This study aims to explore the effect and related molecular mechanism of miR-153-3p on high glucose-stimulated human glomerular mesangial cells.
Materials and methodsThe quantitative real-time polymerase chain reaction (qPCR) assay was employed to check miR-153-3p and PAQR3 expression levels in diabetic nephropathy patients. (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) MTT assay was applied to investigate the effects of miR-153-3p transfection or PAQR3 administration on mesangial cell (MC) activity. ELISA assays were used to check the expression levels of extracellular matrix (ECM) related proteins. The bioinformatics method and dual-luciferase reporter assay were employed together to anticipate and check the targeting relationship between miR-153-3p and PAQR3. Western blot assays were applied to check the PAQR3, PI3K and AKT expression after miR-153-3p transfection or PAQR3 administration.
ResultsThe expression level of miR-153-3p was lower in diabetic nephropathy patients, while the expression of PAQR3 was concomitantly higher. Upregulation of miR-153-3p can reduce MC proliferation and ECM accumulation. Further research indicated that miR-153-3p directly regulated PAQR3 expression via coupling with the 3’-UTR of PAQR3. Finally, the fact that miR-153-3p regulates the PI3K/AKT pathway by PAQR3 was confirmed.
ConclusionMiR-153-3p regulates the PI3K/AKT pathway through PAQR3, thereby playing a role in regulating cell proliferation and ECM accumulation in high glucose-stimulated MCs.
El presente estudio tiene como objetivo analizar el efecto y el mecanismo molecular relacionado de miR-153-3p en células mesangiales glomerulares (CM) humanas estimuladas con glucosa alta.
Materiales y métodosSe empleó el ensayo cuantitativo de reacción en cadena de la polimerasa en tiempo real (qPCR) para comprobar los niveles de expresión de miR-153-3p y PAQR3 en pacientes con nefropatía diabética. Se aplicó el ensayo MTT (bromuro de 3-(4,5-dimetiltiazol-2-il)-2,5-difeniltetrazolio) para investigar los efectos de la transfección de miR-153-3p o la administración de PAQR3 en la actividad de las CM. Se utilizaron ensayos ELISA para comprobar los niveles de expresión de las proteínas relacionadas con la matriz extracelular (MEC). Se emplearon conjuntamente un método bioinformático y un ensayo indicador de luciferasa dual para prever y verificar la relación de focalización entre miR-153-3p y PAQR3. Se aplicaron ensayos de Western blot para comprobar la expresión de PAQR3, PI3K y AKT tras la transfección de miR-153-3p o la administración de PAQR3.
Resultadosel nivel de expresión de miR-153-3p fue menor en los pacientes con nefropatía diabética, mientras que la expresión de PAQR3 fue simultáneamente mayor. El aumento de miR-153-3p puede reducir la proliferación de CM y la acumulación de MEC. Otras investigaciones indicaron que miR-153-3p regulaba directamente la expresión de PAQR3 a través del acoplamiento con la 3’-UTR de PAQR3. Por último, se confirmó el hecho de que miR-153-3p regula la vía PI3K/AKT mediante PAQR3.
ConclusiónMiR-153-3p regula la vía PI3K/AKT a través de PAQR3, por lo que desempeña una función en la regulación de la proliferación celular y la acumulación de MEC en CM estimuladas con glucosa alta.
Diabetic nephropathy (DN) typically occurs in patients with poor glycaemic control, hypertension, hyperfiltration of the glomeruli, or genetic predisposition. It is typically characterised by proteinuria.1,2 With its rising incidence, DN has become a global public health issue.3 According to estimates by the World Trade Organisation, by 2025, the number of diabetics worldwide will reach at least 370 million, of which 30% will develop into DN.4 Unique pathological changes of DN include proliferation of mesangial cells (MCs), mesangial enlargement of vessels and accumulation of abnormal extracellular matrix (ECM).5,6 Brosius et al. reported that high glucose (HG) induces MC proliferation and ECM protein synthesis, eventually leading to diabetic nephropathy.5 Therefore, inhibition of MC proliferation and ECM accumulation may be a possible treatment for DN.
The progestin and adipoQ receptor (PAQR) family consists of a series of different membrane receptors.7 PAQR3, a transmembrane protein, is specifically targeted at the Golgi body.8,9 PAQR3 was initially thought to be a regulator of Raf kinase. More and more evidence showed that PAQR3 was involved in many biological processes, including energy metabolism, autophagy, cholesterol, insulin sensitivity homeostasis and obesity.10–12 In DN, high glucose can induce increased PAQR3 expression, while a knockdown of PAQR3 can reduce MC cell proliferation and reduce extracellular matrix precipitation.13 However, the cause of PAQR3 maladjustment in DN remains unclear.
In this study, the results revealed that increased expression level of PAQR3 in the tissues of DN patients was related to the down-regulation of miR-153-3p expression level. MiR-153-3p expression was suppressed in the chronic renal disease model,14 but its effect on DN has not been reported. This study explored the causes of PAQR3 maladjustment in DN, identified the mechanism by which miR-153-3p plays a role in DN, and provided potential new targets and possible mechanisms for the treatment of DN.
