The prevalence of neonatal hyperthyroidism (HN) due to maternal Graves Disease (GD) ranges from 0.1 to 2.7%. It may occur in pregnant women with the following: active DG, after treatment with radioactive iodine, anti-thyroid or thyroidectomy or with a previous child with hyperthyroidism. The aim of our observational study was to evaluate the follow-up of infants born to mothers with GD at a Tertiary Hospital prior to the implementation of a follow-up protocol.
MethodsThis was a retrospective observational study using data from the medical records of mothers with a diagnosis of GD and their newborns from January 2013 until May 2018. Newborns were divided into two groups: high and low risk for NH according to maternal TRAb, third trimester treatment and signs of fetal hyperthyroidism.
ResultsWe identified 31 newborns, 58% female; 87% high risk. In none of the newborns was umbilical cord blood collected. In the high risk group, 22% had thyroid function evaluation at day-1, one patient presented with hyperthyroidism and 82% were asymptomatic. Considering the cases with an insufficient blood sample for analysis, 9 consultations would have been spared. We found a significant delay in obtaining the high-risk group results which would have spared 10 appointments. A positive correlation was found between age at outpatient clinic discharge and the number of appointments and the maternal TRAb titer.
ConclusionThe correct surveillance of pregnancy and newborns with identification of those at high risk is essential to avoid unnecessary consultations and blood analyses that increase parental anxiety and hospital costs. Consequently, a multidisciplinary protocol was created to standardize the approach.
La prevalencia del hipertiroidismo neonatal (HN) debido a la enfermedad de Graves (EG) materna varía del 0,1 al 2,7%. Puede ocurrir en mujeres embarazadas con EG activa, después del tratamiento con yodo radioactivo, antitiroideo o tiroidectomía, o en las que ya tienen un hijo con hipertiroidismo. El objetivo de nuestro estudio observacional fue evaluar el seguimiento de los bebés nacidos de madres con EG en un hospital terciario previamente a la implementación del protocolo de seguimiento.
MétodosEstudio observacional retrospectivo, utilizando datos de los registros médicos de madres con diagnóstico de EG y sus recién nacidos desde enero del 2013 hasta mayo del 2018. Estos se dividieron en dos grupos: alto y bajo riesgo de HN según thyrotropin stimulating receptor antibodies (TRAb) materno, tratamiento del tercer trimestre y signos de hipertiroidismo fetal.
ResultadosIdentificamos 31 recién nacidos, 58% niñas; 87,1% de alto riesgo. Ninguno de ellos recolectó sangre del cordón umbilical. En el grupo de alto riesgo, 22% tuvo evaluación de la función tiroidea en el día uno, un paciente presentó hipertiroidismo y 81,5% era asintomático. Considerando los casos en los que la muestra de sangre era insuficiente para el análisis, se habrían ahorrado nueve consultas. Encontramos un retraso significativo en la obtención de los resultados del grupo de alto riesgo, que habría ahorrado 10 citas. Se identificó una correlación positiva entre la edad al alta de la consulta externa y el número de citas y el título de TRAb materno.
ConclusiónLa vigilancia correcta del embarazo y de los recién nacidos con identificación de las personas de alto riesgo es esencial para evitar consultas y análisis innecesarios que aumentan la ansiedad de los padres y los costos hospitalarios. Fue posible elaborar un protocolo multidisciplinario para estandarizar el enfoque.
Maternal Graves’ Disease (GD) affects 0.1–2.7% of pregnancies. Studies estimate that 2% of these newborns present with neonatal GD, which refers to a state of congenital transient hyperthyroidism.1 This pathology is associated with mortality rates up to 25% and with immediate and long-term morbidities if not timely diagnosed and treated.2–5
Thyroid hormones play an essential role in the development of the fetus. Maternal state of hyperthyroidism or hypothyroidism, even subclinical, during the first half of gestation is a risk factor for adverse pregnancy outcomes such as miscarriage, preterm delivery, and motor and cognitive impairment in early childhood.1–3,6 After the 20th week of gestation transplacental passage of maternal thyrotropin stimulating receptor antibodies (TRAb) is possible and stimulation of fetal TSH receptors can occur.2,3
Well known risk factors for fetal and neonatal hyperthyroidism are: high level of maternally transmitted TRAb, maternal treatment with anti-thyroid drugs (ATD) during pregnancy and previous iodine therapy.1,2 These require a close and challenging follow-up during pregnancy, balancing between the risk of fetal hypothyroidism (excessive placental transfer of maternal ATDs, which is dose-dependent) and the risk of fetal hyperthyroidism (placental transfer of maternal TRAb).2,3
Concerning ATD treatment during pregnancy, propylthiouracil (PTU) is preferred in the first trimester as it is less teratogenic and acts faster by blocking the peripheral conversion of free Thyroxine (FT4) to free Triiodothyronine (FT3); however, the risk of hepatotoxicity by the second trimester imposes a shift to methimazole.3,5,7,8
Since fetal thyroid dysfunction precedes neonatal hyperthyroidism, fetal thyroid ultrasound is crucial for early detection and prediction of symptoms. Fetal ultrasound monitoring should be performed after the 18th to 22nd weeks of gestation in case of current or past history of GD (actual maternal ATD therapy, maternal GD with high TRAb titer, whether hyperthyroidism is active or even when ablative therapy has been performed).2,9,10 Ultrasonographic findings in case of fetal hyperthyroidism include: goiter, tachycardia, cardiomegaly, hydrops, advanced bone maturation, craniosynostosis and growth retardation. Fetal hypothyroidism can present with goiter, bradycardia, delayed bone maturation and growth retardation. Fetal goiter can be a cause of fetal head hyperextension, respiratory and swallowing obstruction and polyhydramnios.2,4,9,10
Since it is required a multidisciplinary approach to those infants and their mothers we have elaborated a protocol in order to standardize this approach. This protocol was implemented in 2018 and according to actual international guidelines a close follow-up during pregnancy is provided for women with current or past history of GD.1,10–12
The aim of our observational study was to evaluate the follow-up of infants born to mothers with GD in a Tertiary Hospital previously the implementation of the follow-up protocol.
