Saponins are the main bioactive substances with anti-hyperglycemic activities of Momordica charantia. This study aimed to verify the effects of M. charantia saponins on insulin secretion and explore the potential underlying mechanisms in INS-1 pancreatic β-cells. We injured INS-1 cells with 33.3mM glucose and then treated them with saponins. Saponins improved cell morphology and viability as demonstrated by inverted microscopy and CCK8 detection and significantly increased insulin secretion in a concentration-dependent manner as shown by ELISA. Thus, we obtained the optimal concentration for the subsequent experiments. Potential mechanisms were explored by immunofluorescence, western blotting, and RT-qPCR techniques. First, saponins increased the mRNA and protein levels of IRS-2 but decreased the serine 731 phosphorylation level of IRS-2. Moreover, saponins increased the phosphorylation of Akt protein and decreased the protein level of FoxO1, which were both reversed by the PI3K inhibitor ly294002. Furthermore, saponins increased the protein level of the downstream molecule and insulin initiating factor PDX-1, which was also reversed by ly294002. Saponins also increased Akt and PDX-1 mRNA and decreased FoxO1 mRNA, which were both reversed by ly294002. Saponins increased glucose-stimulated insulin secretion (GSIS) and intracellular insulin content, which were reversed by ly294002, as determined by ELISA. The immunofluorescence results also confirmed this tendency. In conclusion, our findings improve our understanding of the function of saponins in INS-1 pancreatic β-cells and suggest that saponins may increase insulin secretion via the PI3K/Akt/FoxO1 signaling pathway.
Las saponinas son los principales principios bioactivos con actividad antihiperglucémica de Momordica charantia. El objetivo de este estudio era comprobar los efectos de las saponinas de M. charantia sobre la secreción de insulina y explorar los posibles mecanismos subyacentes en las células β pancreáticas INS-1. Lesionamos células INS-1 con 33,3mM de glucosa y las tratamos luego con saponinas. Las saponinas mejoraron la morfología y la viabilidad celulares, como mostraron la microscopia invertida y la detección de CCK8, y aumentaron significativamente la secreción de insulina de forma proporcional a la concentración, como se demostró mediante ELISA. Se obtuvo así la concentración óptima para los experimentos posteriores. Se exploraron los posibles mecanismos mediante técnicas de inmunofluorescencia, inmunotransferencia y RT-qPCR. En primer lugar, las saponinas aumentaban los niveles de ARNm y proteínas de IRS-2, pero reducían el nivel de fosforilación de serina 731 de IRS-2. Además, las saponinas aumentaban la fosforilación de la proteína Akt y disminuían la concentración de proteínas de FoxO1, revertidas ambas por el inhibidor de PI3K ly294002. Las saponinas aumentaban también la concentración de proteínas de la molécula anterógrada y el factor iniciador de la insulina PDX-1, algo que también revirtió ly294002. Además, las saponinas aumentaban el ARNm de Akt y PDX-1 y disminuían el ARNm de FoxO1, efectos ambos revertidos por ly294002. Las saponinas aumentaban también la secreción de insulina estimulada por la glucosa (SIEG) y el contenido de insulina intracelular, efectos ambos revertidos por ly294002, según la determinación mediante ELISA. Los resultados de inmunofluorescencia también demostraron esta tendencia. En conclusión, nuestros resultados mejoraron los conocimientos de la función de las saponinas en las células β pancreáticas INS-1 y sugirieron que las saponinas pueden aumentar la secreción de insulina a través de la vía de señalización PI3K/Akt/FoxO1.
Type 2 diabetes mellitus (T2DM) is a metabolic disease characterized by chronic hyperglycemia caused by relative or absolute insulin deficiency.1 At present, pancreatic β-cell dysfunction and insulin resistance (IR) are the two main links to its occurrence and development.2 The main function of pancreatic β-cells is to synthesize and secrete insulin, which is the key substance for the maintenance of normal glucose metabolism in the body. Traditional Chinese medicine (TCM) is effective in the treatment of diabetes and has fewer side effects. Momordica charantia is natural food with hypoglycemic effects used in modern medicine.3–5 Saponins are an important active component extracted from M. charantia that can reduce hyperglycemia in many ways, such as by increasing the sensitivity of the insulin receptor, protecting pancreatic β-cells, and correcting the dysregulation of fat protein metabolism.6,7 It has been found that total saponins of M. charantia can repair pancreatic β-cells and increase pancreatic β-cell secretory granules.8 So far, it is not clear how saponins improve insulin secretion and whether their function is mediated by the insulin signaling pathway. Furthermore, little information is available about the effects of saponins on high glucose-induced cell dysfunction. In this study, the effects of saponins on insulin secretion and the exact mechanism of their hypoglycemic effect were studied by various experimental techniques, which provided a theoretical basis for the research and development of the hypoglycemic effect of a single drug.
