To evaluate the association between three obesity markers, body mass index (BMI), abdominal circumference (AC), waist to height ratio (WHtR), and albuminuria in adults seen in a primary health care center specialized in chronic diseases in Lima, Perú.
MethodsA cross-sectional, descriptive, retrospective study in adults who attended a primary health care center specialized in chronic diseases in 2011. Patients were divided into four categories: healthy subjects and patients with high blood pressure, with type 2 diabetes mellitus (T2DM), and with both diseases (HBP+T2DM). The main outcome was presence of albuminuria, defined as urine albumin levels higher than 30mg/day. Exposure variables included the following obesity markers: body mass index (BMI), waist-to-height ratio (WHtR), and abdominal circumference (AC). Other covariates considered included sex and age. Crude and adjusted Poisson regressions were performed to estimate prevalence ratios (PRs) and their respective 95% confidence intervals (95% CIs). Areas under the curve were calculated for each indicator, stratified by sex.
ResultsData from 1214 patients, 41% of them male, were analyzed, and albuminuria was found in 14.2%. Albuminuria was found to be associated to AC and WHtR, but not to BMI. All three parameters assessed had similar areas under the curve. The optimum cut-off points found for BMI and AC in females were higher than conventional (32.7kg/m2 and 93cm respectively), while the values in males were lower than conventional (27.9kg/m2 and 100cm respectively). For WHtR, however, the optimum cut-off point was higher in both sexes. The higher index in females was for BMI, followed by AC and WHtR. In males, the higher index was for WHtR, followed by AC and BMI.
ConclusionsAC and WHtR were found to be directly associated to albuminuria, while BMI was not associated to albuminuria. Areas under the curve were similar for all three markers. The optimum cut-off points for BMI and AC were higher than the conventional ones in females and lower in males.
Evaluar la asociación entre tres marcadores de obesidad: índice de masa corporal (IMC), perímetro abdominal (PA) e índice cintura/talla (ICT) con albuminuria, en adultos de un centro de atención primaria especializado en enfermedades crónicas de Lima, Perú.
MétodosEstudio transversal descriptivo retrospectivo de adultos atendidos en un centro de atención primaria especializado en enfermedades crónicas en el 2011. Se incluyeron participantes con hipertensión arterial, con diabetes mellitus tipo 2 (DM2), con ambas condiciones (hipertensión arterial y DM2) y participantes sin hipertensión arterial ni DM2. El desenlace de interés fue el tener albuminuria, definido como albuminuria en orina >30mg/día. Las variables de exposición fueron los siguientes marcadores de obesidad: IMC, ICT y PA. Otras covariables consideradas fueron sexo y edad. Se realizaron regresiones de Poisson crudas y ajustadas para estimar razones de prevalencia y sus respectivos intervalos de confianza al 95% (IC95%). Se calcularon las áreas bajo la curva para cada indicador y se hallaron los puntos de corte con óptimos con el índice de Younden, estratificando por sexo.
ResultadosSe analizaron datos de 1.214 pacientes, el 41.0% fueron varones y el 14,2% tuvo albuminuria. El PA y el ICT estuvieron significativamente asociados con tener albuminuria, mas no el IMC. Los tres parámetros evaluados tuvieron áreas bajo la curva similares. Los puntos de corte óptimos encontrados para IMC y PA en mujeres fueron mayores a los convencionales (32,7kg/m2 y 93cm respectivamente), en tanto que para varones los puntos de corte óptimos fueron menores a los convencionales (27,9kg/m2 y 100cm respectivamente). Sin embargo, para el ICT el punto de corte óptimo fue mayor al convencional en ambos sexos.
ConclusionesSe encontró una asociación directa entre PA e ICT con albuminuria, pero no entre IMC y albuminuria. Las áreas bajo la curva fueron similares para los tres marcadores. Los puntos de corte óptimos para IMC y PA fueron mayores a los convencionales en mujeres, y menores a los convencionales en varones. El punto de corte óptimo para ICT fue mayor al convencional en ambos sexos.