MethodsStudy design and patientsUntil 2016, there was a lack of international guidelines to guide the follow-up of pregnant women with GD and their newborns, so there was a great heterogeneity in clinical practice, as occurred in our hospital. In 2016, a state-of-the-art review article published by American Academy of Pediatrics defined the principles of management of neonates born to mothers with GD.13
We performed a retrospective observational study using data from the medical records of mothers with diagnosis of GD and their newborns in the past five years (from January 2013 until May 2018) in Braga's Hospital. Based on the international guidelines from 2016, we collected the data from clinical records from mothers with GD and their newborns, analyzed the current state of the art to recognize what we needed to update in order to improve the medical care.
The Pathology Laboratory provided all the results from the selected patients. Exclusion criteria were: women with other thyroid pathologies, women whose pregnancy was followed in other centers without any possibility to access their clinical reports and those whose babies were born in other health facilities.
A total of 31 newborns and their respective mothers were included.
Data collection was conducted according to the Hospital's Ethical Committee proceedings and the Declaration of Helsinki.
Clinical data of the motherData collected from clinical records were: age at delivery, date of delivery, type of delivery, year and time of diagnosis in relation with pregnancy (preconception or during pregnancy), parity, previous miscarriage, pregnancy complications, presence of gestational diabetes, presence of auto-immune diseases, thyroid function and TRAb titer at the preconception period and during pregnancy (first, second and third trimester), presence of other positive antibodies, results of fetal ultrasonography and GD treatment before and during pregnancy.
Clinical data of the newbornData collected from clinical records were: gender, gestational age, birth anthropometry and respective z-scores, thyroid function and TRAb titer in umbilical cord sampling, day 2–5, day 10–14 and in first, second and third months of age; also we verified the need for drug therapy, clinical surveillance during hospital stay and after discharge.
Newborns were classified according to their birth weight on Fenton Curves as: adequate for gestational age (AGA – between 10th and 90th percentile), small for gestational age (SGA – under 10th percentile) and large for gestational age (LGA – over 90th percentile).