Insulin receptor and its downstream signaling proteins constitute a complex signal transduction pathway in pancreatic β-cells, regulating insulin secretion and maintaining β-cell growth, proliferation and survival.9–11 IRS-2 is an important signaling protein for the maintenance of the function of pancreatic β-cells,12 and its lack leads to a decrease in the number of pancreatic β-cells and insulin secretion disorders.13 Overexpression of IRS-2 leads to the activation of the PI3K/Akt pathway, which is essential for regulating pancreatic β-cell function and survival.14 Activated PI3K/Akt subsequently decreases the level of the transcription factor FoxO1, which leads to the inhibition of pancreatic β-cell growth. Therefore, evaluating these pathways will provide strong evidence to support the improvement of insulin secretion.
Pancreatic and duodenal homeobox factor-1 (PDX-1) is a member of the homeobox family and is also a downstream molecule of the PI3K/Akt/FoxO1 signaling pathway. FoxO1 causes transcriptional repression of PDX-1. Detection of PDX-1 expression could reflect β-cell function to some extent.
This paper determined that the mechanism of action of the saponins of M. charantia on the insulin signaling pathway in INS-1 pancreatic β-cells was to enhance the expression of IRS-2, which was associated with the activation of the PI3K/Akt/FoxO1 signaling pathway and the downstream molecule PDX-1. Our study provides a theoretical basis for the screening of natural hypoglycemic functional factors.
Materials and methodsPlant materials and reagentsPowder of M. charantia was purchased from Peiyu Biotechnology (Guangzhou, China), prepared from the green, unripe fresh fruits of M. charantia which were dried at low temperature and ultra-fine grinded with no added pigments and preservatives, and identified by Dr. Xueshun Zhang of Shandong provincial hospital of TCM, Shandong Province, China. The preparation of M. charantia saponins was as follows15: powder of M. charantia were powdered and sieved (60 mesh). M. charantia powder (200g) was extracted with 2000ml of ethanol–water (60:40, v/v) solution in an ultrasonic bath for 2h, repeated three times. The filtered solutions were gathered and concentrated to dryness by removing the ethanol solvent using a rotary evaporator device. The extracts obtained were diluted to the concentration of 15mg/ml with distilled water as sample solution, and then subjected to an AB-8 macroporous resin column. After reaching adsorptive saturation, the column was first washed by distilled water, and then eluted by ethanol-water (80:20, v/v) solution. The 80% ethanol-eluted fraction was concentrated by drying under vacuum. The purity of M. charantia saponins was calculated on the basis of the standard curve of ginsenoside Rg1: y=0.0026x+0.0107 (R2=0.9994), and the purity of M. charantia saponins was 76.63%. The other components of the MCS were H2O2 (8%), protein (10%), ash (2%), pectin, cellulose and so on. The M. charantia saponins were saturated with anhydrous ethanol before dissolving in deionized water as a stock solution. The stock was diluted to three concentrations (80μg/ml, 160μg/ml, 320μg/ml). After filtration sterilization, they were used for subsequent experiments.
Cell culture RPMI-1640 medium, fetal bovine serum, trypsin 0.25% EDTA, and TRIzol were obtained from Gibco (NY, USA). l-Glutamine, penicillin–streptomycin, sodium pyruvate, β-mercaptoethanol, DAPI, and radio immunoprecipitation assay (RIPA) buffer were purchased from Solarbio (Beijing, China). PBS, and SDS-PAGE were obtained from KeyGEN BioTECH (Nanjing, China). Cell Counting Kit-8 solution, and ly294002 (PI3K inhibitor) were from MedChemExpress (New Jersey, USA). The rat INS (insulin) ELISA kit was purchased from Elabscience Biotechnology (Wuhan, China). Bicinchoninic acid assay (BCA), and rabbit anti-guinea Pig IgG anti-insulin antibody were obtained from Thermo Fisher Scientific (NY, USA). Anti-IRS-2, anti-pIRS-2, anti-Akt, anti-pAkt, anti-FoxO1, anti-PDX-1, anti-gapdh, and anti-β-actin were from Abcam (Cambridge, USA). Reverse transcriptase, and SYBR Premix Ex Taq II Kit were obtained from TaKaRa Biotechnology (Japan).