Albuminuria is defined by the National Kidney Foundation in the Kidney Disease Outcomes Quality Initiative as urine albumin >30mg/24h,1 and is an indicator of vascular permeability secondary to an intraglomerular hemodynamic anomaly.2,3 Different studies have found the presence of albuminuria in the general population to be a predictor of cardiovascular diseases and of chronic kidney disease.4–7
The main risk factors for the appearance and development of albuminuria are arterial hypertension (AHT), type 2 diabetes mellitus (T2DM) and obesity.4,5,8 However, the physiopathology of albuminuria varies among different disease conditions. In this regard, albuminuria in AHT is essentially related to endothelial dysfunction, while in T2DM it is related to damage to the glomerular filtration membrane, intraglomerular hypertension and hyperfiltration that leads to diabetic neuropathy.9,10
Obesity is usually evaluated by means of different markers such as the body mass index (BMI), the waist-to-height ratio (WHtR) and abdominal circumference (AC). In obese individuals, albuminuria can appear as a consequence of structural alterations to the glomerular filtration barrier secondary to hyperfiltration.9,11 Such potential damage underscores the need for screening initiatives and early interventions in order to avoid albuminuria and thus prevent cardiovascular diseases or the progression of chronic kidney disease in obese patients.
Although the association between obesity and albuminuria has been studied in European, North American and Asian populations with their particular anthropometric characteristics,12–14 few studies have evaluated and compared the usefulness of obesity markers as risk measures of albuminuria in Latin American populations. It is therefore difficult to establish precise recommendations for these populations regarding obesity control with a view to reducing the risk of albuminuria.
The present study was thus carried out to evaluate the association between three obesity markers (BMI, AC and WHtR) and albuminuria in adults seen in a primary care center specializing in chronic diseases in Lima (Peru).
Materials and methodsStudy designA secondary analysis was made of the case histories database of the Diabetes and Hypertension Center (Centro de Diabetes e Hipertensión [CEDHI]) of the national Public Health system (Seguro Social [EsSalud]) in Lima (Peru). This center receives patients ≥18 years of age referred from the primary care centers belonging to the Rebagliati – EsSalud healthcare network for annual control.
Population and sampleWe analyzed all the case histories of the patients attending the CEDHI between 1 January and 31 August 2011, since in this period all the histories were registered in electronic format. We included those histories whose results referred to albuminuria, and excluded patients with laboratory test results which suggested urinary infection (>10 leukocytes per field in urinary sediment), patients with confirmed urinary infection receiving antibiotic treatment for urinary infection, and pregnant women.
ProceduresDuring the study period, all the CEDHI patients underwent measurements of body weight, height, AC and blood pressure, carried out by four trained nurses. Weight and height were recorded using a standard scale and measuring rod (Detecto®, USA), with the patient in the standing position and in light clothing without shoes. Abdominal circumference in turn was measured with an inextensible metric tape fitted around an imaginary line corresponding to a horizontal plane at the height of the midpoint between the lower margin of the ribcage and the anterior superior iliac spine on the abdomen. The measurements were obtained with the patient either naked to the waist or wearing very light clothing, in the standing position, at rest and at the end of a normal expiration. Blood pressure was recorded using a mercury sphygmomanometer (Nova-Presameter®, Riester, Jungingen, Germany) with the patient in the standing position after a 15-min resting period, and with coffee being avoided prior to evaluation. Hypertensive patients were instructed to continue taking their blood pressure-lowering medication.
Furthermore, all the CEDHI patients underwent laboratory testing to record the following parameters: glucose, creatinine, hemoglobin, albuminuria (based on urine collection corresponding to the 24h before the visit to the CEDHI) and full urine parameters (first morning urine). The patients were then evaluated by the CEDHI specialists in the cardiology, endocrinology and internal medicine consulting rooms as indicated, according to the reason for reference to the center.
Albuminuria and all the biochemical tests were carried out on an automated basis in the home care program laboratory (PADOMI) – Essalud, using a Konelab™ PRIME 60 Clinical Chemistry Analyzer (Thermo Fisher Scientific, Vantaa, Finland).
Definition of variablesThe study endpoint was albuminuria, defined as albumin >30mg/day1 in a 24-h urine sample, using the methodology described above. The exposure variables were the WHtR, the BMI and AC. The waist-to-height ratio was obtained by dividing AC by height (both in cm). The BMI in turn was calculated as body weight divided by height, squared (kg/m2).
Patient age and gender were also evaluated. Systolic blood pressure was stratified as <140, 140 to <160, and ≥160mmHg. Diastolic blood pressure was stratified as <60, 140 to <90, and ≥90mmHg. Anemia was defined by hemoglobin <11mg/dl in females and <12mg/dl in males. The glomerular filtration rate was calculated using the Modification of Diet in Renal Disease Study-4 (MDRD 4)15 formula, and was stratified as <30, 30 to <60, and ≥60ml/min.
Statistical analysisA descriptive analysis was made, absolute and relative frequencies being calculated, as well as the mean and standard deviation (SD). A bivariate analysis was carried out to determine differences in the main variables according to gender, using the chi-squared test.
