To evaluate the effectiveness and safety of sensor-augmented insulin pump therapy (SAP) in addition to a comprehensive diabetes program on glycated hemoglobin (HbA1c), severe hypoglycemia, ketoacidosis, and the hospital admission rate in patients with type 1 diabetes under real-world settings during a 2-year follow-up.
MethodsThis was a retrospective real-life study comparing diabetes control before and after SAP therapy initiation. Patients ≥18 years old with type 1 diabetes were included. They were followed for 2 years with clinical assessments at months 3, 6, 12, 18, and 24. Effectiveness was estimated by difference in medians of HbA1c from baseline and at each follow-up visit. Safety was assessed by comparing the annual rates of severe hypoglycemia, hyperglycemic crisis, and hospital admission related to diabetes.
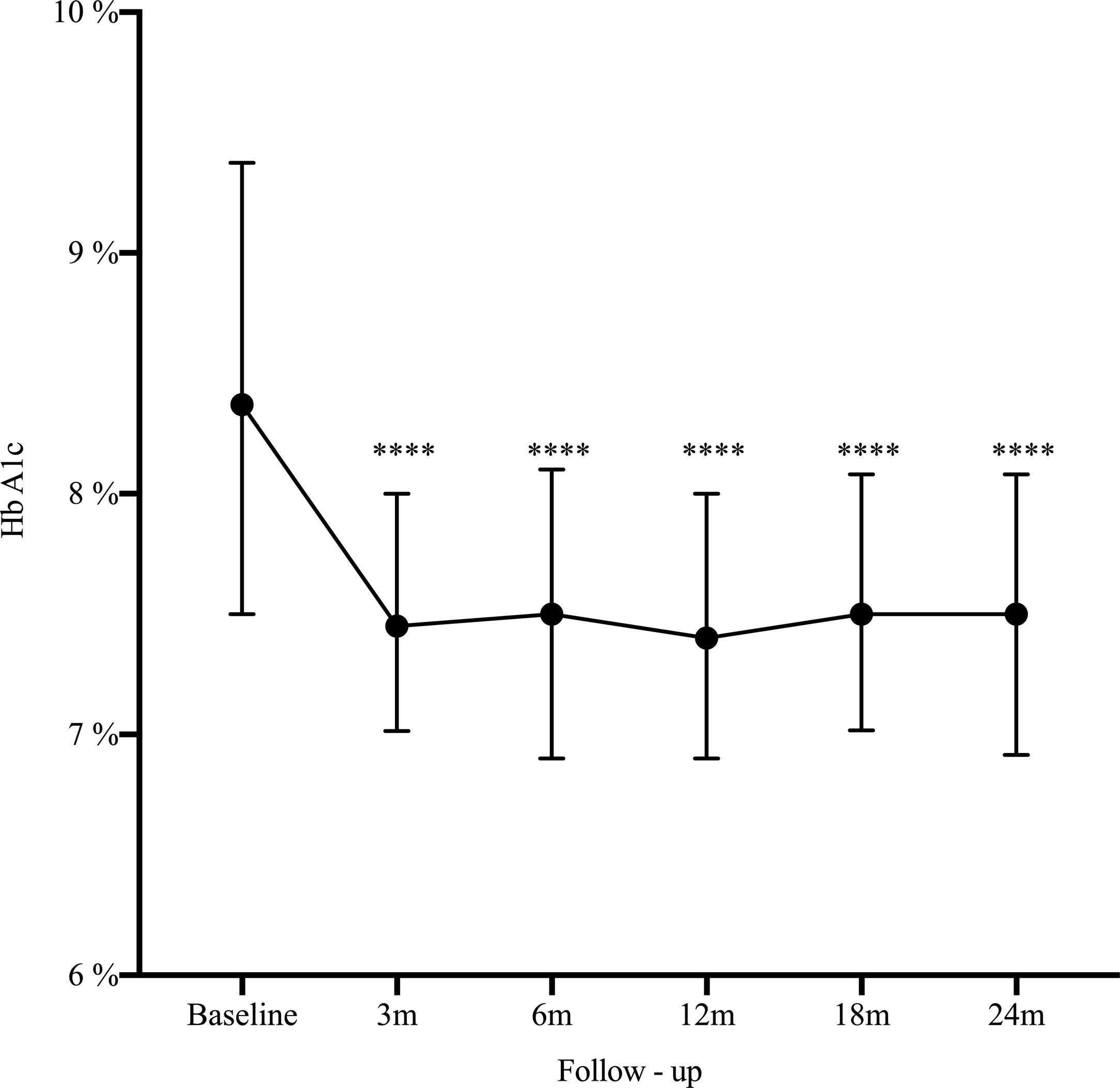
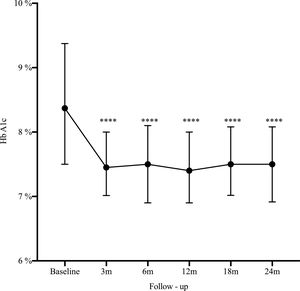
Results162 patients were included, median age 32 years, women 73%). The main indication for SAP was poor metabolic control (51.2%). At 2 years HbA1c decreased from 8.4% to 7.5% (−0.9%, 95% CI: 0.5–1.2; p<0.0001), HbA1c ≤7% improved from 14.2% to 25.3% (11.1%, 95% CI: 19.7–2.5; p=0.006), and severe hypoglycemia decreased from 22.2% to 14.1% (−8.1%, 95% CI: −16.5 to 0.3; p=0.03).
