In response to the findings of possible increased cardiovascular (CV) risk with rosiglitazone, the U.S. Food and Drug Administration (FDA) issued guidance mandating that all new glucose lowering drugs rule out excess CV risk by conducting large cardiovascular outcomes trials (CVOTs).1 Since that time, large numbers of patients with Type 2 Diabetes (T2D) have participated in trials of different agents, including dipeptidyl peptidase 4 inhibitors,2–4 glucagon-like peptide 1 receptor agonists (GLP-1 RA)5,6 and sodium–glucose cotransporter 2 inhibitors (SGLT-2 i).7 Some trials have shown CV benefits for GLP-1 RA and SGLT-2. However, the fact that a majority of patients included until now in CVOTs had a history of previous CV disease means that they are not representative of the larger populations of patients with T2D who are usually treated in primary health care centers.8 In contrast, the REWIND trial (Researching cardiovascular Events with a Weekly INcretin in Diabetes) testing the GLP-1 RA dulaglutide9 recruited a high proportion of patients without prior CV disease. We therefore sought to compare the baseline features of REWIND participants to the clinical characteristics of a large population with T2D typically attending primary care centers of the Catalan Health Institute in Spain.
Data were obtained from the trial design publication of REWIND. This international, multicenter, randomized placebo-controlled trial was designed to determine whether the addition of dulaglutide to the glucose lowering conventional treatment of patients with T2D is safe in terms of the incidence of CV events. Information regarding a representative patient population with T2D usually treated in primary health setting was obtained from a cross-sectional study of an unselected primary care population by Vinagre et al.10 This aimed to determine clinical characteristics and metabolic/CV risk factor control of patients with T2D attending primary care centers of the Catalan Health Institute in Spain. The major strength of this study was the inclusion of every patient with T2D from a population database of 3,755,038 people, with data from 286,791 patients with diabetes.
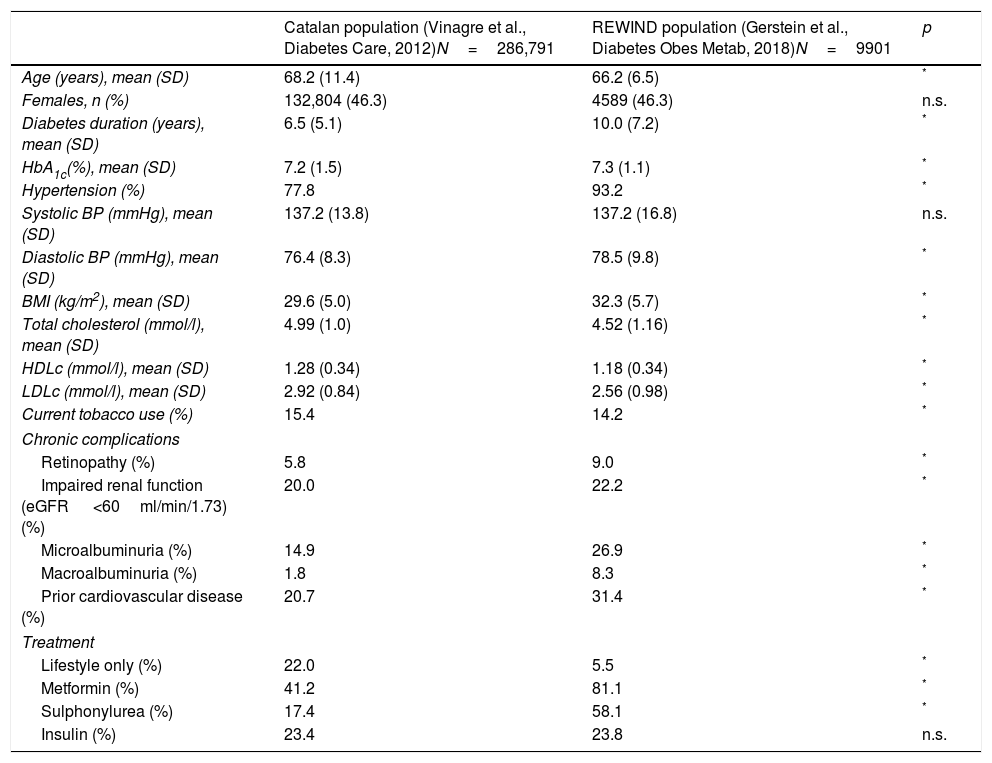
As noted in Table 1, in spite of some similarities in the clinical features of participants who were included in REWIND to the T2D patients within the primary health care setting, the population included in the CVOT does not fully represent the general population. Whereas several of the differences are statistically significant most are clinically irrelevant while the statistical significance is mainly driven by the large numbers of Catalan participants. Conversely, the clinically relevant differences in diabetes duration, hypertension and glucose lowering drug use, are likely due, at least in part, to the fact that the manuscript of Vinagre et al.10 included all people with diabetes in Catalonia from age 31 to 90 years and the population recruited in the REWIND trial included participants aged from 50 and above. Unfortunately, we could not restrict the