INS-1 cell cultureINS-1 cells were donated by Dr. Ling Gao of Shandong Institute of Clinical Medicine, Shandong Province, China. Cells were cultured in RPMI-1640 medium containing 11.1mM glucose supplemented with 10% fetal bovine serum, 0.11g/L l-glutamine, 100U/ml penicillin–streptomycin, 50μM β-mercaptoethanol, and 0.132g/L sodium pyruvate, in a humidified incubator in a 5% CO2 and 95% air atmosphere at 37°C. The medium was changed every 2–3 days, and the cells were passed every 5–6 days. The cells used in the research were between the 10th and 20th passages. After cells were treated with different concentrations of saponins, they were placed under an inverted microscope for observation.
INS-1 cell treatmentTo compare the effects of different concentrations of saponins on insulin secretion, cells were divided into five groups: (1) NC group: treated with culture medium only; (2) HG group: treated with culture medium containing 33.3mM glucose; (3) HG+S (80μg/ml) group: treated with culture medium containing 33.3mM glucose and 80μg/ml saponins; (4) HG+S (160μg/ml) group: treated with culture medium containing 33.3mM glucose and 160μg/ml saponins; (5) HG+S (320μg/ml) group: treated with culture medium containing 33.3mM glucose and 320μg/ml saponins.
To detect whether saponins can improve insulin secretion through the PI3K/Akt/FoxO1 signaling pathway and downstream molecule PDX-1, cells were divided into four groups: (1) NC group: treated with culture medium only; (2) HG group: treated with culture medium containing 33.3mM glucose; (3) HG+S group: treated with culture medium containing 33.3mM glucose and 320μg/ml saponins; (4) HG+S+ly294002 group: treated with culture medium containing 33.3mM glucose, 320μg/ml saponins and 25μM ly294002 (PI3K inhibitor).
Cell viability assayCells seeded at 2×104/well onto 96-well dishes were divided into five groups. Each group was plated in triplicate wells, and 10μl of Cell Counting Kit-8 solution was added to each well for 2h. Absorbance values were measured at a wavelength of 450nm,and the average value was used to calculate the cell survival rate. The experiment was repeated 3 times, and the average value was obtained.
Detection of glucose-stimulation insulin secretion by ELISATo measure insulin secretion, cells after different treatments in 24-well dishes were incubated with Krebs-Ringer Bicarbonate buffer (KRB; 125mM NaCI, 4.74mM KCI, 1.2mM KH2PO4, 1.2mM MgSO4, 2.5mM CaCl2, 10mM HEPES, 5mM NaHCO3, and 0.1% BSA, pH 7.4) containing 2.8mM glucose for 2h to reduce insulin secretion to basal glucose levels. After washing with PBS three times, cells were incubated with KRB containing basal (2.8mM) or stimulatory (16.7mM) glucose at 37°C for 1h. The supernatant was carefully collected and centrifuged at 4°C at 800rpm for 5min. The supernatant was subsequently tested for insulin levels. The same groups of cells were incubated accordingly, and intracellular insulin was measured after cell lysis with 50μl of acidified ethanol (75% absolute ethyl alcohol, 1.5% hydrochloric acid, and 23.5% water steamed three times). The experiment was repeated three times. Glucose-stimulated insulin secretion (GSIS) and intracellular insulin content were measured by the Rat INS (insulin) ELISA kit.
Immunofluorescence microscopyCells adhered to coverslips in 24-well dishes were fixed with 4% formaldehyde for 30min after different treatments, washed with PBS and permeabilized with PBS/0.5% Triton-X-100 for 10min. Then, the cells were incubated with goat serum (ZSGB-Bio, China) for 1h to block nonspecific binding of the antibodies. Next, the cells were incubated with primary anti-insulin antibody (Cat: ab181547),16 which was diluted 1:1000 with PBS. The cell nuclei were stained with DAPI. Cells were imaged with a confocal laser microscope (Molecular Devices, USA).