The BMI was classified as normal (<25kg/m2), overweight (≥25kg/m2 to <30kg/m2) or obese (≥30kg/m2).16 The AC and WHtR values were stratified into tertiles, since no validated cut-off points are available for Latin America.
Crude and adjusted Poisson regressions with robust variances were performed to estimate the prevalence ratios (PRs) of the association between each of the obesity markers (WHtR, BMI, AC) and the presence of albuminuria. The models were adjusted for diagnosis, gender and age.
Comparisons were made of the receiver operating characteristic (ROC) curves, sensitivity, specificity, Youden's index and the percentage of patients correctly classified as presenting albuminuria with respect to each of the obesity markers, on an independent basis for males and females.
Youden's index is defined as the sum of sensitivity and specificity, with the subtraction of 1. We obtained Youden's index for each cut-off point with the aim of establishing the cut-off point offering the best sensitivity/specificity balance (optimum cut-off point). The values obtained were compared with the commonly used cut-off points. All the statistical analyses were carried out using the Stata version 13.0 package (StataCorp® LP, College Station, USA).
Ethical considerationsThe present study comprises a retrospective data analysis; as a result, there was no contact with human subjects. In such situations, the possible risks for the subjects are minimal and mainly concern breaches of confidentiality. We faced no such problem, however, since no personal identifiers were compiled (such as names, identification card numbers, case history numbers, addresses or telephone numbers).
The study was approved by the research and Ethics Committee of the Rebagliati – EsSalud healthcare network (resolution number: 379-GRAR ESSALUD-2016).
ResultsDescriptive analysisWe evaluated a total of 1476 case histories of patients who attended the CEDHI between January and August 2011. A total of 228 patients were excluded due to a lack of albuminuria results; a further 33 patients were excluded due to the presence of urinary infection as evidenced from the case history or urine sediment findings; and one pregnant patient was also excluded. This left a total of 1214 patients who met the eligibility criteria and who were included in the analysis.
The mean patient age was 65.8 (SD: 12.7) years; 498 were males (41.0%). The distribution of patients according to the diagnosis was as follows: 661 presented AHT but no T2DM (54.5%), 105 had T2DM but no AHT (8.6%), 242 had both T2DM and AHT (19.9%), and 206 presented neither T2DM nor AHT at the time of the evaluation (17.0%). In turn, 14.2% of the patients presented albuminuria.
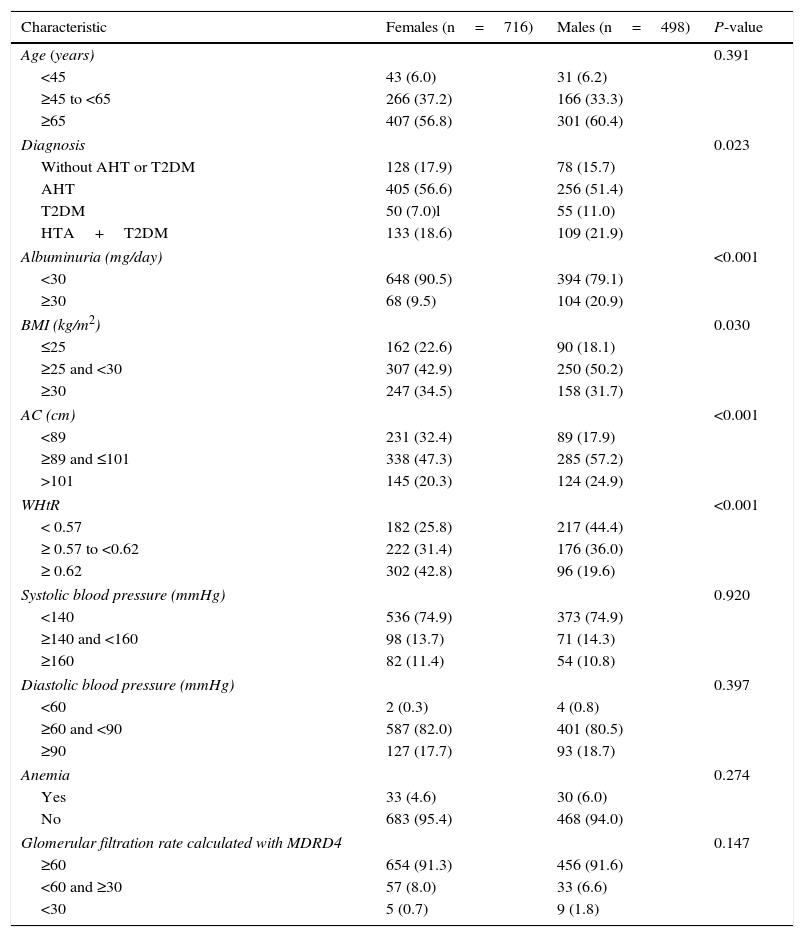
In terms of the BMI, 45.9% of the patients were overweight and 33.4% were obese. The mean WHtR was 0.6 (SD: 0.7). The mean AC was 96.9cm (SD: 10.3). Obesity as determined by the BMI was slightly more prevalent in females (34.5% vs 31.7%), while AC>101cm was less frequent in women (20.3 vs 24.9%) and the WHtR≥0.62 was more prevalent among females (42.8% vs 19.6%) (Table 1).
