Endocrinology and nutrition departments are among the departments that care for patients with high or very high cardiovascular risk, such as patients with diabetes mellitus (DM) and patients with familial hypercholesterolaemia (FH). DM is an independent risk factor for atherosclerotic cardiovascular disease.1 One of the main causative factors in these diseases is low-density lipoprotein cholesterol (LDL-C).2 On this point, controlled studies have provided robust scientific evidence on the importance of reducing LDL-C to decrease cardiovascular risk.3
The development and clinical availability of PCSK9 inhibitors have represented a breakthrough in the management of hypercholesterolaemia, as they help patients with high or very high vascular risk achieve the treatment goals recommended by the guidelines of various scientific associations.4,5 The FOURIER study found that evolocumab reduced LDL-C levels by more than 60% and also significantly reduced the number of cardiovascular events in patients with prior atherosclerotic cardiovascular, cardiac, cerebral or peripheral disease over a period of 2.2 years.6 The reduction was similar in patients with or without T2DM, but the absolute risk of reductions was higher in patients with T2DM.7 In addition, the ODYSSEY OUTCOMES study with alirocumab found a reduction in the relative risk of cardiovascular events in patients with recent coronary syndrome.8
The objective of this study was to report the clinical characteristics (LDL-C levels and history of DM and/or FH), demographic characteristics and changes over time in lipid levels (including total cholesterol, LDL-C, high-density lipoprotein cholesterol [HDL-C], triglycerides and non-HDL cholesterol) of the first patients to be treated with evolocumab in clinical practice in endocrinology and nutrition departments in Spain.
A multicentre, retrospective, observational study was designed with a consecutive review of all medical records for patients who were seen. Patients who started treatment with evolocumab in 21 endocrinology and nutrition departments (between February 2016 and April 2017) in Spain, as per routine clinical practice, who met the screening criteria (patients ≥18 years, with at least one dose of evolocumab in the above-mentioned period and with an LDL-C test) were included. The latest laboratory parameters within the 12 weeks prior to starting treatment with evolocumab were considered baseline values and clinical data were collected up to 12±4 weeks after starting evolocumab.
The study protocol was approved by the ethics committees and all the patients signed the informed consent form. A descriptive statistical analysis was performed for all the variables.
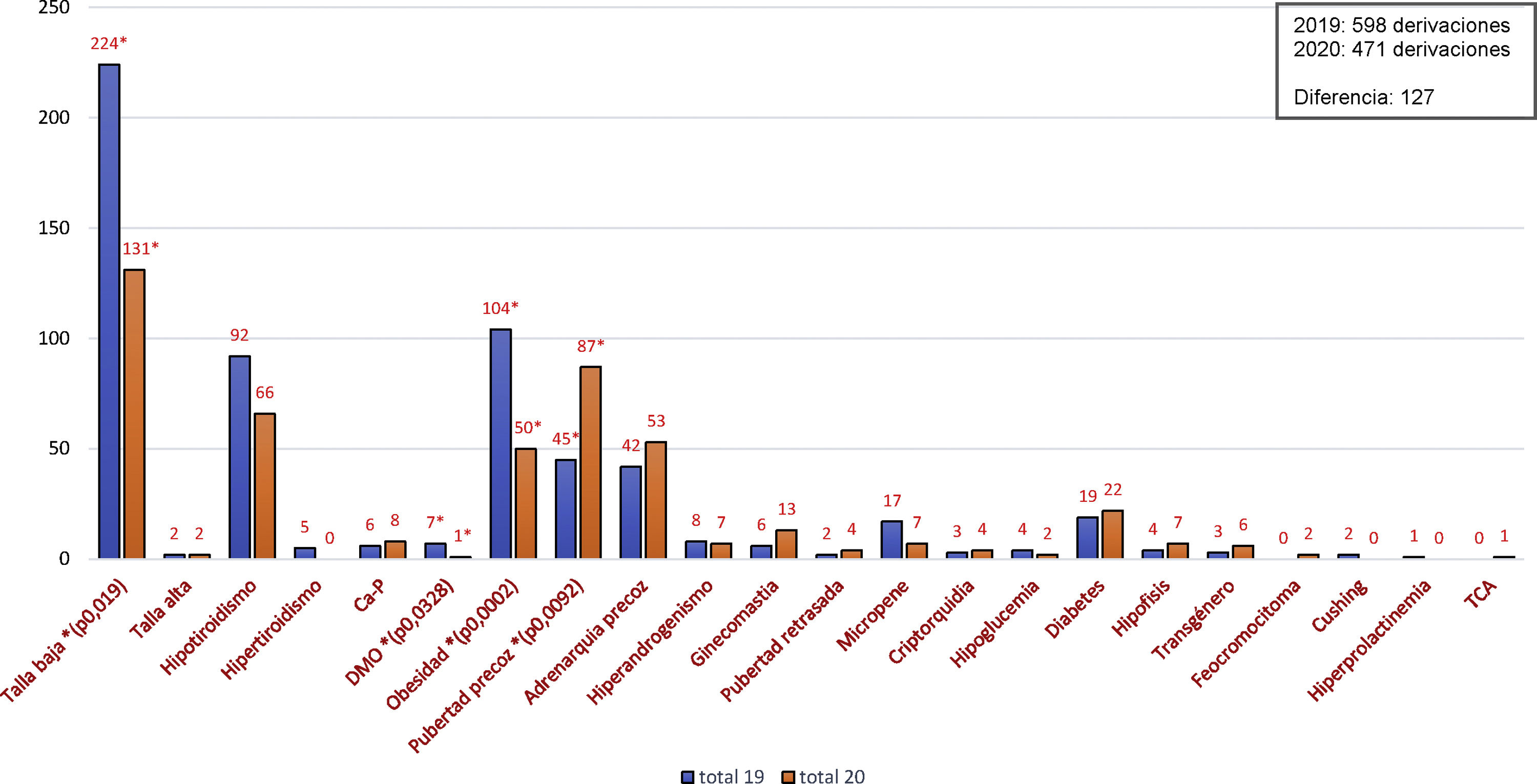