ConclusionsSAP therapy improved glycemic control after the third month of use and for up to 2 years of follow-up, with lower rates of hospital admission and severe hypoglycemia. More studies are needed to assess the add-on impact of education programs and technologies for diabetes care.
Evaluar la efectividad y seguridad de la terapia con bomba de insulina aumentada por sensor (SAP), junto con un programa integral de diabetes en hemoglobina glicosilada, hipoglucemia severa, cetoacidosis y tasa de admisión hospitalaria en pacientes con diabetes tipo 1 bajo condiciones del mundo real durante el seguimiento a dos años.
MétodosEste es un estudio retrospectivo de vida real comparando el control de la diabetes, antes y después de iniciar terapia SAP. Se incluyeron pacientes ≥18 años de edad con diabetes tipo 1. Fueron seguidos por dos años con evaluación clínica en los meses tres, seis, 12, 18 y 24. La efectividad fue estimada por diferencia en medianas de HbA1c entre la línea de base y en cada visita del seguimiento. La seguridad se evaluó comparando las tasas anuales de hipoglucemia severa, crisis hiperglucémica y hospitalización relacionada con diabetes.
ResultadosSe incluyeron 162 pacientes con diabetes tipo 1, mediana de edad 32 años, 73% mujeres. La indicación principal para terapia SAP fue el pobre control glucémico (51,2%). A los dos años, la mediana de HbA1c disminuyó de 8,4 a 7,5% (−0,9%, IC 95%: 0,5 a 1,2; p<0,0001), HbA1c ≤7% mejoró del 14,2 al 25,3% (11,1%, IC 95%: 19,7 a 2,5; p=0,006), y las hipoglucemias severas disminuyeron de 22,2 a 14,1% (−8,1%, IC 95%: −16,5 a 0,3; p=0,03).
ConclusionesLa terapia SAP mejoró el control glucémico después del tercer mes de uso y hasta los dos años de seguimiento, con menores tasas de admisión hospitalaria e hipoglucemias severas. Se necesitan más estudios para evaluar el impacto adicional de los programas de educación en las tecnologías para el cuidado de la diabetes.
Continuous Subcutaneous Insulin Infusion (CSII) therapy and Continuous Glucose Monitoring (CGM) have been associated with better glycemic control without an increase in hypoglycemia when compared with multiple daily insulin injections (MDI) without CGM, mostly in Type 1 Diabetes (T1D).1–3 Clinical practice guidelines recommend their use over MDI in patients with either type 1 or 2 diabetes who have not achieved an A1C goal or who have achieved their A1C goal but continue to experience severe hypoglycemia or high glucose variability.4–7 Among these technologies, Sensor-augmented insulin pump therapy (SAP) which combines CSII with CGM has demonstrated better glycemic control with a comparable reduction in hypoglycemic episodes than CSII alone in short-term studies, however, long-term efficacy has been less described.3,8–12 In latin America there is very scarce evidence of the effectiveness and safety of the addition of SAP in a group of diabetic patients who are being followed in a structured diabetes program with continuous education and support.
This study is an up-date on the long-term follow-up of our real-world experience which was previously reported in 2016.13 The present study aimed to determine the long-term effectiveness and safety of SAP therapy within a comprehensive diabetes program in two specialized centers at Medellín, Colombia.