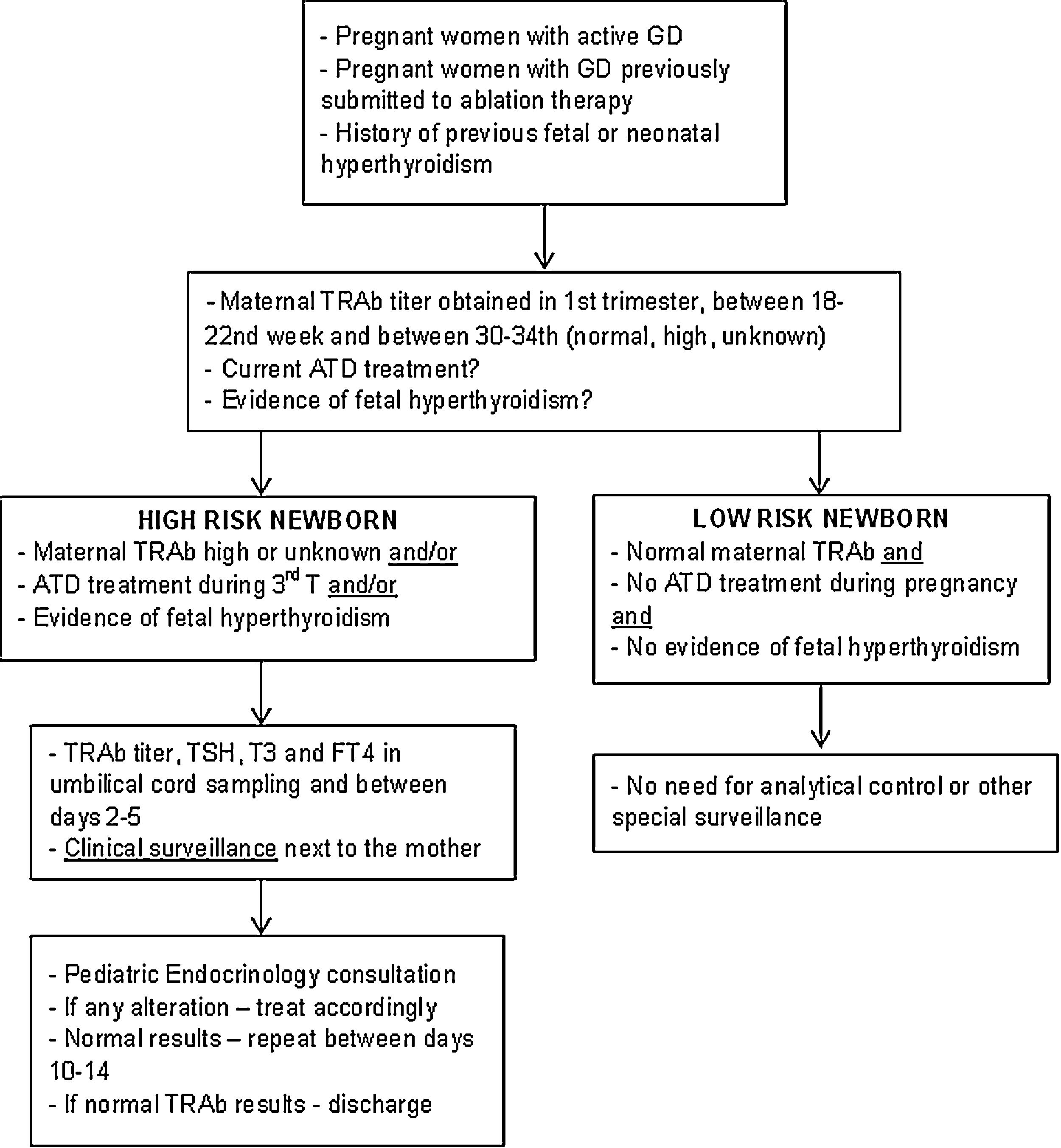
Newborns at high risk for neonatal hyperthyroidism were identified if their mother's TRAb titer was high or unknown, if their mother received treatment with ATD during the third trimester or if there was evidence of fetal hyperthyroidism. If mother's TRAb titer was normal, pregnancy occurred without treatment and in the absence of clinical manifestations of fetal hyperthyroidism, newborns were classified as low risk.
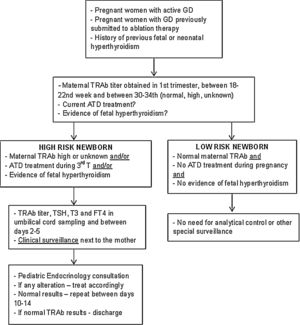
Description of surveillance protocolBased on the data collected in this study and following the international guidelines, a multidisciplinary protocol was elaborated in our hospital for the follow-up of infants born from a mother with GD – Fig. 1. It involved professionals from different areas: Pediatric Endocrinology, Endocrinology, Obstetrics and Neonatology. It was established that ideally, before conception, women should achieve a normal thyroid function and treatment options be discussed.
Biochemical dataTRAbs were measured using immunoenzimatic assay, detection limit was 1UI/L.13 The peak TRAb titer was defined as the patient's maximum value during pregnancy.
Serum FT4 and T3 were measured by chemiluminescence technique and TSH by immunochemiluminometric assay.
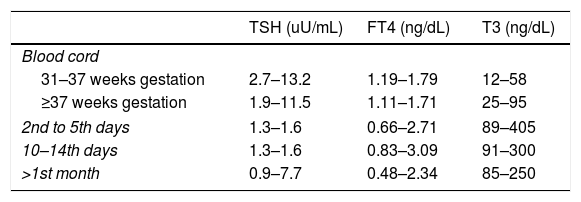
Mother's thyroid hormones levels considered in normal range were: serum T3 80–185ng/dL, FT4 0.82–1.77ng/dL and TSH 0.5–4.8uU/mL.13 Newborn and infants normal thyroid levels are described in Table 1.
Thyroid hormone levels in newborns and infants according to age.
| TSH (uU/mL) | FT4 (ng/dL) | T3 (ng/dL) | |
|---|---|---|---|
| Blood cord | |||
| 31–37 weeks gestation | 2.7–13.2 | 1.19–1.79 | 12–58 |
| ≥37 weeks gestation | 1.9–11.5 | 1.11–1.71 | 25–95 |
| 2nd to 5th days | 1.3–1.6 | 0.66–2.71 | 89–405 |
| 10–14th days | 1.3–1.6 | 0.83–3.09 | 91–300 |
| >1st month | 0.9–7.7 | 0.48–2.34 | 85–250 |
Categorical variables were compared using x2 tests and continuous variables using t student test. The demographic and clinical characteristics were summarized as counts and percentages, mean±standard deviation (SD) for normally distributed continuous variables, and median and interquartile range for other continuous variables. A p-value of <0.05 was considered statistically significant.
ResultsCharacterization of the mothersWe have identified 31 pregnancies in 30 mothers.