A total of 120 patients were included with a mean age of 57.0 years (standard deviation [SD] 11.5); 51.7% were women and 88.3% had FH (72.5% heterozygous FH). In total, 55.0% had at least one prior cardiovascular event, primarily coronary revascularisation (54.5%), carotid atherosclerotic disease (47.0%), angina (45.5%), myocardial infarction (39.4%), peripheral arterial disease (22.7%) or ischaemic stroke (15.2%), and 25.0% had DM (23.3% T2DM). The mean baseline LDL-C level was 180.2mg/dl (SD 62.2). After eight weeks of treatment, the mean LDL-C level fell to 83.0mg/dl (SD 63.8), representing a 54.3% reduction from baseline (95% CI: 62.8%, 45.8%), and 33.3% achieved levels <50mg/dl. Fig. 1 shows changes over time in the different lipid parameters at eight weeks. In patients with DM, the baseline mean LDL-C level was 176.9mg/dl (SD 79.4), with a 74.0% reduction, which enabled 66.7% of patients to achieve LDL-C levels <50mg/dl.
As they were the first patients to receive this therapy, it is not surprising that their baseline LDL-C level was unusually high (180mg/dl). However, despite such high starting levels, the patients achieved significant LDL-C reductions (−54.3%; 95% CI: −62.8%, −45.8%) after just eight weeks of treatment with evolocumab, which were greater in the subgroup with T2DM (−74.9%).
According to the new treatment goals set out in the recent guidelines, 83% of patients with DM would be under the target set for high-risk patients (<70mg/dl) and 67% would be under the new target set for very high-risk patients (<55mg/dl) after treatment with evolocumab. The results of this study were obtained in a population that did not achieve the treatment goals and could not be offered an alternative treatment. This was reflected in the fact that 36% of the patients were intolerant to statins, 50% were treated with ezetimibe, 47% were treated with high-intensity statins and 5.8% were treated with moderate-intensity statins.
It is important to highlight the high rate of short-term treatment adherence (98.2%), with a 96.7% rate of self-administration; just six patients stopped treatment with evolocumab.
The study had some limitations inherent to its design, with a limited observation period. It also had a small sample size, which limited evaluation by subgroups.
This first study of evolocumab in routine clinical practice in endocrinology and nutrition departments confirmed the results obtained in both randomised clinical trials and other routine clinical practice studies.9,10
In conclusion, it was seen that, in endocrinology and nutrition departments, the first patients prioritised for treatment with evolocumab under routine clinical practice conditions were patients with FH who, in more than half of cases, had already experienced one or more prior vascular events. Of the patients included, 23% had T2DM. In this initial phase of availability of the therapy, the use of evolocumab was adjusted to the established regimens for the use of inhibitors, but with initial LDL-C levels significantly higher than the recommended thresholds, perhaps because evolocumab was administered in patients with no other treatment options. Despite this, and consistent with the results of prior clinical trials, LDL-C levels fell significantly (54% compared to baseline and 74% in the subgroup of patients with DM) very quickly: eight weeks after starting treatment with evolocumab.