. Moreover, prolonged hypoglycaemia over time can cause cognitive problems, convulsions, and even death. Additionally, hypoglycaemic events can constitute a major cost for the NHS, making prevention especially important.30 The use of CSII and SAP with low glucose suspend both enable improved glycaemic control in patients, and reduce the number and severity of hypoglycaemic events.3,7,8 Notably, SAP with low glucose suspend is reportedly more effective than CSII alone.7,8 Lastly, the recent consensus document mentions numerous countries that already fund RT-CGM in specific situations and criteria relevant for each setting are consistent across Europe.6
This study has several limitations, starting with the theoretical nature of modelling, which is sometimes not representative of standard clinical practice. Another limitation of this study is that the patient baseline characteristics and efficacy data were obtained from a cohort of Australian patients with hypoglycaemia unawareness and HbA1c levels of ≤8.5%.8 Additionally, the effectiveness data obtained from that study were collected after only 6 months of follow-up8 and data were obtained with a mean sensor wear of 70%. A sensor use below this figure may preclude cost-effectiveness results. These limitations may reduce the generalizability of the findings. Moreover, our present analysis did not consider the extra costs required to train patients in SAP therapy vs. CSII therapy. It must also be emphasized that the efficacy data used in our analysis was for the previous pump model (MiniMed™ Veo™) rather than the model currently available on the market (MiniMed™ 640G). The prior model does not include the option of predictive low glucose suspend due to hypoglycaemia. However, based on both clinical evidence published to date and clinical experience, it can be suggested that the results would be even more favourable if the predictive low glucose suspend feature had been included.3
ConclusionsThe outcomes of our present analysis reveal that SAP with low glucose suspend was associated with greater clinical benefits than CSII alone. Despite the greater costs of SAP, it is undoubtedly a cost-effective alternative26 for the treatment of T1DM patients at high risk of hypoglycaemia from both the Spanish NHS perspective and the societal perspective.
Conflicts of interestThis study was funded by Medtronic. I Conget, FJ Ampudia-Blasco, and P Martín-Vaquero disclose receipt of financial support from Medtronic for consulting services related to this study. I Elías, C Pineda, and M Álvarez are employees of Medtronic Ibérica, S.A. A Delbaere is an employee of Medtronic International Trading Sarl. S Roze served as a paid consultant for Heva Heor Sarl.
The authors hereby declare that this economic support did not interfere with the performance of this study.