There is currently no consensus among the different scientific societies on screening for thyroid dysfunction in the first trimester of pregnancy. Indeed, diagnosis and treatment of subclinical hypothyroidism during pregnancy are controversial, as no cut-off value for thyrotropin (TSH) is universally accepted. TSH measurement may be influenced by different factors throughout pregnancy, but especially during the first trimester. The association between overt hypothyroidism during pregnancy and obstetric and perinatal complications is well established. It is also accepted that thyroid hormones are important for neurodevelopment of the offspring. However, there is no scientific evidence available about the impact of subclinical hypothyroidism and its treatment during the first trimester of pregnancy on children's neurodevelopment. In recent years, studies conducted in the offspring of mothers with subclinical hypothyroidism have reported new biochemical parameters which may eventually serve as biomarkers of offspring neurodevelopment and which are more reproducible and are measured at an earlier time than the conventional clinical tests.
En la actualidad no existe un consenso entre las diferentes sociedades científicas para la detección de la disfunción tiroidea en el primer trimestre del embarazo. De hecho, el diagnóstico y tratamiento del hipotiroidismo subclínico durante el embarazo es controvertido, ya que no se acepta universalmente el valor límite para la tirotropina (TSH). La determinación de TSH puede estar influenciada por diferentes factores durante todo el embarazo, pero especialmente durante el primer trimestre. La asociación entre el hipotiroidismo clínico durante el embarazo y las complicaciones obstétricas y perinatales está bien establecida. También se acepta que las hormonas tiroideas son importantes para el desarrollo neurológico del feto. Sin embargo, falta evidencia científica sobre el impacto en el neurodesarrollo infantil del tratamiento del hipotiroidismo subclínico en el primer trimestre de gestación. En los últimos años, los estudios realizados en hijos de madres con hipotiroidismo subclínico han descrito nuevos parámetros bioquímicos que eventualmente pueden servir como biomarcadores del neurodesarrollo fetal, siendo más reproducibles y pudiendo determinarse en un período anterior al de las pruebas clínicas clásicas.
Thyroid hormones are directly and indirectly involved in multiple metabolic and cell tropism processes during intrauterine life. They are crucial for a correct somatic growth and neurologic development of offspring.1 During pregnancy, important modifications on thyroid economy occur, mainly due to the increased hormonal needs and to the transplacental trafficking of thyroid hormones to the fetus.2
The studies published in recent years about perinatal effects of maternal thyroid dysfunction during pregnancy have produced a growing debate on the need to establish strategies for universal or selective screening of these alterations in pregnant women.3 After an exhaustive analysis of the current evidence, our group has advocated the implementation of a systematic screening of thyroid function in the first trimester of pregnancy as it provides a more efficient way to detect gestational thyroid dysfunction.4 However, the introduction of universal screening of thyroid function in pregnancy in clinical practice carries multiple difficulties of interpretation and management, mainly related to both the specific physiologic characteristics of the thyroid gland in the earliest stages of pregnancy and to the technical limitations of available hormone detection methods.
Thyroid physiology changes during pregnancyIn normal pregnancy, the maternal hypothalamic-pituitary-thyroid system undergoes physiological changes to adapt to the new situation. A progressive increase in human chorionic gonadotropin (hCG) concentrations occurs in early gestation, whose “thyrotropin (TSH)-like” effect determines, firstly, a direct stimulation of the thyroid gland secretion which induces a preferential thyroxine (T4) production, and, secondly, causes a substantial decreasing of serum TSH.3,5 It has been described an inverse correlation between both hormones during the first 14–15 weeks of gestation. However, this classic relationship between TSH and hCG has been recently challenged by new studies indicating a weaker mutual influence than previously described.6 Moreover, other also recent studies have shown that there are other variables that play a significant role in the variability of TSH in the first trimester of pregnancy, which difficult the interpretation of the serum concentrations of TSH in the first weeks of gestation.7 Plasma TSH concentrations are strongly influenced by several parameters such as body mass index,8,9 age, ethnicity3 and iodine deficiency in pregnancy.10 It is also known that TSH values in the first trimester are significantly higher in patients with anti-thyroid peroxidise (aTPO) antibodies.11 All these factors should be considered at the time of interpreting a given TSH value and its particular significance for a given woman.
As mentioned above, the TSH concentration is currently the most accepted marker of gestational thyroid status but, in specific situations, it could be also necessary to determine free T4 and/or aTPO antibodies. In fact, what really determines the offspring outcomes regarding thyroid function, is the concentration of free T4 reaching the fetus during intrauterine life. For this reason, maternal hypothyroxinemia, regardless of the maternal TSH value, has been recently considered as a relevant issue.12–15 However, the interpretation of free T4 concentrations during pregnancy is a very difficult matter due to the enhanced circulating level of T4-binding globulin compared to the non-pregnant condition and the decreased albumin concentration, which could decrease immunoassays’ reliability. The measurement of free T4 by using immunoassay techniques during pregnancy maybe strongly influenced by modifications of T4 binding proteins concentrations and composition.16 In the last years, it has been suggested that free T4 measurement with dialysis or ultrafiltration using online solid phase extraction liquid chromatography tandem mass spectrometry is the gold standard method in pregnancy.10,17–19 Unfortunately, this method is labor-intensive, technically demanding, time-consuming and expensive, and it is not readily available in most clinical laboratories.