MethodologyStudy designThis is a retrospective real-life study analyzing effectiveness and safety of SAP therapy in two reference centers for diabetes management at Medellín – Colombia (Clínica Integral de Diabetes – CLID and Endocrino SA), between January 2008 to December 2017.
Eligibility criteriaPatients ≥18 years old with type 1 diabetes who were using SAP for at least two years, were included. Patients with missing data or lost during the 2-year follow-up were excluded.
InterventionPatients were on SAP (MiniMed® 640G or 530G, Guardian™ 2 Link transmitter and Enlite™ sensor; Medtronic, Northridge, CA), following standardized protocols for diabetes care. All the patients were using automation of insulin infusion depending on sensor glucose readings, individualized threshold for 530G users and predictive low glucose suspend feature on 640G pumps. Patients follow up included structured diabetes education, nutritional assessment, and psychological assistance. Structured education included a 6-month training course prior to SAP therapy and then continuing education with monthly out-patient appointments. The components of education and training comprise: instructions for managing daily living and sick-day rules, using CSII, adjusting insulin doses, carbohydrate counting, preventing and managing hypoglycemia and diabetic ketoacidosis, and understanding the role of either self-monitoring blood glucose (SMBG) or rtCGM. The indications for starting the insulin pump were established in accordance with institutional protocols: (1) poor glycemic control (HbA1c>7.5%) with MDI insulin regimen for the last six months at either institution, (2) dawn phenomenon, (3) frequent and/or asymptomatic hypoglycemia, (4) diabetic gastroparesis, and (5) episodes of severe hypoglycemia. Clinical data was extracted from a prespecified and systematically registered electronic medical record, and included sociodemographic variables (age, sex, educational level), anthropometric (height, weight, body mass index), comorbidities (hypertension, dyslipidemia, micro and macrovascular complications, heart failure, coronary artery disease, peripheral artery disease and cerebrovascular disease), diabetes (duration, and treatment).
Follow-upPatients were followed for 2 years since the initiation of SAP. Data on glycemic control and safety outcomes was analyzed retrospectively. Outcomes were analyzed at baseline, 3, 6, 12, 18, and 24 months by electronic medical record review.
OutcomesHbA1c was obtained at baseline and every 3 months to determine metabolic control. Safety outcomes included were: severe hypoglycemia (neuroglycopenic symptoms due to low blood sugar that require third party assistance), hospital admission related to diabetes (severe hypoglycemia, diabetic ketoacidosis and hyperosmolar hyperglycemic state defined by ADA standards), sensor or infusion set allergy or infection, or infusion set obstruction. At the end of follow-up additional information was obtained regarding the type of insulin, total daily insulin dose, basal/bolus percentage, type of bolus used (wizard or correction) and number of glucose readings per day. Adherence to real-time CGM was defined as the percentage of time using the sensor with its equivalence in days (i.e. 0–20%=0–3 days, 21–40%=3.1–6.1 days, 41–60%=6.1–9.1 days, 61–80%=9.1–12.7 days, and >80%=12.7 days) was also estimated.
Statistical analysisQualitative variables were analyzed descriptively and presented as relative and absolute frequencies. For the quantitative variables, the mean and median were obtained, as well as the standard deviation and the interquartile ranges (IQR 25% to 75%) depending on whether the variable did or did not have normal distribution. A repeated measures analysis for HbA1c was applied with the Friedman test and Wilcoxon post hoc tests with Bonferroni adjustment. The difference in medians was estimated to assess the effectiveness in changing HbA1c of each period with respect to baseline, 95% confidence interval for the median was estimated with Bonett–Price test. The research protocol was approved by the institutional review board of the Universidad Pontificia Bolivariana and conducted in accordance with the ethical standards of the Declaration of Helsinki.