Materials and methodsTissue sampleA total of 40 cases of renal tissue from DN patients and 32 cases of renal tissue from healthy patients were randomly collected at the second affiliated hospital of Nantong University. Informed written consents were obtained from all patients for sample collection. Study protocols were approved by the Ethics Committee of the second affiliated hospital of Nantong University and conducted according to the principles expressed in the Declaration of Helsinki.15
Cell cultureThe MCs were obtained from Lonza (Basel, Switzerland). They were cultured in culture medium (DMEM; Gibco, Carlsbad, CA, USA) with 10% foetal bovine serum (Gibco), and 1% penicillin/streptomycin (Gibco) in a humidified 5% CO2 incubator at 37°C. For the DN model, MCs were divided into two groups: (1) NG (normal glucose) group, in which MCs were cultured in DMEM with normal glucose (5.5mM, Gibco) as a control; and (2) HG (high glucose) group, in which MCs were cultured in DMEM with high glucose (30mM, Gibco) according to the protocol.16,17
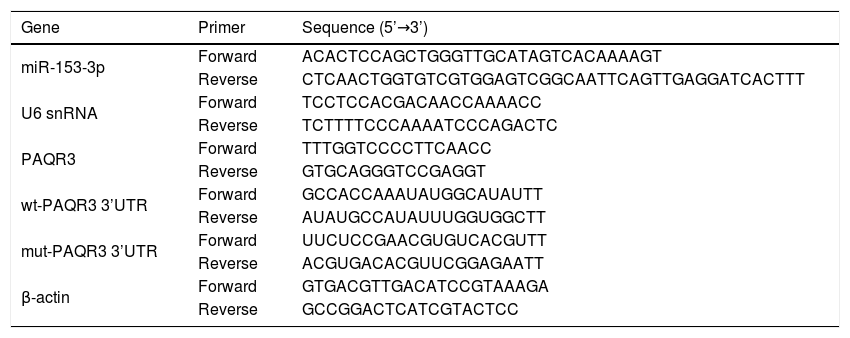
qPCRA TRizol reagent (Invitrogen) was used to extract the total RNA from collected cells. The quantity and integrity of extracted total RNA were evaluated by a Nano Drop 1000 spectrophotometer (Thermo Fisher Scientific, Inc.). A MiRNA-specific TaqMan miRNA assay kit (Applied Biosystems, Foster City, CA, USA) was applied to reverse transcribe the whole RNA into cDNA. The expression levels of miR-153-3p were detected through qRT-PCR by the SYBR Premix EX Taq (Takara, Japan). The PCR primers were synthesised by Sangon Biological Engineering Technology (Shanghai, China). U6 snRNA or β-actin were used as the endogenous reference genes to normalise miRNA or mRNA expression levels, respectively. The relative expression levels of miR-153-3p and PAQR3 in each experimental group were analysed using the 2−△Ct method. All reactions were performed in triplicates. Primer sequences are shown in Table 1.
Primers for miR-153-3p, PAQR3 and reference genes.
| Gene | Primer | Sequence (5’→3’) |
|---|---|---|
| miR-153-3p | Forward | ACACTCCAGCTGGGTTGCATAGTCACAAAAGT |
| Reverse | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGATCACTTT | |
| U6 snRNA | Forward | TCCTCCACGACAACCAAAACC |
| Reverse | TCTTTTCCCAAAATCCCAGACTC | |
| PAQR3 | Forward | TTTGGTCCCCTTCAACC |
| Reverse | GTGCAGGGTCCGAGGT | |
| wt-PAQR3 3’UTR | Forward | GCCACCAAAUAUGGCAUAUTT |
| Reverse | AUAUGCCAUAUUUGGUGGCTT | |
| mut-PAQR3 3’UTR | Forward | UUCUCCGAACGUGUCACGUTT |
| Reverse | ACGUGACACGUUCGGAGAATT | |
| β-actin | Forward | GTGACGTTGACATCCGTAAAGA |
| Reverse | GCCGGACTCATCGTACTCC |
Briefly, cells were washed with pre-cooling PBS buffer three times, and the total protein was extracted by RIPA buffer (Beyotime, Shanghai, China). A BCA protein assay kit (CoWin Biotechnology) was applied to detect the protein concentration. An equal amount of total proteins were electrophoresed to SDS-PAGE. Then, they were transferred to the polyvinylidene difluoride (PVDF) membranes (Millipore) and blocked by 5% non-fat milk for 1h. The protein was identified by incubating with specific primary antibodies, including PAQR3 (rabbit anti-PAQR3 antibody, ab174327, 1:3000; Abcam, Cambridge, MA, USA), p-PI3K (rabbit anti-PI 3 kinase p85 alpha (phospho Y607) antibody, ab182651, 1:3000; Abcam), PI3K (rabbit anti-PI 3 kinase p85 alpha antibody, ab86714, 1:3000; Abcam), p-AKT (rabbit anti-AKT (phospho T308) antibody, ab38449, 1:3000; Abcam), AKT (rabbit anti-pan-AKT antibody, ab8805, 1:3000; Abcam), PCNA (rabbit anti-PCNA antibody, ab29, 1:3000; Abcam), Collagen IV (rabbit anti-collagen IV antibody, ab6586, 1:3000; Abcam), Fibronectin (rabbit anti-fibronectin antibody, ab2413, 1:3000; Abcam), and β-actin (rabbit anti-beta actin antibody, ab8227, 1:3000; Abcam) overnight at 4°C. Then, the membranes were further incubated with secondary HRP-conjugated goat anti-rabbit immunoglobulin G secondary antibody (ab205718, 1:2000; Abcam) and the bands on the membranes were visualised using enhanced chemiluminescence (ECL) reagent (Beyotime). The analysed samples were normalised by β-actin and protein bands were quantified by grey value analysis by ImageJ software (National Institutes of Health).
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) MTT assayThe MTT method was used to assess the extent of cell proliferation. Briefly, 2.5×102 cells were plated into 96-well plates in triplicates and treated in different conditions as indicated in each experiment. Following treatment, a final concentration of 0.5mg/ml MTT solution (Beyotime) was applied and incubated for another 4h. Subsequently, 100μl dimethyl sulfoxide (DMSO, Sigma) was added to visualise after discarding the culture medium. The optical density (OD) value of each sample was checked at 490nm through a microplate reader (BioTek, Winooski, VT, USA).
ELISAThe content of fibronectin and collagen IV of cells in the supernatant was assessed by ELISA Kit according to the instruction. The Collagen IV ELISA Kit was purchased from Biocompare (South San Francisco, CA, USA) and Fibronectin ELISA Kit was purchased from Abcam (ab38449). The optical density (OD) values of each sample were detected using a microplate reader (BioTek).