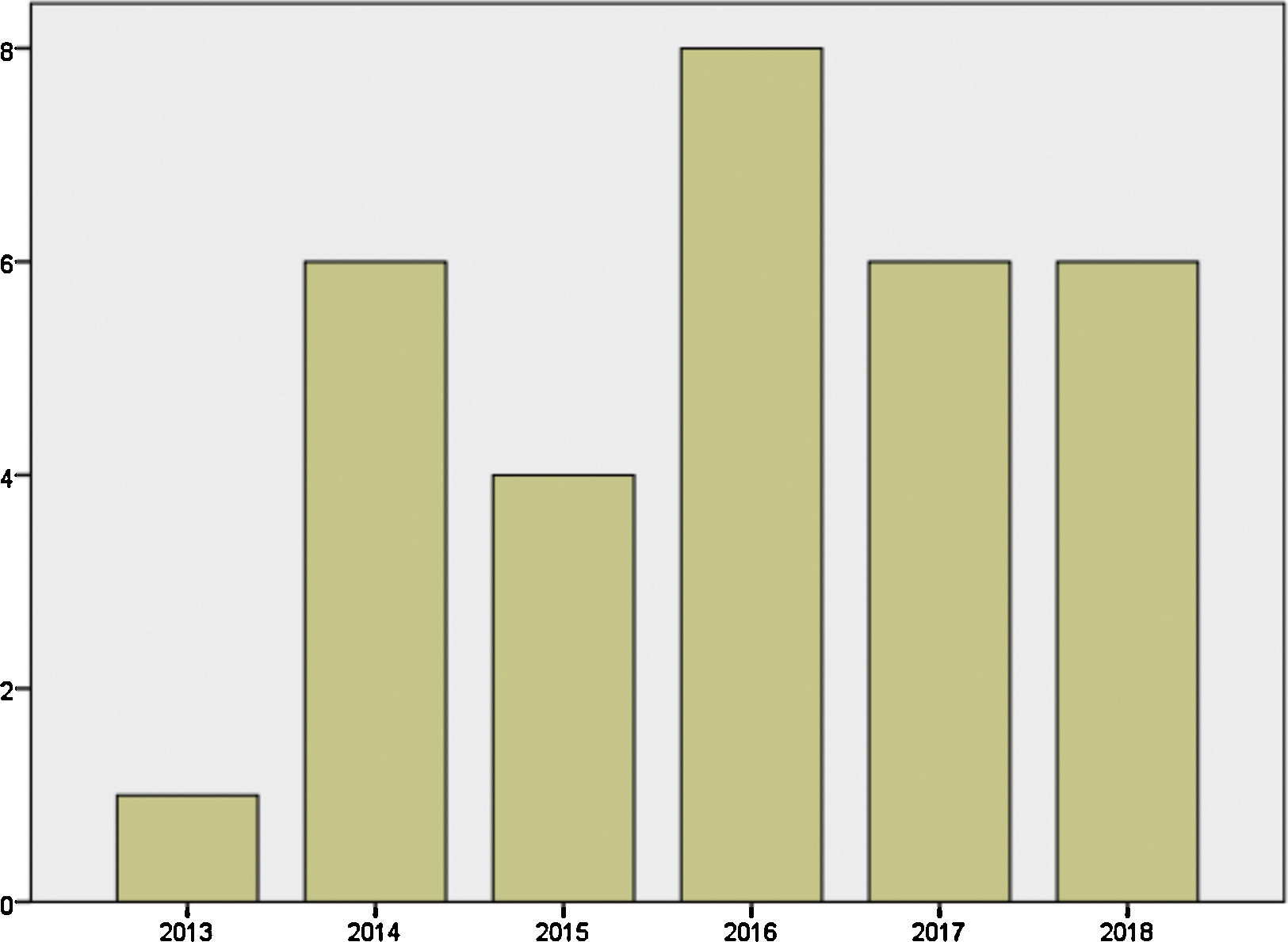
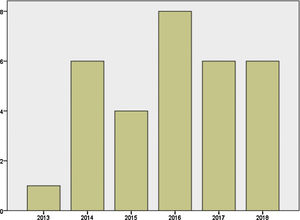
Mother's mean age at delivery was 32.29±4.44 years (range 23.0–44.0); median parity until current pregnancy was 2 (range 1–3). GD was diagnosed before pregnancy in 87.09% of the pregnancies (n=27) and during pregnancy in 9.67% (n=3), one in each trimester. Graph 1 shows distribution of deliveries through the past five years.
A past medical history of another auto-immune disease was present in 10% (n=3) of the mothers: 2 had type 1 diabetes and the other had chronic discoid lupus. Ten mothers (32.2%) developed gestational diabetes during pregnancy. Twenty six percent (n=7) of those with preconceptional diagnosis of GD had a previous history of miscarriage and 18.5% (n=5) presented with complications during current pregnancy such as threatened miscarriage in first trimester (n=2), vesicular lithiasis (n=1) and intrauterine growth restriction (IUGR) (n=2).
Preconception evaluation and treatmentPreconceptional thyroid function was available in 17 subjects; 41.93% (n=13) of them were euthyroid, 9.67% (n=3) presented with hyperthyroidism and one with hypothyroidism. Only one woman had a preconceptional TRAb titer. Before pregnancy, 38.71% (n=12) of women were under medical treatment (19.35% (n=6) with methimazole; 9.67% (– n=3) with PTU and 9.67% (– n=3) with levothyroxine) and 41.9% (n=13) had no treatment. The three women under levothyroxine were previously submitted to 131-iodine treatment.