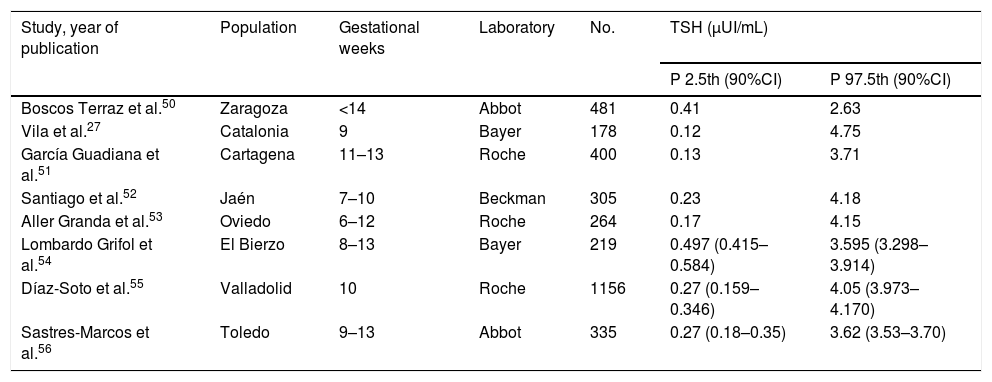
Thyroid function screening in pregnant womenThe aim of thyroid function screening is to identify any alteration susceptible of treatment that otherwise could not be detected, with the purpose of avoiding and preventing potential obstetric and perinatal complications associated to thyroid dysfunction from the beginning of pregnancy.3,20 Some studies have shown that the universal screening of thyroid function in pregnant population is cost-effective.21 Different considerations should be taken into account regarding universal screening: (A) Screening timing: It is recommended, or at least it seems very recommendable, to perform it in the preconceptional period and, if that is not possible, in the very early stages of gestation (<10 weeks of amenorrhea), as treatment in such an earlier period may have a higher potential of preventing irreversible sequels in the fetus.22 In fact, the initiation of thyroxine replacement therapy after the first trimester of pregnancy has not shown benefits on child neurodevelopment.23 (B) Variables studied: Studies have shown that the determination of TSH is the most sensitive parameter for thyroid dysfunction detection in the general population.24 TSH is also recommended for screening of pregnant women and, if it is abnormal, other parameters should be considered as the free or total T4, and the presence of aTPO antibodies. In some situations of high obstetric risk, a full study of thyroid hormones should be performed.25 (C) Reference values: Thyroid function parameters vary significantly depending on gestational age in most of published studies5 but not in all of them.6 For this reason, it is recommended to establish reference ranges for each trimester of pregnancy and for each population, as local conditions including iodine status may influence results, and for each laboratory, as depending on the measurement technique used for both TSH and free and total T4, the obtained values may vary substantially.26 In the absence of local reference values of TSH, the last American Thyroid Association (ATA) guidelines recommend the cut-off value of 4μUI/mL in the first trimester when using immunoassays.10 The cut-off of 4μUI/mL represents a decrease of about 0.5–1μUI/mL compared to the non-pregnant TSH reference range. However, the reference ranges published so far in different countries, as Spain for example and other (see Table 1), show significant differences even within the same country.27 Indeed, in this regard, the 2017 ATA guideline emphasises the need to establish own local criteria in the diagnosis and management of thyroid dysfunction during pregnancy.
TSH reference values in Spain.
| Study, year of publication | Population | Gestational weeks | Laboratory | No. | TSH (μUI/mL) | |
|---|---|---|---|---|---|---|
| P 2.5th (90%CI) | P 97.5th (90%CI) | |||||
| Boscos Terraz et al.50 | Zaragoza | <14 | Abbot | 481 | 0.41 | 2.63 |
| Vila et al.27 | Catalonia | 9 | Bayer | 178 | 0.12 | 4.75 |
| García Guadiana et al.51 | Cartagena | 11–13 | Roche | 400 | 0.13 | 3.71 |
| Santiago et al.52 | Jaén | 7–10 | Beckman | 305 | 0.23 | 4.18 |
| Aller Granda et al.53 | Oviedo | 6–12 | Roche | 264 | 0.17 | 4.15 |
| Lombardo Grifol et al.54 | El Bierzo | 8–13 | Bayer | 219 | 0.497 (0.415–0.584) | 3.595 (3.298–3.914) |
| Díaz-Soto et al.55 | Valladolid | 10 | Roche | 1156 | 0.27 (0.159–0.346) | 4.05 (3.973–4.170) |
| Sastres-Marcos et al.56 | Toledo | 9–13 | Abbot | 335 | 0.27 (0.18–0.35) | 3.62 (3.53–3.70) |
CI, confidence interval; P, percentile; TSH, thyroid-stimulating hormone.