Cell transfectionThe synthetic miR-153-3p mimics and NC mimics were obtained from GenePharma (Shanghai, China). After cells were cultured in plates for one day, miR-153-3p mimics and NC mimics were transfected into cells by Lipofectamine 2000 (Invitrogen).
Dual-luciferase reporter assayThe fragments of 3’-UTRs of PAQR3 mRNA containing the wild-type and the mutant-type binding sites of miR-153-3p anticipated by using TargetScan were cloned into pmirGLO luciferase report vector (Promega, Madison, WI, USA). The constructed luciferase reporters were named as PAQR3-WT and PAQR3-MUT, respectively. For luciferase assay, miR-153-3p mimics and NC mimics (control) were co-transfected with reporter plasmids into cells. The luciferase activities were tested by the dual-luciferase kit (Promega) after two days of transfection.
Statistical analysisAll data are shown as the mean±standard error of the mean from three independent experiments. Comparisons between two groups were performed using Student's t-test. The linear relationship between levels of miR-153-3p and PAQR3 in DN patients was investigated by Spearman's correlation coefficient. p values of <0.01 (two-tailed) were considered to indicate a statistically significant difference. GraphPad Prism 5 (GraphPad Software, Inc.,) was used for analysis.
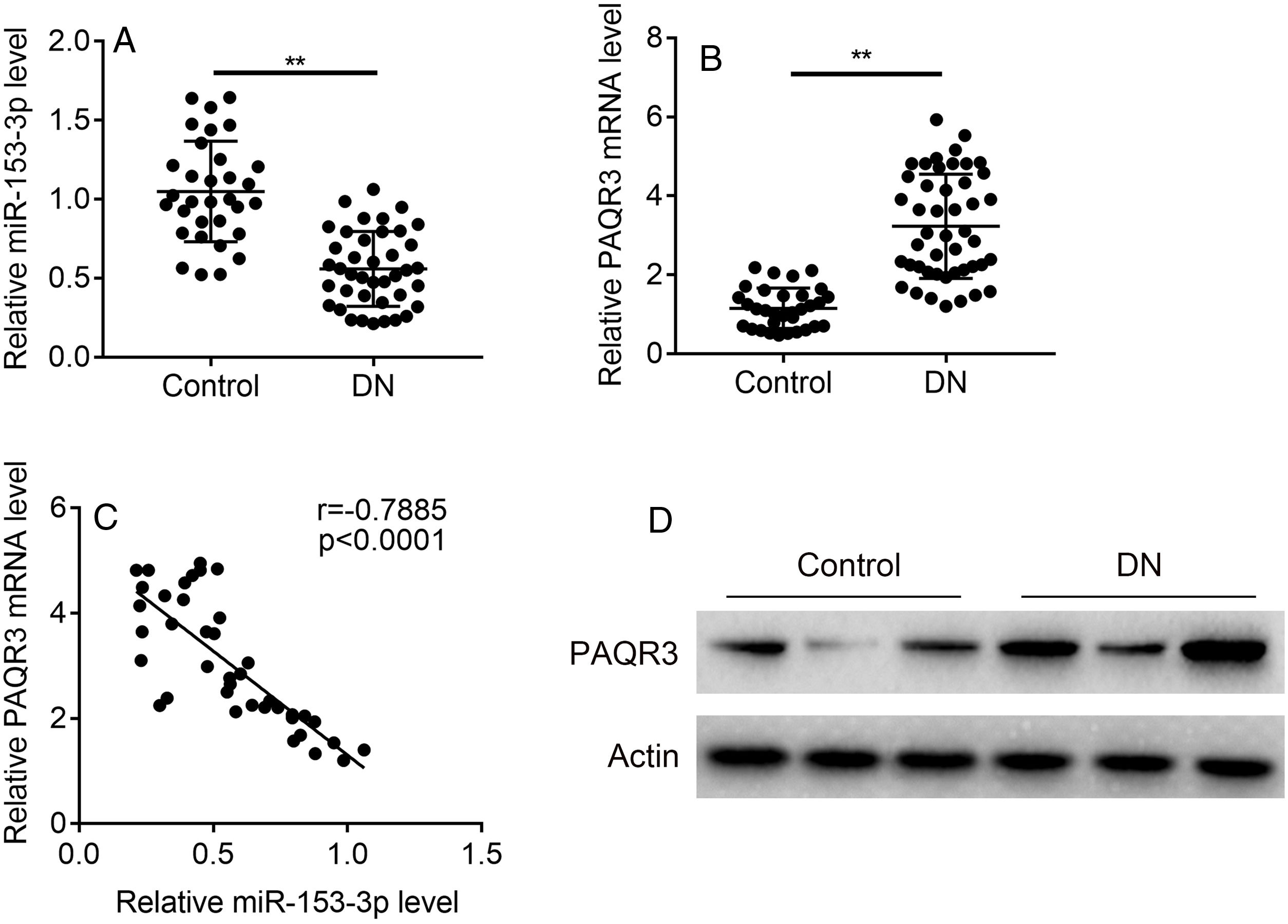
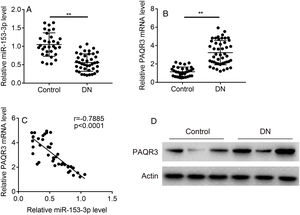
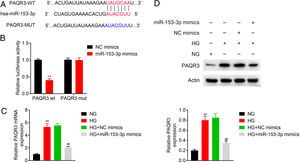
ResultsThe expression levels of miR-153-3p and PAQR3 in diabetic nephropathy (DN) tissues were correlatedIn order to understand the correlation between the expression levels of miR-153-3p and PAQR3 in DN, 40 DN patients and 32 non-DN patients were recruited and their renal tissues were collected as experimental samples in this study. The expression level of miR-153-3p was clearly lower in renal tissue of DN patients compared with the normal group (Fig. 1A). Interestingly, the mRNA expression level of PAQR3 was significantly increased in renal tissue of DN patients compared with the normal group (Fig. 1B). Furthermore, we conducted a statistical correlation analysis of miR-153-3p and PAQR3 mRNA expression levels in renal tissue of DN patients. The result indicated that there was a negative correlation (r=−0.7885) between expression levels of miR-153-3p and PAQR3 in renal tissue of patients with DN (p<0.0001) (Fig. 1C). Moreover, we randomly checked the protein expression level of PAQR3 in renal tissue of three DN patients and three patients without DN. The result revealed that the protein expression level of PAQR3 in renal tissue of DN patients was markedly higher than that of non-DN patients (Fig. 1D).