General characteristics of the study population according to gender.
| Characteristic | Females (n=716) | Males (n=498) | P-value |
|---|---|---|---|
| Age (years) | 0.391 | ||
| <45 | 43 (6.0) | 31 (6.2) | |
| ≥45 to <65 | 266 (37.2) | 166 (33.3) | |
| ≥65 | 407 (56.8) | 301 (60.4) | |
| Diagnosis | 0.023 | ||
| Without AHT or T2DM | 128 (17.9) | 78 (15.7) | |
| AHT | 405 (56.6) | 256 (51.4) | |
| T2DM | 50 (7.0)l | 55 (11.0) | |
| HTA+T2DM | 133 (18.6) | 109 (21.9) | |
| Albuminuria (mg/day) | <0.001 | ||
| <30 | 648 (90.5) | 394 (79.1) | |
| ≥30 | 68 (9.5) | 104 (20.9) | |
| BMI (kg/m2) | 0.030 | ||
| ≤25 | 162 (22.6) | 90 (18.1) | |
| ≥25 and <30 | 307 (42.9) | 250 (50.2) | |
| ≥30 | 247 (34.5) | 158 (31.7) | |
| AC (cm) | <0.001 | ||
| <89 | 231 (32.4) | 89 (17.9) | |
| ≥89 and ≤101 | 338 (47.3) | 285 (57.2) | |
| >101 | 145 (20.3) | 124 (24.9) | |
| WHtR | <0.001 | ||
| < 0.57 | 182 (25.8) | 217 (44.4) | |
| ≥ 0.57 to <0.62 | 222 (31.4) | 176 (36.0) | |
| ≥ 0.62 | 302 (42.8) | 96 (19.6) | |
| Systolic blood pressure (mmHg) | 0.920 | ||
| <140 | 536 (74.9) | 373 (74.9) | |
| ≥140 and <160 | 98 (13.7) | 71 (14.3) | |
| ≥160 | 82 (11.4) | 54 (10.8) | |
| Diastolic blood pressure (mmHg) | 0.397 | ||
| <60 | 2 (0.3) | 4 (0.8) | |
| ≥60 and <90 | 587 (82.0) | 401 (80.5) | |
| ≥90 | 127 (17.7) | 93 (18.7) | |
| Anemia | 0.274 | ||
| Yes | 33 (4.6) | 30 (6.0) | |
| No | 683 (95.4) | 468 (94.0) | |
| Glomerular filtration rate calculated with MDRD4 | 0.147 | ||
| ≥60 | 654 (91.3) | 456 (91.6) | |
| <60 and ≥30 | 57 (8.0) | 33 (6.6) | |
| <30 | 5 (0.7) | 9 (1.8) | |
T2DM: type 2 diabetes mellitus; AHT: arterial hypertension; WHtR: waist-to-height ratio; BMI: body mass index; MDRD4: Modification of Diet in Renal Disease; AC: abdominal circumference.