Analytic evaluation and treatment during PregnancyThere was no significant variation between biochemical values through trimesters (p>0.005); 67.77% (n=21) of women had no other positive antibody, 25.81% (n=8) were positive for Thyroid Peroxidase Antibodies (Anti-TPO) and one patient (3.22%) was positive for both Anti-TPO and Thyroglobulin antibodies. Nineteen women (61.29%) presented TRAb titer three times above the normal value.
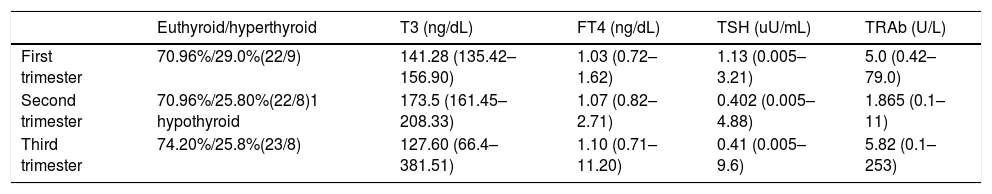
Table 2 describes mean values of FT4, T3, TSH and TRAb in each pregnancy trimester. In Table 3 it is summarized medical treatment for each trimester.
Mean values of FT4, T3, TSH and TRAb values in each pregnancy trimester.
| Euthyroid/hyperthyroid | T3 (ng/dL) | FT4 (ng/dL) | TSH (uU/mL) | TRAb (U/L) | |
|---|---|---|---|---|---|
| First trimester | 70.96%/29.0%(22/9) | 141.28 (135.42–156.90) | 1.03 (0.72–1.62) | 1.13 (0.005–3.21) | 5.0 (0.42–79.0) |
| Second trimester | 70.96%/25.80%(22/8)1 hypothyroid | 173.5 (161.45–208.33) | 1.07 (0.82–2.71) | 0.402 (0.005–4.88) | 1.865 (0.1–11) |
| Third trimester | 74.20%/25.8%(23/8) | 127.60 (66.4–381.51) | 1.10 (0.71–11.20) | 0.41 (0.005–9.6) | 5.82 (0.1–253) |
Medical treatment in each pregnancy trimester.
| No treatment | Methimazole | Propylthiouracil | Levothyroxine | |
|---|---|---|---|---|
| First trimester | 51.61% (n=16) | 19.35% (n=6) | 12.90% (n=4) | 16.13% (n=5) |
| Second trimester | 51.61% (n=16) | 9.67% (n=3) | 22.58% (n=7) | 16.13% (n=5) |
| Third trimester | 51.61% (n=16) | 19.35% (n=6) | 12.90% (n=4) | 16.13% (n=5) |
Ultrasonography pregnancy schedule was the same to all pregnant women despite their TRAb titer. The only alteration registered in ultrasonography was IUGR (n=2).
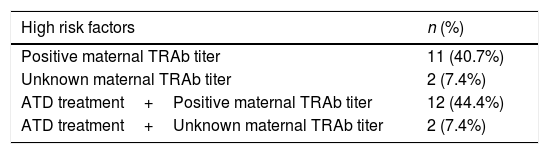
Characterization of the newbornsFrom a total of 31 newborns, 58.06% girls (n=18). Twenty seven newborns (87.09%) were considered of high risk for neonatal hyperthyroidism (Table 4). There were no statistical differences between high and low risk group considering newborn gender (p=0.462).
Distribution of high risk factors for neonatal hyperthyroidism. TRAb – thyrotropin stimulating receptor antibodies; ATD – anti-thyroid drugs.
| High risk factors | n (%) |
|---|---|
| Positive maternal TRAb titer | 11 (40.7%) |
| Unknown maternal TRAb titer | 2 (7.4%) |
| ATD treatment+Positive maternal TRAb titer | 12 (44.4%) |
| ATD treatment+Unknown maternal TRAb titer | 2 (7.4%) |
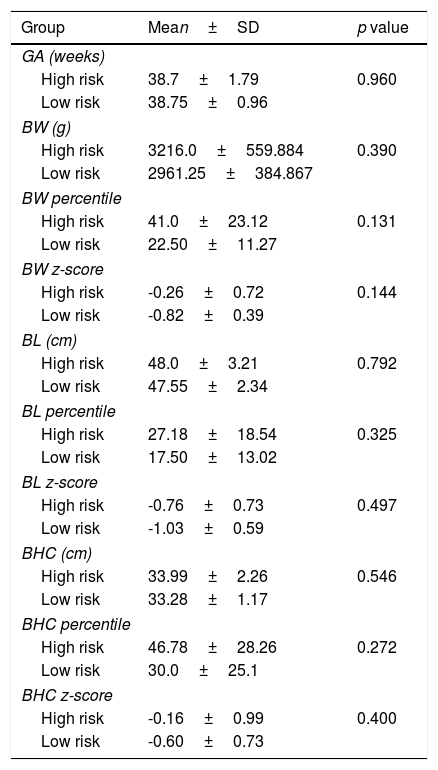
Both groups were similar according to gestational age, birth weight, length and head circumference (Table 5). Considering Fenton curves, in the high risk group there were 2 SGA, 1 LGA and 24 AGA and in the low risk group all newborns were AGA (p=0.782). In the high risk group there were 15 eutocic deliveries, 8 caesarians and 4 instrumented deliveries; in the low risk group all births were eutocic; there were no differences between both groups (p=0.407).