comparisons only to people >50 years from the Spanish database.10 Differences in the prevalence of chronic complications stated in Table 1 could be related to the inclusion criteria used to recruit patients to the clinical trial. However, these differences in microangiopathy/macroangiopathy frequencies limit the extent to which the results of the study can be generalized to general T2D population. Unfortunately we did not get access to the general catalan population database and we do know this fact substantially limited our analysis. We could not perform analysis including: (i) the comparison of the participants actually enrolled in REWIND with the representative population with respect key patient characteristics, (ii) the analysis estimating the percentage of individuals in the representative population who matched each of the specific characteristics of people recruited into the CVOT and (iii) to examine how each of the REWIND inclusion/exclusion criteria affected the number of patient included in the T2D reference population.
Clinical characteristics of participants in the REWIND study and the Catalan study.
| Catalan population (Vinagre et al., Diabetes Care, 2012)N=286,791 | REWIND population (Gerstein et al., Diabetes Obes Metab, 2018)N=9901 | p | |
|---|---|---|---|
| Age (years), mean (SD) | 68.2 (11.4) | 66.2 (6.5) | * |
| Females, n (%) | 132,804 (46.3) | 4589 (46.3) | n.s. |
| Diabetes duration (years), mean (SD) | 6.5 (5.1) | 10.0 (7.2) | * |
| HbA1c(%), mean (SD) | 7.2 (1.5) | 7.3 (1.1) | * |
| Hypertension (%) | 77.8 | 93.2 | * |
| Systolic BP (mmHg), mean (SD) | 137.2 (13.8) | 137.2 (16.8) | n.s. |
| Diastolic BP (mmHg), mean (SD) | 76.4 (8.3) | 78.5 (9.8) | * |
| BMI (kg/m2), mean (SD) | 29.6 (5.0) | 32.3 (5.7) | * |
| Total cholesterol (mmol/l), mean (SD) | 4.99 (1.0) | 4.52 (1.16) | * |
| HDLc (mmol/l), mean (SD) | 1.28 (0.34) | 1.18 (0.34) | * |
| LDLc (mmol/l), mean (SD) | 2.92 (0.84) | 2.56 (0.98) | * |
| Current tobacco use (%) | 15.4 | 14.2 | * |
| Chronic complications | |||
| Retinopathy (%) | 5.8 | 9.0 | * |
| Impaired renal function (eGFR <60ml/min/1.73)(%) | 20.0 | 22.2 | * |
| Microalbuminuria (%) | 14.9 | 26.9 | * |
| Macroalbuminuria (%) | 1.8 | 8.3 | * |
| Prior cardiovascular disease (%) | 20.7 | 31.4 | * |
| Treatment | |||
| Lifestyle only (%) | 22.0 | 5.5 | * |
| Metformin (%) | 41.2 | 81.1 | * |
| Sulphonylurea (%) | 17.4 | 58.1 | * |
| Insulin (%) | 23.4 | 23.8 | n.s. |
HbA1c, glycated hemoglobin, BMI, body mass index, LDLc, low density lipoprotein cholesterol, HDLc, high density lipoprotein cholesterol.
Until now, most of the CVOTs included patients with T2D with a high frequency of established CV disease.8 Although all of them have provided strong evidence regarding the CV effects of the tested drugs, there is always room for hesitancy regarding the generalizability of findings to “typical” T2D patients seen in contemporary clinical practice. Despite some similarities in baseline clinical features, applicability of the REWIND trial to unselected Catalan T2D population is clearly limited.