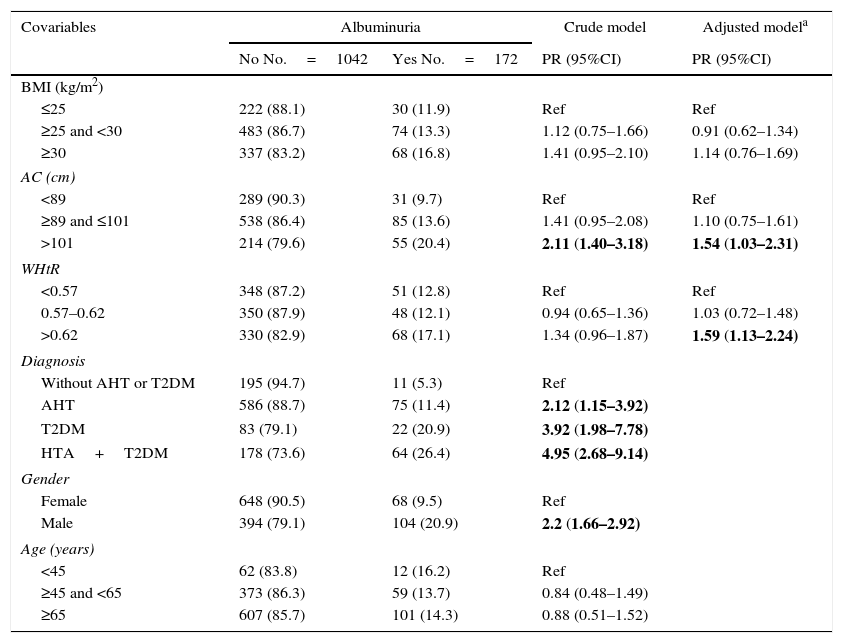
The crude model identified male gender, T2DM, AHT or both disorders combined as risk factors for albuminuria. On adjusting for diagnosis, gender and age, both AC and WHtR were seen to be associated with albuminuria, but not the BMI (Table 2). These associations persisted when the sensitivity analysis was performed, stratified according to diagnosis (T2DM, AHT, neither and both disorders).
Crude and adjusted association between obesity markers and albuminuria.
| Covariables | Albuminuria | Crude model | Adjusted modela | |
|---|---|---|---|---|
| No No.=1042 | Yes No.=172 | PR (95%CI) | PR (95%CI) | |
| BMI (kg/m2) | ||||
| ≤25 | 222 (88.1) | 30 (11.9) | Ref | Ref |
| ≥25 and <30 | 483 (86.7) | 74 (13.3) | 1.12 (0.75–1.66) | 0.91 (0.62–1.34) |
| ≥30 | 337 (83.2) | 68 (16.8) | 1.41 (0.95–2.10) | 1.14 (0.76–1.69) |
| AC (cm) | ||||
| <89 | 289 (90.3) | 31 (9.7) | Ref | Ref |
| ≥89 and ≤101 | 538 (86.4) | 85 (13.6) | 1.41 (0.95–2.08) | 1.10 (0.75–1.61) |
| >101 | 214 (79.6) | 55 (20.4) | 2.11 (1.40–3.18) | 1.54 (1.03–2.31) |
| WHtR | ||||
| <0.57 | 348 (87.2) | 51 (12.8) | Ref | Ref |
| 0.57–0.62 | 350 (87.9) | 48 (12.1) | 0.94 (0.65–1.36) | 1.03 (0.72–1.48) |
| >0.62 | 330 (82.9) | 68 (17.1) | 1.34 (0.96–1.87) | 1.59 (1.13–2.24) |
| Diagnosis | ||||
| Without AHT or T2DM | 195 (94.7) | 11 (5.3) | Ref | |
| AHT | 586 (88.7) | 75 (11.4) | 2.12 (1.15–3.92) | |
| T2DM | 83 (79.1) | 22 (20.9) | 3.92 (1.98–7.78) | |
| HTA+T2DM | 178 (73.6) | 64 (26.4) | 4.95 (2.68–9.14) | |
| Gender | ||||
| Female | 648 (90.5) | 68 (9.5) | Ref | |
| Male | 394 (79.1) | 104 (20.9) | 2.2 (1.66–2.92) | |
| Age (years) | ||||
| <45 | 62 (83.8) | 12 (16.2) | Ref | |
| ≥45 and <65 | 373 (86.3) | 59 (13.7) | 0.84 (0.48–1.49) | |
| ≥65 | 607 (85.7) | 101 (14.3) | 0.88 (0.51–1.52) | |
T2DM: type 2 diabetes mellitus; AHT: arterial hypertension; WHtR: waist-to-height ratio; BMI: body mass index; AC: abdominal circumference; PR: prevalence ratio.
The statistically significant results are shown in boldface.