Expression levels of miR-153-3p and PAQR3 in diabetic nephropathy (DN) tissues.
(A and B) The mRNA expression levels of miR-153-3p and PAQR3 were detected in renal tissue of patients with DN (n=40) and normal group (n=32). (C) The linear correlation of miR-153-3p and PAQR3 mRNA expression levels in renal tissue of patients with DN was analysed. (D) The protein expression level of PAQR3 was detected in renal tissue of patients with DN (n=3) and normal group (n=3). Data were presented as the mean±SD with three independent experiments. **p<0.01.
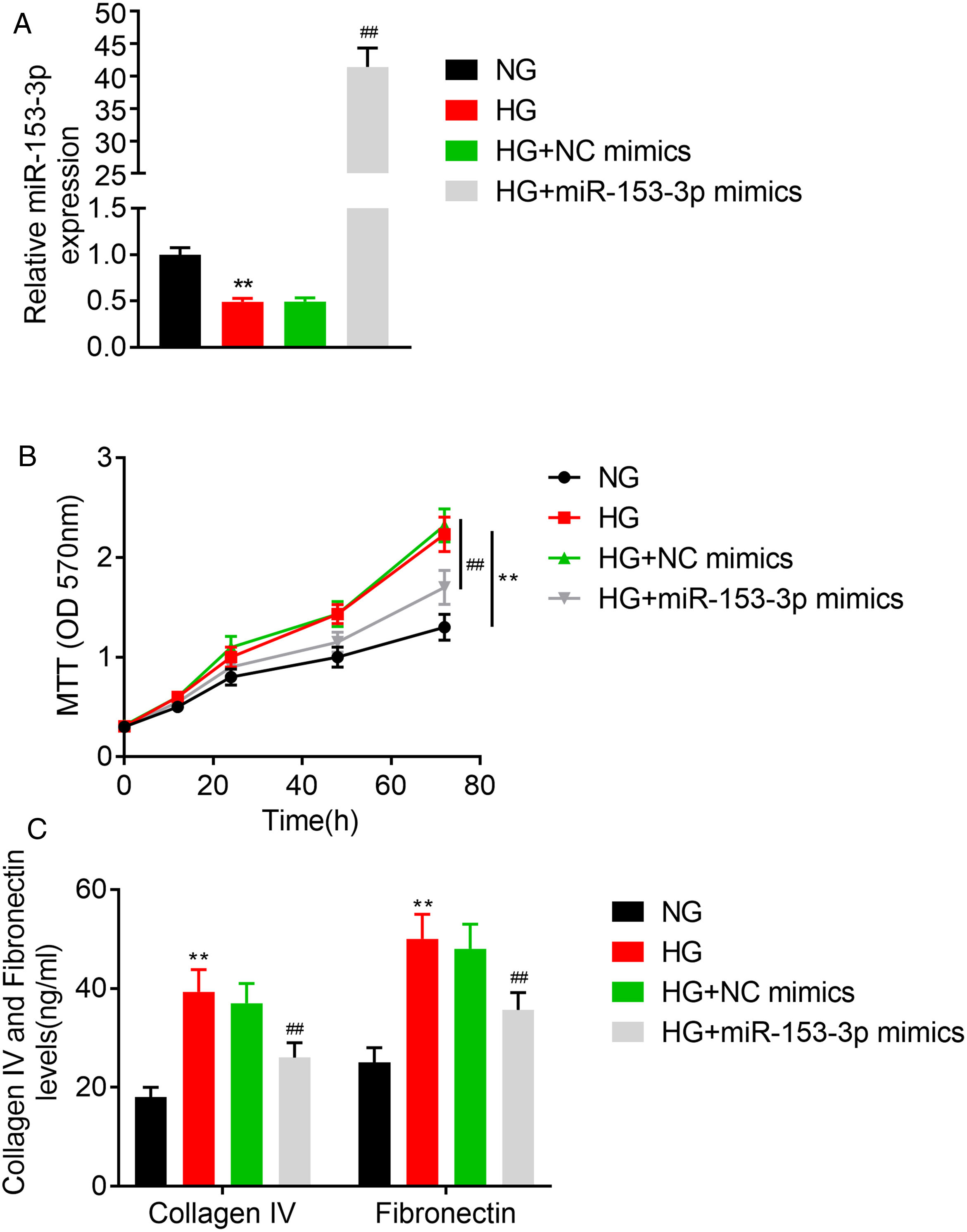
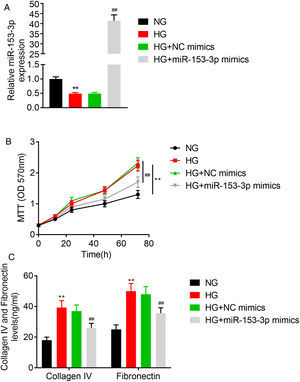
In this study, we studied the regulatory role of miR-153-3p and PAQR3 in cell proliferation and extracellular matrix accumulation in MCs. The expression level of miR-153-3p was decreased in cells cultured under high glucose conditions compared with cells cultured under normal glucose conditions. To understand the effect of miR-153-3p on MCs stimulated by high glucose, miR-153-3p mimics or NC mimics were transfected into MCs and then the cells were cultured under high glucose conditions. After transfection, the expression level of miR-153-3p in the cells transfected with miR-153-3p mimics was significantly higher than that of the NC mimics transfected cells or the untreated cells, indicating that the expression level of miR-153-3p was successfully upregulated by cell transfection (Fig. 2A). Then, an MTT assay was carried out to examine the effect of miR-153-3p on the cell proliferation of MCs. The MTT assay results showed that compared with the normal glucose group, high glucose treatment significantly promoted cell proliferation, while miR-153-3p mimic markedly inhibited the cell proliferation ability of MCs (Fig. 2B). Subsequently, the expression level of ECM proteins, collagen IV and fibronectin, were assessed by ELISA to determine the effects of miR-153-3p on extracellular matrix deposition of MCs. Collagen IV and fibronectin were clearly increased in the high glucose group, and decreased in MCs transfected with miR-153-3p mimics, indicating that upregulation of miR-153-3p can reduce extracellular matrix accumulation (Fig. 2C).