Characterization of high and low risk groups according to gestational age (GA), birth weight (BW), birth length (BL) and birth head circumference (BHC).
| Group | Mean±SD | p value |
|---|---|---|
| GA (weeks) | ||
| High risk | 38.7±1.79 | 0.960 |
| Low risk | 38.75±0.96 | |
| BW (g) | ||
| High risk | 3216.0±559.884 | 0.390 |
| Low risk | 2961.25±384.867 | |
| BW percentile | ||
| High risk | 41.0±23.12 | 0.131 |
| Low risk | 22.50±11.27 | |
| BW z-score | ||
| High risk | -0.26±0.72 | 0.144 |
| Low risk | -0.82±0.39 | |
| BL (cm) | ||
| High risk | 48.0±3.21 | 0.792 |
| Low risk | 47.55±2.34 | |
| BL percentile | ||
| High risk | 27.18±18.54 | 0.325 |
| Low risk | 17.50±13.02 | |
| BL z-score | ||
| High risk | -0.76±0.73 | 0.497 |
| Low risk | -1.03±0.59 | |
| BHC (cm) | ||
| High risk | 33.99±2.26 | 0.546 |
| Low risk | 33.28±1.17 | |
| BHC percentile | ||
| High risk | 46.78±28.26 | 0.272 |
| Low risk | 30.0±25.1 | |
| BHC z-score | ||
| High risk | -0.16±0.99 | 0.400 |
| Low risk | -0.60±0.73 | |
No newborn had overt clinical manifestations of thyroid dysfunction. There was no relation between maternal TRAb titer during pregnancy and mean gestational age and birth anthropometry.
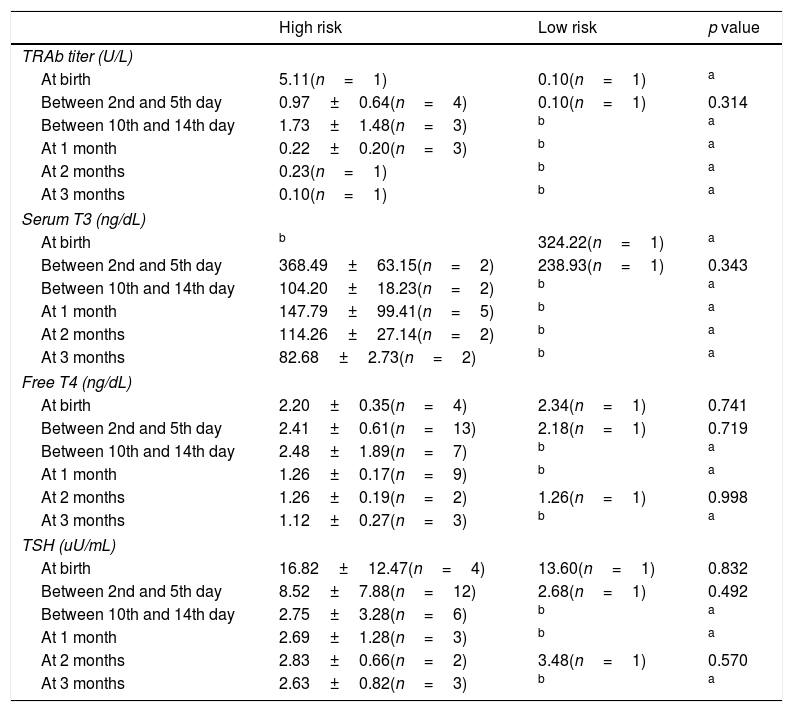
Clinical and analytic surveillance of newbornsConsidering our recent Unit's protocol for high risk newborns and according to international guidelines, 41.93% (n=13) had no analytic evaluation and 2 infants in the low risk group did. There were no differences between high and low risk group considering the performance of unnecessary analytic control (p=0.945).
At birth, umbilical cord sample was not performed in any patient. 22.22% (n=6) of the high risk newborns had an evaluation of thyroid function during the first day of life; in two of them, the blood sample was not sufficient for analysis and one patient presented hyperthyroidism. TRAb titers at birth were available in two patients.
Between the 2nd and the 5th day of life, 13 high risk newborns were submitted to blood analysis and hyperthyroidism was detected in one patient. One low risk patient was analyzed and was euthyroid. Between the 10th and the 14th day of life no low risk patient was tested and among the 10 high risk newborns evaluated two were hyperthyroid.
There was no correlation between thyroid function at birth and thyroid function between the 2nd and 5th day of life and the 10–14th day of life (p>0.005).
In high risk group, 40.74% (n=11) had TRAb titer results and in low risk group 50.00% (n=2) had at least one TRAb titer result. Three high risk patients (11.11%) were tested only for thyroid function and they were euthyroid. The first TRAb titer determination was performed later in the high risk group (11.64±12.36 vs 1.0±1.41 days, maximum in the 30th day of life – p=0.019).