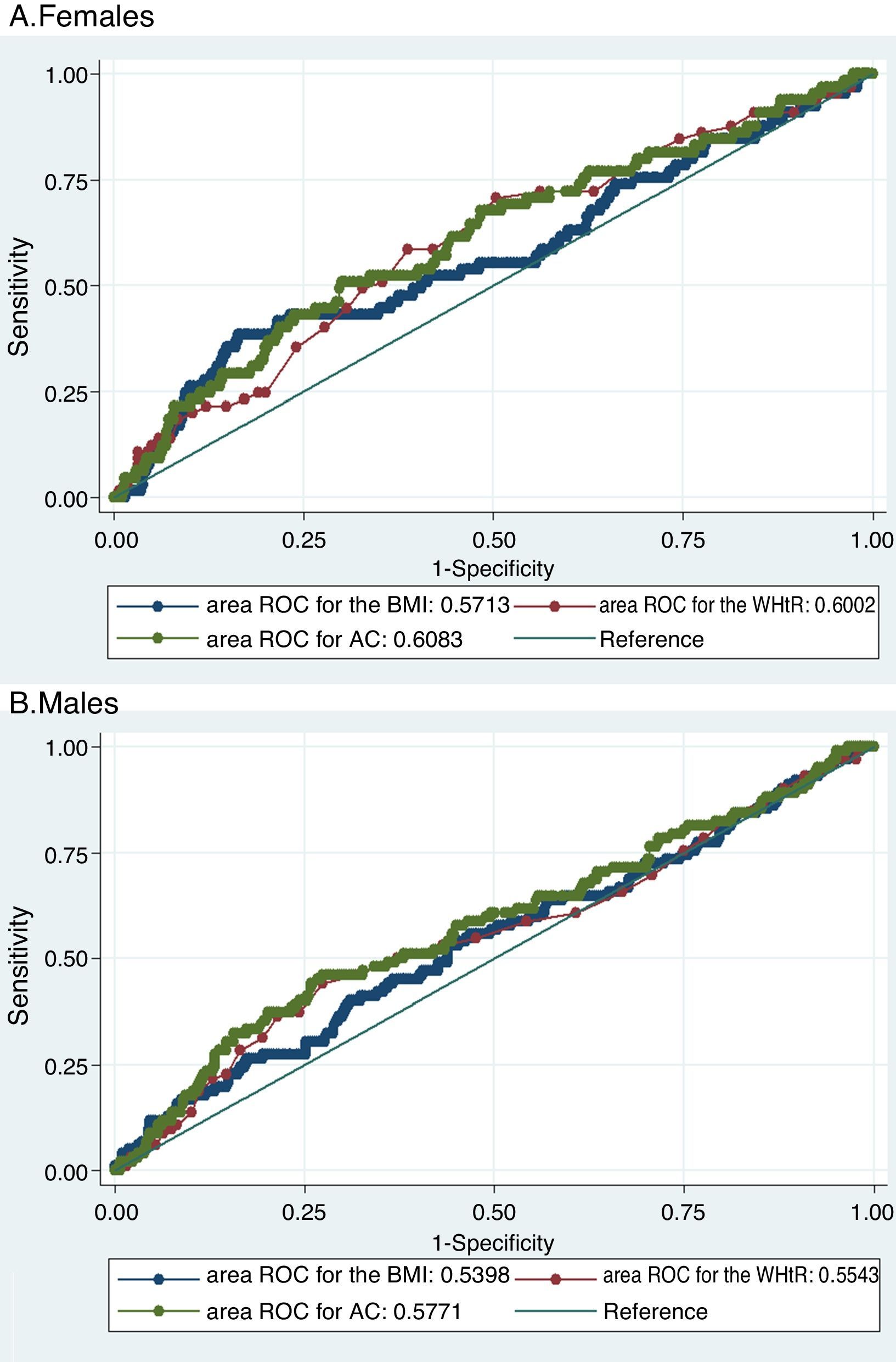
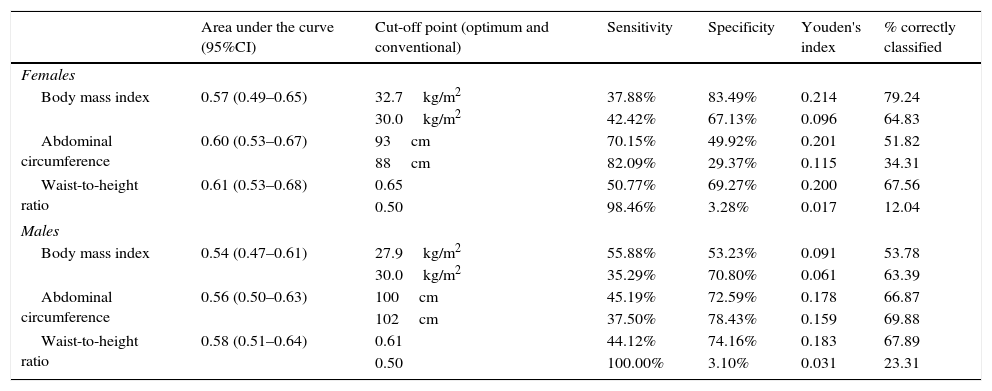
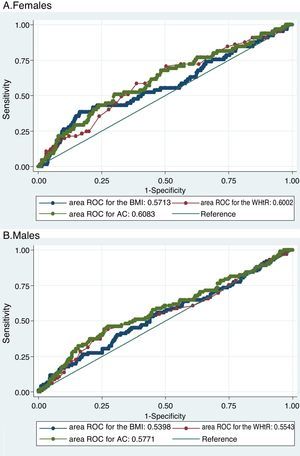
The three evaluated obesity markers showed similar areas under the ROC curves (AUC) in relation to albuminuria, in both males and females (Table 3 and Fig. 1).