High expression of miR-153-3p prevents cell proliferation and extracellular matrix accumulation of mesangial cells. (A) MiR-153-3p level was measured using real-time PCR. Cells were divided into four groups, including NG, HG, HG+NC mimics, and HG+miR-153-3p mimics. (B) Cell proliferation ability of MCs was detected using MTT assay. (C) Protein expressions levels of collagen IV and fibronectin were detected using ELISA kit. Data were presented as the mean±SD with three independent experiments. **p<0.01 versus control group and ##p<0.01 versus NC mimics group.
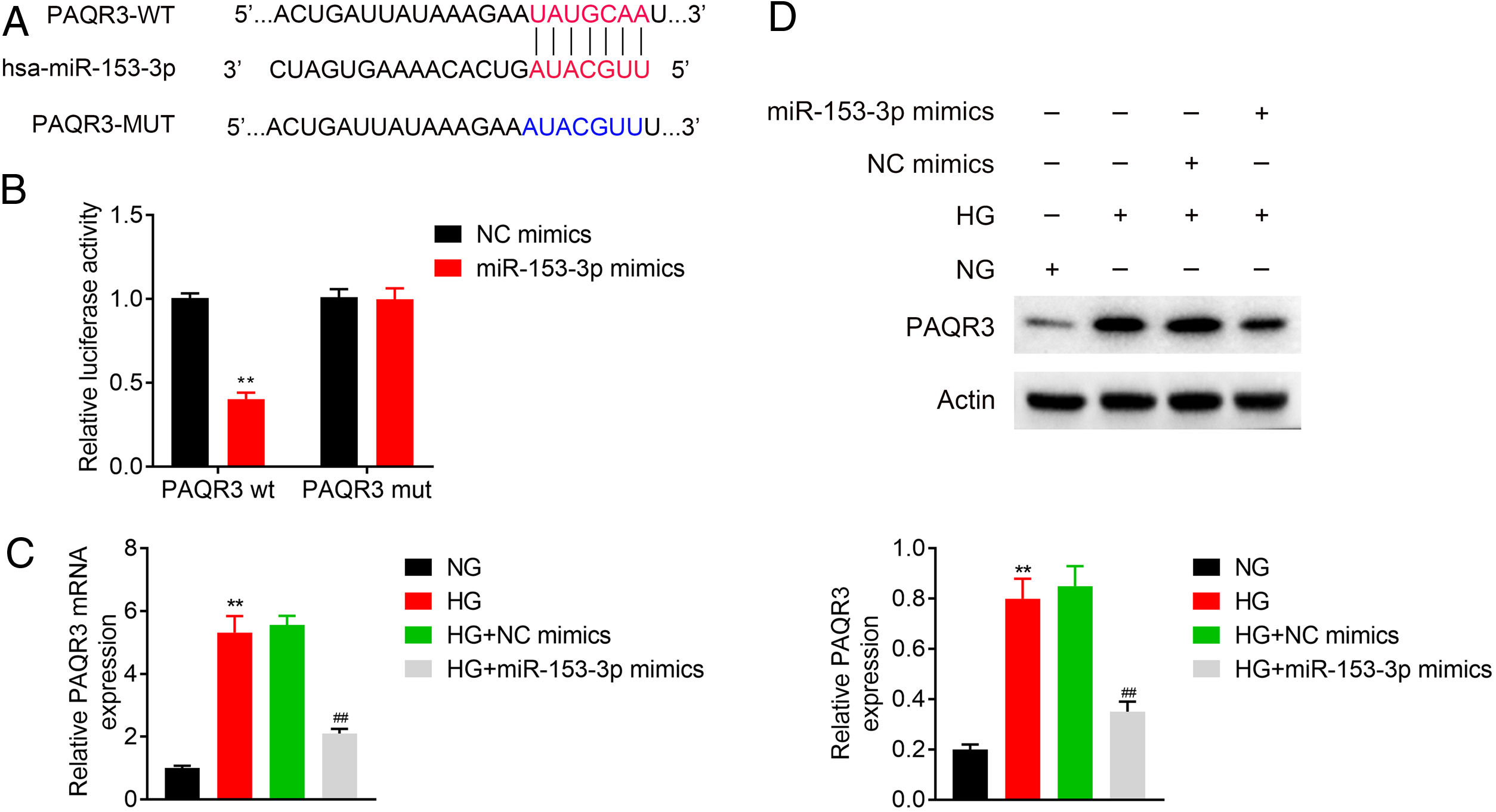
To explore the biomechanism of miR-153-3p in renal tissue of DN patients, the mRNA binding sites were further anticipated in TargetScan (http://www.targetscan.org/vert_71/). The results indicated that PAQR3 mRNA was a binding target of miR-153-3p. The anticipated 3’-UTRs of PAQR3 mRNA binding to miR-153-3p are presented in Fig. 3A. To investigate whether PAQR3 was a potential target of miR-153-3p, PAQR3 WT and MUT fragments were cloned into the downstream of the firefly luciferase coding region. The results of dual luciferase reporter assay indicated that the overexpression of miR-153-3p strikingly decreased the luciferase activity of the PAQR3-WT reporter gene, but had no effect on the PAQR3-MUT control (Fig. 3B). To further examine whether miR-153-3p regulated PAQR3 expression, the expression levels of PAQR3 protein were detected in the four types of cells described above. The qPCR and western blot assay results showed that high glucose treatment significantly promoted PAQR3 mRNA and protein expression compared with that in the normal glucose group. Moreover, a high expression level of miR-153-3p significantly suppressed PAQR3 mRNA and protein expression compared with that in the NC group (Fig. 3C and 3D). These data showed that PAQR3 is a target of miR-153-3p and its protein expression is repressed by miR-153-3p.