Adapted from Díaz-Soto, et al.55
Different guidelines on the management of thyroid dysfunction during pregnancy have been published lately10,23,24 and one specifically devoted to hypothyroidism in adults including pregnancy,28 which exemplifies the difficulty to achieve a consensus in this area and the need of continuous updated reviews of all available forcoming evidence on neonatal effects of different degrees of maternal hypothyroidism. Thus, considering the current lack of consistent information, it remains awaiting the formulation of clear and solid criteria useful for the clinical practice.
Thyroid dysfunction management in pregnancyEven with the aforementioned controversy, in the last years, scientific evidence has come about the decisive role of thyroid hormones in crucial stages of intrauterine life as the placentation or neurodevelopment.3,29 Numerous studies have shown significant associations between any degree of thyroid dysfunction and obstetrical and/or perinatal complications, as infertility, repeated miscarriages, preterm delivery or intrauterine growth restriction.29,30
The need to provide diagnostic criteria allowing to identify the affected population and offer an early treatment to reduce the previously described complications, has generated the development and updating of different specific guidelines for the management of thyroid disease during pregnancy.10,24 However, the coexistence of different recommendations has only contributed to increase the debate on which is the better way for detecting thyroid dysfunction during pregnancy.
Although the importance of generating thyroid hormones reference ranges for each laboratory at local level seems reasonable and has been fairly accepted, the recent ATA guidelines have proposed reference ranges that could become entirely arbitrary in some populations, inducing over diagnosis in some and under diagnosis in other. Thus, when universal screening is performed and ATA criteria of thyroid dysfunction are applied, this could determine that the rate of thyroid hypofunction diagnosis maybe close to 50% of the pregnant population.31
Thyroid function evaluation and treatment recommendations during pregnancy should be developed as the consequence of epidemiologic studies aiming to detect at which cut-off of thyroid function the negative maternal and obstetric outcomes are observed in a given population. Obviously, this could vary from population to population and therefore, the generation of reference ranges is important but without clinical interest if the threshold of negative outcomes for the fetus and the mother is not determined in parallel. This will be the only way to avoid unnecessary treatments as well as rending the universal screening cost effective.
Various studies have compared the detection rates of thyroid disease obtained with the two diagnostic criteria currently proposed (local reference ranges versus imported ranges from general recommendations of scientific societies) on the same population; it is concluded that targeted thyroid function testing only those women at high-risk of thyroid dysfunction would miss between 30 and 50% of pregnant women with overt/subclinical hypothyroidism or autoimmune thyroiditis.32,33 However, to date, there are no randomized studies evaluating the effects of treatment of thyroid dysfunction and its effectiveness in reducing obstetric and perinatal complications eventually caused by subclinical hypothyroidism diagnosed by different cut-off values of TSH.
Maternal thyroid dysfunction and offspring neurodevelopmentDuring prenatal development, the thyroid gland completes its formation at the end of the first trimester. The secretion of thyroid hormones by the fetus begins at about 10 weeks of gestation onwards and is particularly active around 16–20 weeks of gestation.5,34 For this reason, an appropriate and sufficient maternal thyroid function is particularly critical in early pregnancy.