Considering the delay to make available TRAb titer results it would be possible to spare a total of 7 neonatology consultations and 3 pediatric endocrinology consultations. We found 7 cases of insufficient blood sample for TRAb titer determination at birth and 6 of them were found to be negative later on. This would spare a total of 2 pediatric endocrinology consultations and 7 neonatology consultations.
Analytic evaluation of both groups is described in Table 6.
Mean analytic results of thyroid function at birth, between 2nd and 5th day, between 10th and 14th day of life, after 1, 2 and 3 months.
| High risk | Low risk | p value | |
|---|---|---|---|
| TRAb titer (U/L) | |||
| At birth | 5.11(n=1) | 0.10(n=1) | a |
| Between 2nd and 5th day | 0.97±0.64(n=4) | 0.10(n=1) | 0.314 |
| Between 10th and 14th day | 1.73±1.48(n=3) | b | a |
| At 1 month | 0.22±0.20(n=3) | b | a |
| At 2 months | 0.23(n=1) | b | a |
| At 3 months | 0.10(n=1) | b | a |
| Serum T3 (ng/dL) | |||
| At birth | b | 324.22(n=1) | a |
| Between 2nd and 5th day | 368.49±63.15(n=2) | 238.93(n=1) | 0.343 |
| Between 10th and 14th day | 104.20±18.23(n=2) | b | a |
| At 1 month | 147.79±99.41(n=5) | b | a |
| At 2 months | 114.26±27.14(n=2) | b | a |
| At 3 months | 82.68±2.73(n=2) | b | a |
| Free T4 (ng/dL) | |||
| At birth | 2.20±0.35(n=4) | 2.34(n=1) | 0.741 |
| Between 2nd and 5th day | 2.41±0.61(n=13) | 2.18(n=1) | 0.719 |
| Between 10th and 14th day | 2.48±1.89(n=7) | b | a |
| At 1 month | 1.26±0.17(n=9) | b | a |
| At 2 months | 1.26±0.19(n=2) | 1.26(n=1) | 0.998 |
| At 3 months | 1.12±0.27(n=3) | b | a |
| TSH (uU/mL) | |||
| At birth | 16.82±12.47(n=4) | 13.60(n=1) | 0.832 |
| Between 2nd and 5th day | 8.52±7.88(n=12) | 2.68(n=1) | 0.492 |
| Between 10th and 14th day | 2.75±3.28(n=6) | b | a |
| At 1 month | 2.69±1.28(n=3) | b | a |
| At 2 months | 2.83±0.66(n=2) | 3.48(n=1) | 0.570 |
| At 3 months | 2.63±0.82(n=3) | b | a |
TRAb, thyrotropin stimulating receptor.
Two high risk newborns started medication with methimazole. One was under methimazole 0.4mg/kg from day-2 until day-36 of life and was discharged from pediatric endocrinology consultation at 8 months-old. The other patient started methimazole 0.25mg/kg at 12th day of life until 90th day and was discharged at 9 months-old. We found no relationship between the presence of maternal TRAb titer and the need for medication or the development of hyperthyroidism in the newborn. Seven patients were submitted to echocardiogram (six high risk patients and one low risk) and no abnormalities were detected.
Five high risk patients were monitored in the Neonatology Intensive Care Unit (NICU) and the other 22 stayed at bedside. Mean duration of hospitalization was similar for both groups (high risk 6.11±9.84 days vs low risk 2.75±0.50 days – p=0506). Comparing patients with insufficient blood sample for TRAb titer determination with the other ones, mean duration of hospitalization was similar between both groups (9.14±14.95 vs 4.67±6.94 days – p=0.266).
Twelve (44.40%) high risk patients were discharged from hospital without any consultation, 10 of them without analytic results; 40.7% (n=11) were discharged with a scheduled Neonatology consultation and 4 patients with a Neonatology and Pediatric Endocrinology consultation. Three low risk patients were discharged with a scheduled Neonatology consultation. The mean number of consultations was significantly higher in high risk patients (3.42±2.60 vs 1.0±0 – p=0.008) and this patients were discharged significantly later (3.42±3.13 vs 0.58 months – p=0.0001).
We found a positive correlation between age at consultation discharge and number of consultations and maternal TRAb titer (r=0.821, p=0.007 and r=0.671, p=0.017). We found no correlation between time until first TRAb titer results and time of discharge.