MiR-153-3p directly regulated PAQR3 expression by binding to the 3’-UTR of PAQR3. (A) Forecast of miR-153-3p binding sites on target gene PAQR3 by TargetScan. (B) Dual-luciferase assays were carried out after cells were co-transfected PAQR3-WT or PAQR3-MUT with miR-153-3p mimics or miR-control for 48h. (C and D) The mRNA and protein expression levels of PAQR3 in cells transfected with miR-153-3p mimics or NC mimics were determined by qPCR and western blot. β-actin was applied as the endogenous reference genes. Data were presented as the mean±SD with three independent experiments. **p<0.01 versus control group and ##p<0.01 versus NC mimics group.
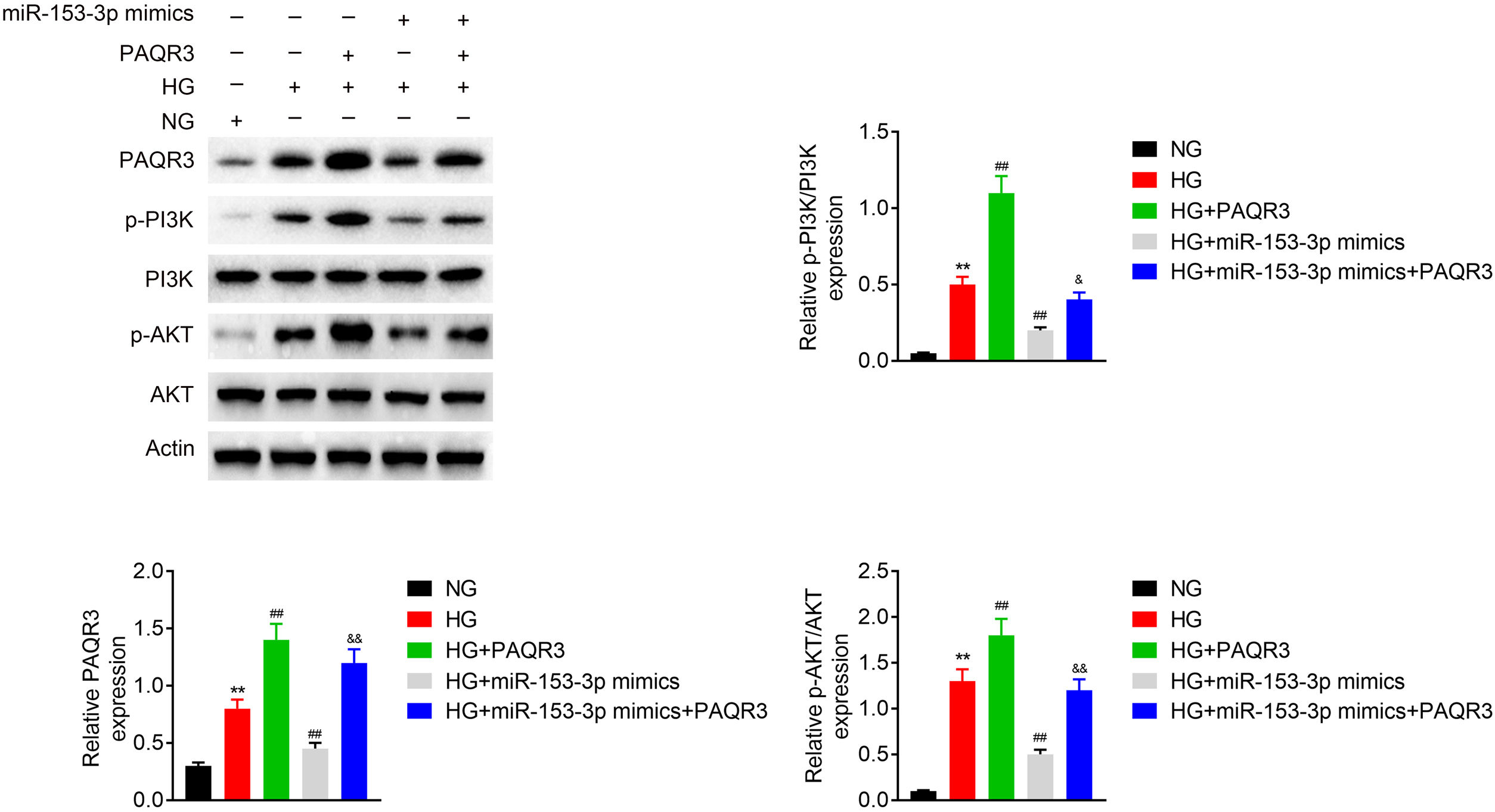
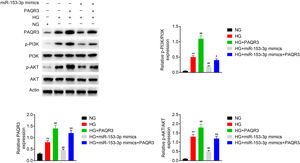
To estimate the molecular mechanism of miR-153-3p regulating PAQR3, western blot assays were applied to detect the expression of PAQR3, PI3K, and AKT in MCs under normal glucose or high glucose treatment. The expression levels of PAQR3 proteins was upregulated in the high glucose group compared to the normal glucose group. Then, the administration of PAQR3 or transfection of miR-153-3p mimics were used to further promote or reverse PAQR3 protein expression, respectively. The results showed that the expression of PAQR3 protein in MCs transfected with miR-153-3p mimics was notably increased after administration with PAQR3. Moreover, the administration of PAQR3 could also promote protein expression of PI3K and AKT. Similarly, the expression of PI3K and AKT protein in MCs transfected with miR-153-3p mimics was notably increased after administration with PAQR3. These data indicated that miR-153-3p regulates the PI3K/AKT signalling pathway through PAQR3 (Fig. 4).