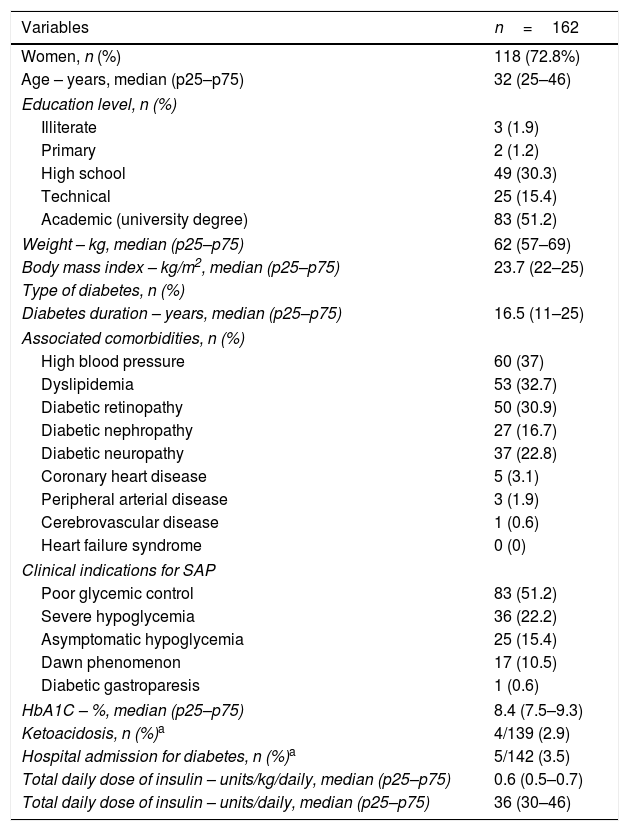
ResultsAmong 253 adults started on SAP, 162 patients were included for the long-term assessment. Baseline characteristics are presented in Table 1. In brief, 73% women, median age was 32 years (IQR: 25–46). All the patients were covered by the government health-care system and 51% had university degree. Anthropometric characteristics were: median BMI 24kg/m2 and none were obese (BMI>30kg/m2). The insulin pumps were either MiniMed 640G or Minimed 530G, coupled to real-time CGM system (Enlite™ sensor, Medtronic). The main indications for SAP were poor glycemic control (51.2%) and severe hypoglycemia (22.2%). Median HbA1C of 8.4% (IQR 7.5–9.3%) and 16.5 years of diabetes duration (IQR 11–25); the main microvascular complications were retinopathy (30.9%), followed by neuropathy (22.8%) and nefropathy (16.7%).
Baseline characteristics of patients with type 1 diabetes prior to initiating SAP therapy.
| Variables | n=162 |
|---|---|
| Women, n (%) | 118 (72.8%) |
| Age – years, median (p25–p75) | 32 (25–46) |
| Education level, n (%) | |
| Illiterate | 3 (1.9) |
| Primary | 2 (1.2) |
| High school | 49 (30.3) |
| Technical | 25 (15.4) |
| Academic (university degree) | 83 (51.2) |
| Weight – kg, median (p25–p75) | 62 (57–69) |
| Body mass index – kg/m2, median (p25–p75) | 23.7 (22–25) |
| Type of diabetes, n (%) | |
| Diabetes duration – years, median (p25–p75) | 16.5 (11–25) |
| Associated comorbidities, n (%) | |
| High blood pressure | 60 (37) |
| Dyslipidemia | 53 (32.7) |
| Diabetic retinopathy | 50 (30.9) |
| Diabetic nephropathy | 27 (16.7) |
| Diabetic neuropathy | 37 (22.8) |
| Coronary heart disease | 5 (3.1) |
| Peripheral arterial disease | 3 (1.9) |
| Cerebrovascular disease | 1 (0.6) |
| Heart failure syndrome | 0 (0) |
| Clinical indications for SAP | |
| Poor glycemic control | 83 (51.2) |
| Severe hypoglycemia | 36 (22.2) |
| Asymptomatic hypoglycemia | 25 (15.4) |
| Dawn phenomenon | 17 (10.5) |
| Diabetic gastroparesis | 1 (0.6) |
| HbA1C – %, median (p25–p75) | 8.4 (7.5–9.3) |
| Ketoacidosis, n (%)a | 4/139 (2.9) |
| Hospital admission for diabetes, n (%)a | 5/142 (3.5) |
| Total daily dose of insulin – units/kg/daily, median (p25–p75) | 0.6 (0.5–0.7) |
| Total daily dose of insulin – units/daily, median (p25–p75) | 36 (30–46) |
A statistically significant decrease in HbA1c was seen as early as the third month after SAP initiation (Δ−0.9%; p<0.0001), and maintained during the long-term follow-up (Fig. 1). There was an increase in the percentage of patients who achieved glycemic control (HbA1C≤7%) from 14.2% at baseline to 25.3% at 2-year follow-up (Δ−11.1%, 95% CI: −19.7 to −25.3; p=0.006). By the end of follow-up, the median total daily insulin dose was 36 international units (IU) (IQR: 30–46) or 0.6IU/kg/daily (IQR: 0.5–0.7). Lispro was the most common insulin used (42%), followed by Aspart (29.6%) and Glulisine (28.4%); the basal/bolus percentage was 40% for the basal rate (IQR 35–50%) and 60% for the prandial component (IQR 50–65%). The average glucose daily fingerstick testing was 5.4 times per day (SD±1.6). The proportion of patients who used the sensor based on percentage of time was: 0–20%, 21–40%, 41–60%, 61–80%, and >80% was 3.5%, 9.7%, 23%, 44.3%, and 19.5% respectively. Among given boluses, the mean of meal bolus wizard and correction wizard was 82% (SD±13.4) and 68.8% (SD±18.9), respectively.