The importance of maternal thyroid hormones in the fetal central nervous system development is well established. Some experimental studies have analyzed the mechanisms of action of thyroid hormones in the development of the brain. Maternal thyroid hormones are necessary and crucial for fetal neural proliferation and migration in early gestation. From mid-gestation onwards, both the mother and the fetus thyroid hormones play an important role in enhancing neurogenesis, neuronal migration, synaptogenesis and myelination.6,35
Several studies have suggested that mild maternal hypothyroidism is associated with adverse fetal neurocognitive outcomes. In a large historic case–control study, Haddow et al. demonstrated that children of mothers with untreated TSH elevations in pregnancy had IQ scores 7 points lower than children of euthyroid mothers at 7–9 years.36 These results have been confirmed in different studies, with worse results on IQ and psychomotricity in children of mothers with overt hypothyroidism and hypothyroxinaemia.13 However, the relationship between subclinical hypothyroidism and neurodevelopment in offspring is controversial. In fact, the first problem is the definition of subclinical hypothyroidism during pregnancy itself, which remains elusive at present. There are only two prospective studies that have evaluated the effects of levothyroxine treatment in pregnant women with subclinical hypothyroidism, without differences in intelligence quotient (IQ) at 3 and 5 years, respectively, between offspring of women treated and untreated.23,37 However, the study of Lazarus et al.23 has multiple limitations, such as that there is not a comparison between children of euthyroid and hypothyroid treated mothers, and that levothyroxine treatment was initiated at the second trimester of gestation. On the other hand, in the study of Casey et al. levothyroxine treatment was initiated in a broad period of time, between 8 and 20 weeks of pregnancy. Therefore, it cannot be discarded from these results that an earlier diagnosis and treatment of subclinical hypothyroidism in pregnant women, specifically before 10 weeks of gestational age, can reduce the risk of lower IQ in children and the incidence of poor obstetric complications. Moreover, regarding the importance of maternal hypothyroxinaemia, the recent data from the Generation R Study suggested that maternal hypothyroxinaemia increased the risk of language delay and nonverbal cognitive delay in offspring,14,15 although this association has not been confirmed by others.37
One of the main limitations of all published data taken together in order to assess the real impact of thyroid dysfunction on fetal neurodevelopment is the difficulty of comparing the results obtained among different studies. Many of the infant neurodevelopment tests (Bayley, Weschler, Brunet-Lezine) are strongly influenced by socio-cultural, economic and educational variables, which limits the reproducibility and comparability of the results observed.15,38 For this reason, there is a need to identify biological markers linked to neurodevelopment that allow a more objective and quantifiable evaluation in the offspring and eventually serving as surrogates predicting future outcome.
Some specific biomarkers of proliferation and neuronal migration have currently been characterized, such as brain-derived neurotrophic factor (BDNF),39 lysophosphatidic acid (LPA)40 and growth-derived neurotrophic factor (GDNF),41 which could be potential useful parameters for an objective a better evaluation of infant neurodevelopment. In this sense, it has been observed that the concentrations of BDNF in cord blood show significant variations in certain conditions such as prematurity42 or preeclampsia,43 and are associated to impaired neuroprotection of the newborn. Recent experimental studies have also identified an association of BDNF with hypothyroidism, even in models of mild thyroid dysfunction.44,45 An inverse relationship between BDNF and TSH and between BDNF and oxidative stress during pregnancy have been found, suggesting that a reduced hippocampal and cerebellar BDNF concentration and an increment of oxidative stress during early development might contribute to the adverse neurodevelopment effects of hypothyroidism during pregnancy.46 In order to eliminate potential confounders in the association between thyroid hormones and neurotrophins, LPA has been selected as a marker of intracellular signaling directly involved in the vascularization of the utero-placental unit and in feto-maternal immunological interactions. Alterations in the mechanisms of LPA-dependent signaling during pregnancy could etiologically be linked to the development of obstetric complications, such as implantation failure, preeclampsia or preterm delivery.47,48 Some authors have highlighted the need to increase the knowledge and interpretation of neurotrophins and LPA levels in the clinical setting,49 in order to evaluate the usefulness of these molecules as neurodevelopmental biomarkers in the early stages of life and as potential predictors of future nervous system disorders.
In conclusion, a vigorous debate among diverse international scientific societies has arisen in latest years and is still ongoing, regarding the recommendation of the universal or selective screening of thyroid function during pregnancy. The guidelines of the different societies agree on the importance of thyroid function evaluation, with TSH determination as the most robust parameter, to be performed earlier in the first trimester of the pregnancy. TSH concentrations should be evaluated according to locally generated own reference values for each population and for each technical method. On the other hand, there are multiple parameters and situations either from the mother and the environment that could influence the TSH value during pregnancy for a given women which are not taken into account at the time of its clinical evaluation. A more accurate and personalized thyroid function evaluation approach will require the inclusion of these factors for a correct interpretation of a given result. In parallel to a more accurate and personalized assessment of TSH results, population-based studies regarding TSH cut-off values at which level negative feto-maternal outcomes are observed are definitely required for every population, allowing a better guidance for the decision of treatment with thyroxine during the pregnancy, mostly in those cases of modest alterations of the thyroid function. Finally, it is yet to be definitely ruled out that alterations of maternal thyroid function in the ranges of what is known as subclinical hypothyroidism does not affect the normal infant – neurodevelopment. As the neurocognitive evaluation of children with classical test has limited reproducibility, it is urgent to introduce the use of new biochemical parameters to build a more reliable and early assessment of neurocognitive development in the progeny; this opens a very interesting field of study both from the standpoint of experimental and clinical medicine.
Conflict of interestsThe authors declare that they have no competing interests regarding the publication of this paper.
Authors’ contributionsMB and SB performed a literature review and drafted the paper. LV, IV, AL and MPD provided advice and revised subsequent drafts of the paper. All authors read and approved the final manuscript.
The authors would like to thank the Universitat Autònoma de Barcelona for its collaboration allowing us to have access to the papers needed for this review.