Protein analysis by western blottingCells after different treatments were washed twice with PBS and placed immediately in RIPA lysis buffer. The cells were lysed for 30min on ice and then centrifuged at 12000×g for 30min at 4°C. The supernatant protein was quantified using BCA and stored at −80°C. Then, protein extracts were separated by SDS-PAGE and electroblotted onto PVDF (Millipore, Germany) membranes. The membranes were incubated with 5% nonfat dry milk in 1× TBST (Beijing ComWin Biotech, China) for 1h at room temperature and then stained with anti-IRS-2 (Cat: ab134101),17 anti-pIRS-2 (Cat: ab3690),18 anti-Akt (Cat: ab89402),19 anti-pAkt (Cat: ab81283),20 anti-FoxO1 (Cat: ab39670),21 anti-PDX-1 (Cat: ab134150),22 anti-gapdh and anti-β-actin (Cambridge, USA) overnight at 4°C. Then, the membranes were incubated with HRP-conjugated AffiniPure goat anti-rabbit IgG (PTG, USA) for 1h at room temperature. Protein band densities were exposed to light-sensitive film and quantified using Image J software.
Quantitative real-time PCR (RT-qPCR)Cells after different treatments were harvested in Trizol, and total RNA was extracted following a protocol for Trizol isolation. cDNA was synthesized from total RNA using reverse transcriptase. RT-qPCR reactions were analyzed using the SYBR Premix Ex TaqII Kit and Roche480 Real-Time PCR System. The primers used for PCR are detailed in Table 1. From the Cq values obtained with qPCR, the expression levels of transcripts were calculated by using the comparative Ct method (2−ΔΔCT formula) after normalization to the internal reference gene (β-actin) values.
Information on the primers used for real-time PCR.
| Genes | Sequences | Product size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| IRS-2 | 5′-CCCTTGACGAGTATGGCTCC-3′ | 75 | 58 |
| 5′-TGGACTCAGGTGTGTTGCTC-3′ | 75 | 58 | |
| AKT | 5′-CCTGGACTACTTGCACTCCG-3′ | 246 | 58 |
| 5′-ATCTCGTACATGACCACGCC-3′ | 246 | 58 | |
| FoxO1 | 5′-GGCAGCCAGGCATCTCATAA-3′ | 150 | 58 |
| 5′-TTGGGTCAGGCGGTTCATAC-3′ | 150 | 58 | |
| PDX-1 | 5′-GTCTGCCTCTGGGACTCTTT-3′ | 96 | 58 |
| 5′-TGTTATGGGACCGCTCAAGT-3′ | 96 | 58 | |
| β-Actin | 5′-CTAGAAGCATTTGCGGTGGA-3′ | 250 | 58 |
| 5′-GAAATCGTGCGTGACATTAAG-3′ | 250 | 58 |
Statistical analysis was performed by the GraphPad Prism 7.0 software. The quantitative data are expressed as the means±standard deviations (SD). P values ≤0.05 were considered to be statistically significant. Differences between two groups were compared using t-tests or ANOVA.