Safety assessmentAt baseline, annual rates of severe hypoglycemia, hospital admission related to diabetes, and ketoacidosis were reported in 22.2%, 3.5% and 2.9% of the patients respectively. After the 2-year follow-up of starting SAP, these safety variables decreased to 14.1% (Δ−8.1%, 95% CI: −16.5 to 0.3; p=0.030), 1.3% (Δ−2.2%, 95% CI: −5.7 to 1.3; p=0.101), and 1.9% (Δ−1%, 95% CI: −4.5 to 2.5; p=0.298). Rates of sensor or infusion set allergy, infection or infusion set obstruction were reported in 7.6%, 1.4%, and 10% respectively.
DiscussionThe present study presents real-world evidence of the effectiveness and safety of SAP therapy. After 2 years of treatment, HbA1c decreased from 8.4% to 7.5% (−0.9%, 95% CI: 0.5–1.2; p<0.0001), glycemic control defined by HbA1c ≤7% improved from 14.2% to 25.3% (11.1%, 95% CI: 19.7–2.5; p=0.006), and severe hypoglycemia decreased from 22.2% to 14.1% (−8.1%, 95% CI: −16.5 to 0.3; p=0.03). These findings are consistent with our previous published data13 and is similar to the experience reported by another institution in Colombia.14–16 These results are not only related to SAP but most likely reflect the results of a multi-intervention approach. They are the reflection of continued education and training of patients in addition to the use of technology. The results are the effect of SAP therapy in addition to a comprehensive diabetes program.
An open-loop insulin delivery system like SAP requires patient input, i.e. carbohydrate counting, use of bolus wizard, adherence to sensor use, sensor calibration, alarm setting and threshold-suspend feature, there is also a motivational factor involved among other variables that may fluctuate in time and depend on each patients behavior. The way to measure the impact of human behavior and its interaction with diabetes care technologies is difficult to assess by classic study designs and this might explain why the results of clinical trials and meta-analyses looking at glycemic control with CSII are conflicting and heterogeneous. Both the U.S. Food and Drug Administration and European Medicines Agency ask pump and CGM manufacturers to provide real-world evidence in combination with RCT to evaluate short- and long-term effectiveness and safety of new medical device submissions.17–19
Meta-analyses have reported benefits of SAP therapy compared to MDI and self-monitoring blood glucose (SMBG),3,20 a statistically and clinically significant greater reduction in HbA1c from −0.68% to −0.7%. In addition, SAP therapy has shown to reduce the frequency of severe and non-severe hypoglycemic events when compared with use of continuous subcutaneous insulin infusion without real-time CGM.21–23 It is possible that the greater and sustained decrease in HbA1C levels and severe hypoglycemic annual rates observed during the 2-year follow-up in our study was due to the “add-on” effect of the comprehensive diabetes program. Structured education programs like DAFNE, INPUT or REPOSE have reported HbA1c reductions in the range of 0.2 and 0.7% over a sustained period of time.24–30 Similarly, real-world evidence from the COMISAIR demonstrated a significant HbA1c reduction from baseline and during the 3-year study period with a mean HbA1c difference of -0.99% (95% CI: −1.45 to −0.52).11,12 In concordance with these studies, our findings support that real time CGM and a structured education program are key elements for the effectiveness of SAP.
Due to the observational nature the present study, the main limitations are lacking of a prospective control group and missing CGM data, hence it was not possible to control for potential confounding factors like non-severe hypoglycemia among others; as a result, these real-world findings related to effectiveness and safety need to be interpreted with caution. However, it is noteworthy that the results are consistent with those of controlled trials, meta-analyses, and real-world studies showing that SAP therapy along with an intensive and comprehensive approach to diabetes care can achieve long-term effective glycemic control with low risks of hypoglycemia and other complications.
Conflict of interestAll authors declare no conflicts of interest.