DiscussionAlthough the approach to children born to GD mothers has some controversial issues, it is well established that those infants require a correct identification and close observation and evaluation after birth. In our Hospital the multidisciplinary follow-up protocol was implemented since March 2018 in order to unify those infant's approach and one year later a national protocol was elaborated by the Portuguese Pediatric Endocrine Society.14
Several studies state the importance of maternal thyroid hormones in the development of the fetus and the placenta specially during the first and second trimesters when the fetus is totally dependent on the maternal hormonal supply.6 The American Thyroid Society (ATA)7 Guidelines strongly recommend pre-pregnancy counseling for women with GD and that euthyroid state should be achieved prior to conception suggesting that they should be offered definitive (131-I or surgery) or medical therapy in order to control disease.2,7 Despite those recommendations, in our cohort we have observed that 12.9% (n=4) women had some thyroid dysfunction prior to conception and we believe these numbers are underestimated since we only had access to 54.84% (n=17) of preconception thyroid function. We realized that irregular attendance to consultations and poor adherence to therapies verified in electronic registries can justify these numbers. We verified an increased percentage of euthyroid mothers during second and third trimesters (41.9%, 61.3% and 67.7% in the first, second and third trimester, respectively) which we believe results from the more frequent analytical and clinical controls that are required along the pregnancy. Also, it is well known that pregnancy can have positive effects in women who have autoimmune diseases.15 In GD, although during the first trimester women can experience worsening of symptoms because of the stimulation of the thyroid gland by human chorionic gonadotropin; as the pregnancy progresses, changes in immunologic responses can lead to improve symptomatology.5
Concerning GD treatment during pregnancy we verified that the recommended shift from PTU to methimazole was done mainly during the second trimester, when the majority of hospital surveillance was initiated. Although methimazole has been linked to poor embryological development, congenital head skin defects, choanal atresia, tracheal-esophageal fistula and nipple hypoplasia, with increased risk during first trimester, no one of our infants presented congenital defects.16
Our cohort showed a higher percentage of previous still births (25.0%) in mothers with GD when compared to other series. In current pregnancy the complications rate was 17.9% with 2 cases of threatened miscarriage and 2 cases of IUGR.17
An interesting result was the high percentage of gestational diabetes found (32.0%); its relation with GD has been reported in other studies proposing multiple mechanisms by which hyperthyroidism may worsen glucose intolerance.18
We also verified that thyroid function at first day of life does not correlates with hormone levels by the 2nd–5th day and by the 10–14th day of life; thus the diagnosis of hyperthyroidism cannot be established by a single measurement, requiring a close analytical and clinical follow-up.1 None of our patients that started medication presented thyroid dysfunction during the first days of life.
Blood cord analysis is proposed by several studies as an effective test to predict neonatal hyperthyroidism.1,3,4,6,16 However these results must be carefully evaluated since TSH and thyroid hormones levels in blood cord reflect fetal thyroid function and do not predict neonatal hyperthyroidism by themselves, they must be analyzed together with seriated serum values.3,4,9,15 In contrast, higher TRAb titers in blood cord are a better risk predictor of neonatal hyperthyroidism. Several studies proved that newborns with negative TRAb titer in blood cord samples did not developed hyperthyroidism, thus confirming its high sensitivity rate (100%) despite very low specificity (35.0%).4,6,19 In our study we failed to confirm these relation because no one of our newborns had these results, either because the blood sample was not collected during labor or because there was no sufficient blood in one case. With the implementation of the new protocol we hope we can overcome these difficulties and spare unnecessary consultations and blood analyses.
According to the most recent guidelines, our protocol states the importance of TRAb titer evaluation during pregnancy at 20–24 weeks as it is a recognized predictor of fetal or neonatal thyrotoxicosis in pregnant women with GD with an estimated predictive value of 42.0%.20,21 In our cohort maternal median TRAb titers increased from the second to the third trimester of pregnancy reaching values that were three times above the normal upper limit (5.82U/L). This fact affected 61.2% (n=19) of mothers and didn’t require any change in the usual ultrasonographic pregnancy schedule as recommended by the Unit's protocol. Although we haven’t found any relation between maternal TRAb titer and newborn's need for medication or development of hyperthyroidism all of them presented maternal TRAb titers three times above the reference value. We believe that if we had less missing values in maternal TRAb titer determinations the results would be concordant with the literature. According to other studies, whenever blood cord sample is not available, the positivity of maternal TRAb titer can be used to identify the subgroups of neonates at risk of neonatal hyperthyroidism.1
Several studies have reported a relationship between low birth weight and maternal thyroid dysfunction.22,23 In our cohort mean z-scores for all measurements were negative and the group of high risk newborns presented 2 SGA and 1 LGA although there were no significant differences between both groups.
Another observation of our study is that low risk patients may not need analytic surveillance and can be discharged home safely without any further consultation. Indeed, in our low risk group no neonate has developed symptoms of hyperthyroidism and those who underwent analytical control presented no alterations. The majority of our high risk patients (81.5%, n=22) were able to stay with their mothers during surveillance which is beneficial for both.
Although further work is needed to validate our protocol, we believe that it represents a balanced approached to those patients, as we tried to identify all the newborns at risk and avoid unnecessary consultations and blood analysis.
We identify as limitations of this study: its retrospective nature impaired measurements to be obtained at defined times and so, patients’ approach was not uniform; blood cord samples were not drawn and the sample size was small. Aware of this problem and to the need to define coordinated actions, and based on the international guidelines, a multidisciplinary protocol was elaborated and approved thereafter.
Our experience highlights the need for a closer interdisciplinary collaboration of endocrinologists, obstetricians, neonatologists and pediatric endocrinologists in the management of maternal GD pregnancies and their newborns. We would like to emphasize that this study is related to the follow-up of infants previously the implementation of the unit's follow-up protocol. We expect in the future to carry out a similar new study to evaluate the follow-up of infants after the implementation of the protocol.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors disclosure no conflicts of interest.