MiR-153-3p regulates the PI3K/AKT signalling pathway through PAQR3. The protein expression levels of PAQR3, PI3K, and AKT in cells transfected with miR-153-3p mimics or/and administration of PAQR3 were determined by western blot. β-actin was applied as the endogenous reference genes. Data were presented as the mean±SD with three independent experiments. **p<0.01 versus control group, ##p<0.01 versus HG group, and &&p<0.01 versus miR-153-3p mimics group.
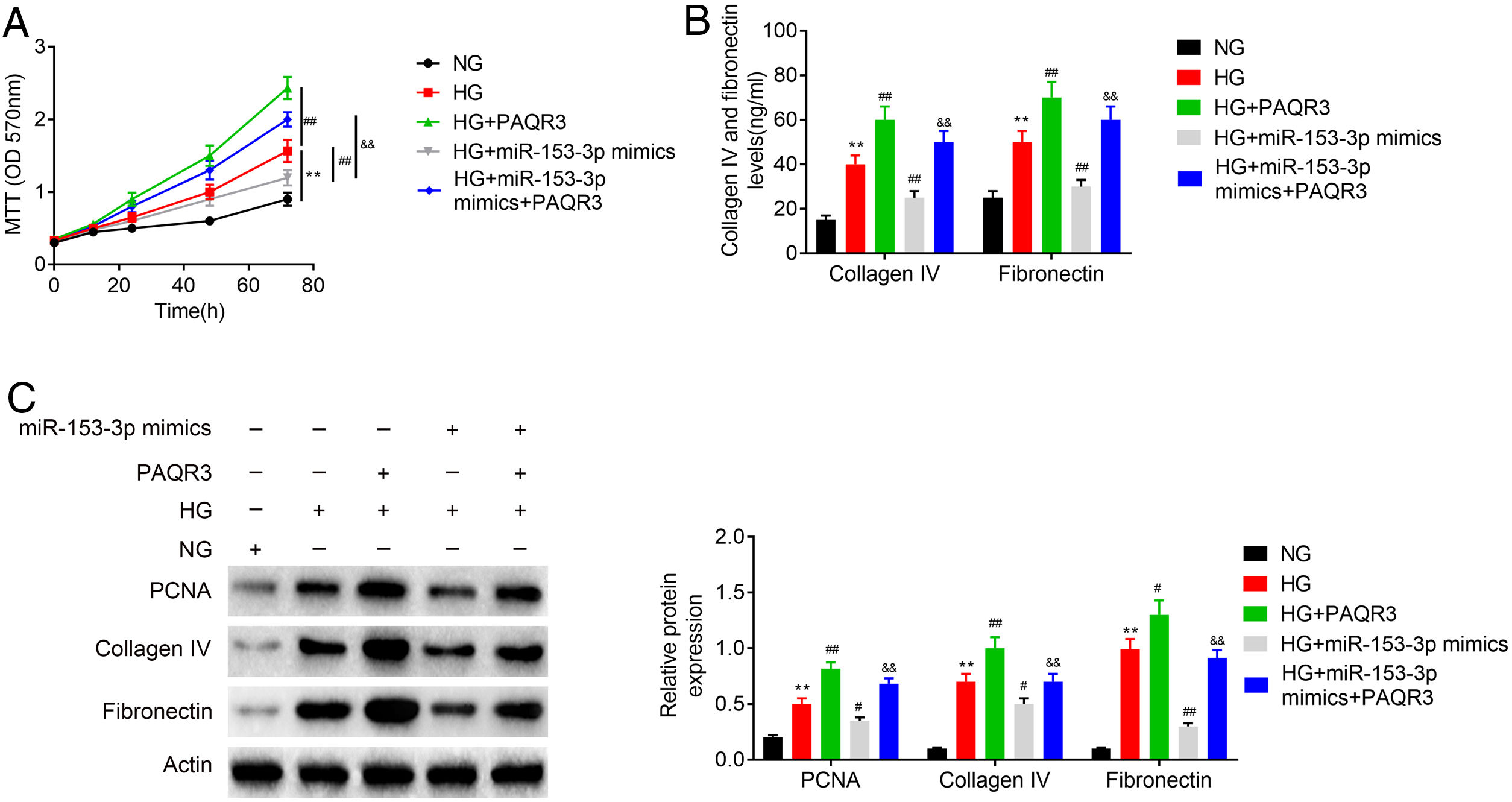
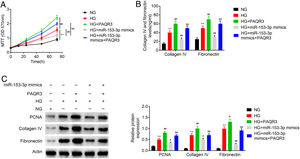
An MTT assay was carried out to examine the effect of miR-153-3p on the cell proliferation of MCs through PAQR3. Previous MTT assays have shown that compared with normal glucose group, high glucose treatment significantly promoted cell proliferation, while miR-153-3p mimic markedly inhibited the cell proliferation ability of MCs. However, the cell proliferation ability of PAQR3-treated MCs was clearly increased compared with the untreated group under high glucose conditions. Interestingly, the cell proliferation ability of MCs transfected with miR-153-3p mimics was also notably increased after administration with PAQR3 (Fig. 5A). Subsequently, the expression levels of ECM proteins, collagen IV and fibronectin, were assessed by ELISA to examine the effects of miR-153-3p on extracellular matrix deposition of MCs through PAQR3. Previous ELISA assays showed that collagen IV and fibronectin were clearly increased in the high glucose group, and decreased in MCs transfected with miR-153-3p mimics. Similar to the result of the MTT assay, the extracellular matrix accumulation of PAQR3-treated MCs was significantly increased compared with the untreated group under high glucose conditions. Interestingly, the extracellular matrix accumulation of MCs transfected with miR-153-3p mimics was also notably increased after administration with PAQR3 (Fig. 5B). Furthermore, a western blot assay was conducted to investigate the regulatory effect of miR-153-3p on cell proliferation and extracellular matrix accumulation of MCs through PAQR3. Similar to the previous MTT and ELISA results, the expression levels of PCNA proteins, which can represent the ability of cell proliferation, and collagen IV as well as fibronectin protein, which can represent the degree of extracellular matrix accumulation, were significantly increased under high glucose conditions compared with the normal glucose group, while miR-153-3p mimic markedly inhibited the expression of these proteins. However, the expression of these proteins of PAQR3-treated MCs was clearly increased compared with the untreated group under high glucose conditions. Interestingly, the expression levels of these proteins in MCs transfected with miR-153-3p mimics was also notably increased after administration with PAQR3 (Fig. 5C). All these data suggested that miR-153-3p regulates cell proliferation and extracellular matrix accumulation of MCs through PAQR3.