Area under the receiver operating characteristic (ROC) curve, and optimum and conventional cut-off points of the obesity markers for predicting albuminuria according to gender.
| Area under the curve (95%CI) | Cut-off point (optimum and conventional) | Sensitivity | Specificity | Youden's index | % correctly classified | |
|---|---|---|---|---|---|---|
| Females | ||||||
| Body mass index | 0.57 (0.49–0.65) | 32.7kg/m2 | 37.88% | 83.49% | 0.214 | 79.24 |
| 30.0kg/m2 | 42.42% | 67.13% | 0.096 | 64.83 | ||
| Abdominal circumference | 0.60 (0.53–0.67) | 93cm | 70.15% | 49.92% | 0.201 | 51.82 |
| 88cm | 82.09% | 29.37% | 0.115 | 34.31 | ||
| Waist-to-height ratio | 0.61 (0.53–0.68) | 0.65 | 50.77% | 69.27% | 0.200 | 67.56 |
| 0.50 | 98.46% | 3.28% | 0.017 | 12.04 | ||
| Males | ||||||
| Body mass index | 0.54 (0.47–0.61) | 27.9kg/m2 | 55.88% | 53.23% | 0.091 | 53.78 |
| 30.0kg/m2 | 35.29% | 70.80% | 0.061 | 63.39 | ||
| Abdominal circumference | 0.56 (0.50–0.63) | 100cm | 45.19% | 72.59% | 0.178 | 66.87 |
| 102cm | 37.50% | 78.43% | 0.159 | 69.88 | ||
| Waist-to-height ratio | 0.58 (0.51–0.64) | 0.61 | 44.12% | 74.16% | 0.183 | 67.89 |
| 0.50 | 100.00% | 3.10% | 0.031 | 23.31 | ||
Receiver operating characteristic (ROC) curves of obesity markers for predicting albuminuria.
Both figures show the ROC curves for each obesity marker to overlap both in females (A) and in males (B), thus showing the capacity of each of the obesity markers to predict albuminuria to be similar.
BMI: body mass index; WHtR: waist-to-height ratio; AC: abdominal circumference; ROC: receiver operating characteristic.
On comparing the cut-off points commonly used in the literature with the optimum cut-off points calculated in our study, we found the optimum cut-off point for the BMI and AC in women to be greater than the conventional cut-off point, while in males the optimum cut-off point was lower than the conventional cut-off point. However, in the case of the WHtR, the optimum cut-off point was higher than the conventional cut-off point for both males and females.
On comparing Youden's index with regard to the optimum cut-off points, the highest index in women corresponded to the BMI, followed by AC and the WHtR. In males the highest index corresponded to the WHtR, followed by AC and the BMI (Table 3).
DiscussionIn our study population AC and the WHtR were seen to be associated with albuminuria, though the BMI was not. In both genders, the AUC values corresponding to the BMI, AC and the WHtR were found to be similar. The optimum cut-off point (i.e., that affording the best sensitivity/specificity balance) for the BMI and AC in women was found to be greater than the conventional cut-off point, while in males the optimum cut-off point was lower than the conventional cut-off point. However, in the case of the WHtR, the optimum cut-off point was higher than the conventional cut-off point for both males and females.
As to the prevalence of albuminuria revealed in our study, 26.4% of the patients with AHT and T2DM, as well as 5.3% of the patients with neither of these disorders, presented albuminuria. These findings are consistent with the prevalences obtained in other population-based studies in the literature.17 However, our results reflect lower prevalences in comparison with other studies in patients from centers specializing in chronic diseases, where the documented prevalences were 36.3% and 50%.18,19
Males showed a higher prevalence of albuminuria than females, in contrast to the findings of studies in Chinese and North American populations.20,21 This is probably because the males of our population showed a higher frequency of T2DM as well as of both T2DM and AHT combined, these being known risk factors for albuminuria. Nevertheless, the different definitions of albuminuria used by other studies must be taken into account. In the Chinese population, Chen et al. used lower cut-off points in defining albuminuria (14mg/g in males and 20mg/g in females),20 while in North America Saydah et al. used an albumin/creatinine ratio of >30mg/g to define albuminuria.21 In Peru, studies have presented different results regarding the association between female gender and albuminuria. Berrios et al., in a multicenter chronic kidney disease screening study among patients participating in “World Kidney Day” in 2010, where 68.4% were females, 51.9% were hypertensive and 19.3% suffered diabetes, identified female gender as a protective factor against albuminuria.22 By contrast, Herrera-Añazco et al., in a study involving diabetic patients subjected to a first nephrological evaluation in hospitals in Lima, where 52% were females, found female gender to be associated with albuminuria.23 The differences with respect to our own findings may be explained by the differences in the frequency of T2DM and AHT in the studied populations, as well as by the way in which albuminuria was evaluated. In effect, while we defined albuminuria as >30mg of albumin in urine per day, Berrios et al. used reactive strips to assess albuminuria, defined as >20mg/l.