PHI ameliorated LPS-induced pathological changes in lung tissue of ALI mice. Cell proliferation ability of MCs was detected using MTT assay. Cells were divided into five groups, including NG, HG, HG+PAQR3, HG+miR-153-3p mimics, and HG+miR-153-3p mimics+PAQR3. (B) Protein expressions levels of collagen IV and fibronectin were detected using ELISA kit. (C) The protein expression levels of PCNA, collagen IV and fibronectin in cells were determined by western blot. β-actin was applied as the endogenous reference genes. Data were presented as the mean±SD with three independent experiments. **p<0.01 versus control group, ##p<0.01 versus HG group, and &&p<0.01 versus miR-153-3p mimics group.
Recently, some studies have suggested that human glomerular mesangial cells, a typical diabetic nephropathy-associated cell line, have the potential for cell proliferation and extracellular matrix accumulation under the high glucose-stimulation in vivo.16 The pathological changes in MCs are accurately mediated by extracellular mechanical and intracellular molecular signals. Understanding the molecular regulatory mechanism of cell proliferation and extracellular matrix differentiation under the high glucose-stimulation of MCs may provide novel therapeutic targets for DN. However, the molecular regulatory mechanism of MCs’ fate determination remains poorly understood.
It has been suggested that MiRNAs play a key role in cell proliferation modulation and extracellular matrix accumulation processes.18,19 Many miRNAs are highly expressed in the kidney system, contributing to the maintenance of normal tissue functions and homeostasis.20 Moreover, many miRNAs are implicated in cell proliferation and ECM accumulation of MCs and dysregulation of miRNAs play a key role in DN.21 Specifically, the relationship between circular RNA circRNA_15698 and the ECM of MCs was confirmed to be regulated by miR-185/TGF-β1.22 Recently, studies have also found that circ_0000491, a novel identified circular RNA, participated in the aggravation of the ECM of MCs by restraining miR-101b through targeting TGFβRI.23 Moreover, miR-382 was found to inhibit MC proliferation and ECM accumulation by regulation of FoxO1 signalling pathways in mice with DN.24 However, the specific molecular regulatory mechanism of cell proliferation and ECM accumulation in MCs under high glucose stimulation in DN by miRNAs requires further investigation. This study disclosed that the expression level of miR-153-3p is lower in DN patients and the degree of cell proliferation and ECM accumulation under high glucose stimulation of MCs could be regulated by upregulating or downregulating the expression level of miR-153-3p. These results suggest that miR-153-3p may act as an inhibiting factor in cell proliferation and ECM accumulation under high glucose stimulation of MCs in DN.
A growing number of studies confirmed that miRNAs carry out their functions by regulating the expression of target mRNAs.25 One previous study proved that overexpressed miR-370 accelerates MC proliferation and ECM accumulation via binding to canopy 1.26 Moreover, miR-29c was reported to be a signature miRNA and participated in the progression of DN via activation of Sprouty homolog 1.27 In addition, lncRNA CTBP1-AS2 was reported to be a mediator of inflammation in DN via miR-155-5p/FOXO1 axis.28 Here, the fact that miR-153-3p could target PAQR3 was demonstrated by predicting the mRNA binding sites in TargetScan and dual-luciferase assays. Moreover, high expression levels of miR-153-3p clearly suppressed PAQR3, PI3K, and AKT protein expression. Furthermore, the results showed that the cell proliferation and ECM accumulation abilities could be promoted under high glucose stimulation while this effect could be amplified by PAQR3 overexpression. Similarly, overexpression of PAQR3 could also abolish the effect of miR-153-3p-suppressed cell proliferation and extracellular matrix accumulation. These data indicated that PAQR3 could aggravate MC proliferation and ECM accumulation of MCs suppressed by miR-153-3p in diabetic nephropathy.
In conclusion, in this study, we found that miR-153-3p and PAQR3 would be downregulated and upregulated in diabetic nephropathy patients, respectively. Moreover, upregulation of miR-153-3p can reduce the MC proliferation and extracellular matrix accumulation. Meanwhile, miR-153-3p regulates the PI3K/AKT pathway through PAQR3. Finally, overexpression of PAQR3 could abolish miR-153-3p-suppressed cell proliferation and extracellular matrix accumulation. Our study demonstrates that miR-153-3p regulates MC proliferation and ECM accumulation through the PAQR3 pathway, which inspires future research on the mechanisms underlying DN pathogenesis and provides new ideas for the diagnosis and treatment of DN.
Availability of data and materialsAll data generated or analysed during this study are included in this published article.
FundingThis work was supported by the Nantong City Science and Technology Project (Grant No. JC2019040).
Authors’ contributionsHongli Yang and Xingxing Fang designed the study, supervised the data collection, Yan Shen and Wubin Yao analysed and interpreted the data, Dongmei Chen and Lianglan Shen prepared the manuscript for publication and reviewed the draft of the manuscript. All authors have read and approved the manuscript.
Conflict of interestsThe authors state that there are no conflicts of interest to disclose.