The AUCs corresponding to all the obesity markers were slightly greater in women than in men. This appears to indicate that albuminuria in males is influenced by gender-specific factors such as sex hormone levels that may explain the inflammatory mechanisms linking central obesity to albuminuria.24
With regard to the BMI, the optimum cut-off points were greater in women than in men. This may be because women usually accumulate more fat in the region of the buttocks and thighs, while males do so at the abdominal level.25,26 Thus, in males and females with the same BMI, the former have a greater volume of visceral adipose tissue. Likewise, for each single-point increment in the BMI, visceral fat increases more in males than in females.26,27
This observation underscores the limitations of the BMI in evaluating different risk factors in males and females, and points to the need for adequate cut-off points for the Latin population, based on the visceral adipose tissue volume distribution in this population. This is because visceral fat is the adipose tissue related to albuminuria28 and to other disorders such as T2DM, AHT, and cardiovascular disease.29
Abdominal circumference and the WHtR are anthropometric parameters based on the measurement of AC, an indicator of visceral fat. Central or visceral adipose tissue is associated with a range of cardiovascular outcomes such as T2DM, AHT, coronary disease and albuminuria, since it secretes inflammatory factors, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNFα), which cause endothelial dysfunction at glomerular level (among other tissues), thereby incrementing urine albumin excretion.30
These parameters showed a significant association with albuminuria, though they were evaluated with non-conventional cut-off points (stratified into tertiles). This reflects the importance of visceral fat in the development of albuminuria, and underscores the usefulness of these evaluations as possible indicators of albumin urine loss. However, AC is more difficult to evaluate than the BMI, and thus requires greater training. This should be taken into account when a diagnostic test is being selected. Of the different parameters, the WHtR exhibits a slightly greater AUC, possibly because it is a more precise indicator, since its calculation incorporates AC.31
Our findings may have clinical implications. Although the T2DM and AHT management guides advocate periodic albuminuria screening,32 such recommendations are often difficult to follow in regions characterized by a lack of resources and healthcare infrastructures, where physicians must prioritize screening and preventive efforts, focusing their attention on higher risk patients.
In this context, our findings show that the obesity indicators are modestly associated with the prevalence of albuminuria. Consequently, they may be factors to be taken into account when patients with and without chronic disorders are being selected for screening and corresponding preventive actions.
Although the three anthropometric indicators studied (BMI, WHtR and AC) showed similar AUCs, we consider that the WHtR and AC are possibly the most useful, since they were significantly associated with albuminuria. The choice of cut-off point remains controversial, due to the lack of data for Latin American populations.
However, since the sensitivity and specificity of the conventional and optimum cut-off points are low, albuminuria screening should not be based on this criterion alone. Further studies are needed in this regard in order to determine the combination of risk factors that may alert the physician to the need for albuminuria screening.
The lack of data regarding the duration of the disease, hip circumference, antihypertensive medication capable of modifying the albuminuria values, and other intrinsic renal disorders, is an obvious limitation of our study.
Nevertheless, our study evaluates different obesity markers and affords information of importance in assessing obesity among the Peruvian population. In this regard, our findings make a contribution to the Latin American literature, which has scant representation in this field of research.
In conclusion, on examining the association between obesity markers and albuminuria, we have identified a direct correlation between AC and the WHtR and albuminuria, but not between the BMI and albuminuria. The AUCs were similar for all three markers. The optimum cut-off points for the BMI and AC were greater than the conventional cut-off points in women, and lower than the conventional cut-off points in men. The optimum cut-off point for the WHtR was greater than the conventional cut-off point in both males and females.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Quintanilla AE, Taype-Rondan Á, Lazo-Porras M, Herrera-Añazco P. Marcadores de obesidad asociados a albuminuria en un centro de atención primaria de Lima, Perú. Endocrinol Diabetes Nutr. 2017;64:295–302.