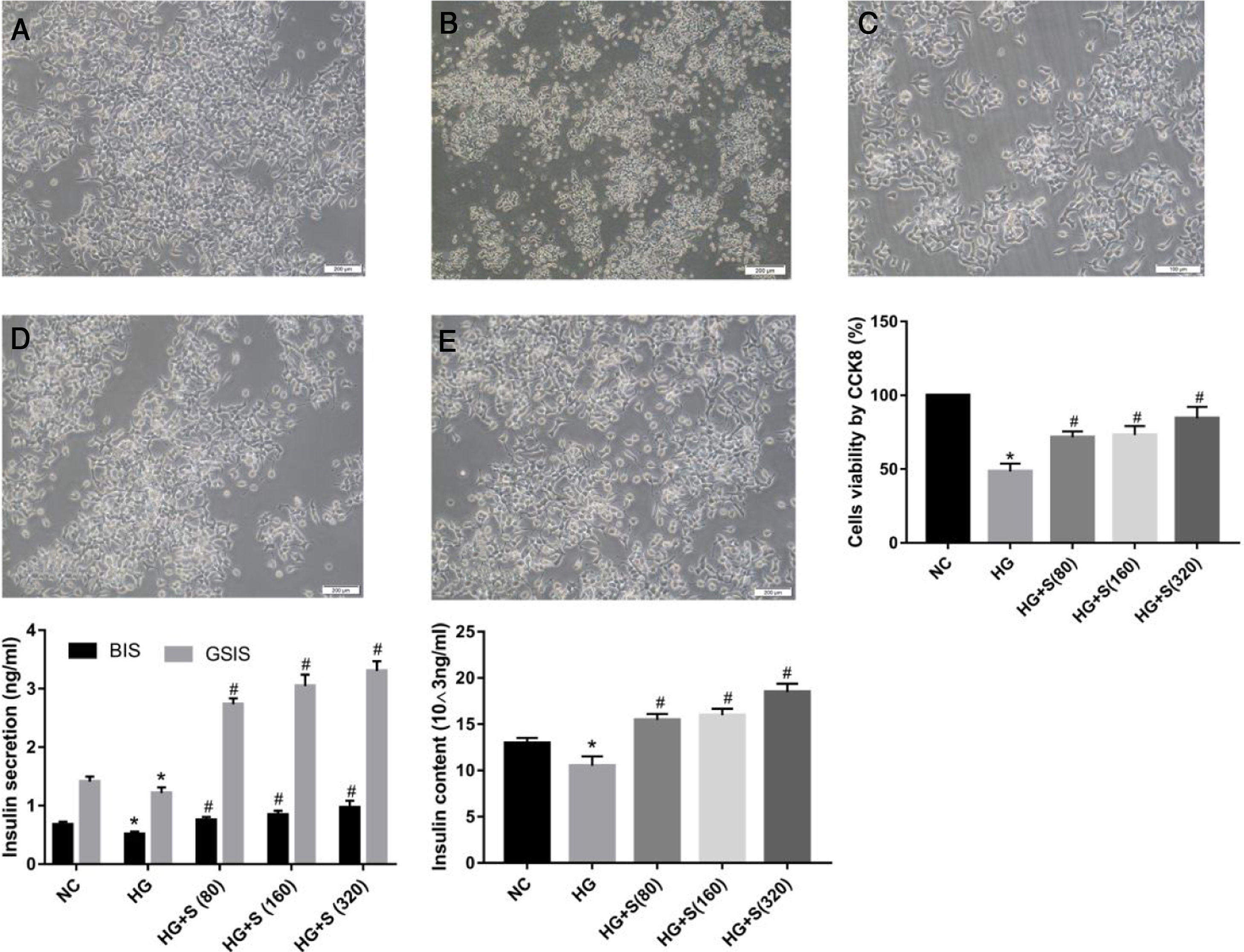
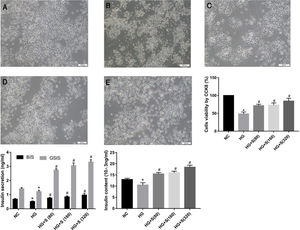
ResultsSaponins improved cell morphology and viability and increased insulin secretionThe normal group was characterized by a large number of cells, monolayer growth, uniform distribution, irregular polygon morphology, and obvious projections (Fig. 1A). Cells in the high glucose group were observed floating and shedding, and their projections were contracted and aggregated, indicating massive cell death (Fig. 1B). The number of injured cells dying under the saponin intervention was reduced, and cell morphology tended to be normal (Fig. 1c-e). Compared with the normal control group, the survival rate of cells stimulated with high glucose for 24h was significantly decreased, as shown by the CCK8 assay. The survival rates of each group were as follows: NC group 100±0%, HG group 48.4±3.127%, HG+S (80μg/ml) group 71.58±2.288%, HG+S (160μg/ml) group 73.07±3.565, HG+S (320μg/ml) group 84.46±4.43. Saponins protected cells from injury caused by high glucose in a concentration-dependent manner (Fig. 1B).
Saponins improved cell morphology and viability and increased insulin secretion. INS-1 cells were treated with or without saponins for 24h. (A) Morphology of cells in different groups under an inverted microscope (20×). Note: a, NC group; b, HG group; c, HG+S (80μg/ml) group; d, HG+S (160μg/ml) group; e, HG+S (320μg/ml) group. (B) Cell viability in different groups measured by CCK8 assay. The unit of saponins is μg/ml, all of the following are in this unit. (C) Effects of saponins on GSIS and intracellular insulin content in cells from different groups. Note: BIS represents basal insulin secretion, GSIS represents glucose-stimulated insulin secretion. Data are shown as the mean±SD of at least three independent experiments. Differences between two groups were compared using t-tests or ANOVA. *P<0.05 compared with the control group; #P<0.05 compared with the HG group.
GSIS and intracellular insulin content were measured by ELISA to evaluate the role of saponins in insulin secretion. The insulin levels of both GSIS and intracellular content in the high glucose group decreased compared to those in the normal control group. However, this injury caused by high glucose was reversed by saponin treatment. We concluded that saponins increased insulin secretion in a concentration-dependent manner. As noted, the most remarkable increase was observed in cells treated with 320μg/ml saponins (Fig. 1C).
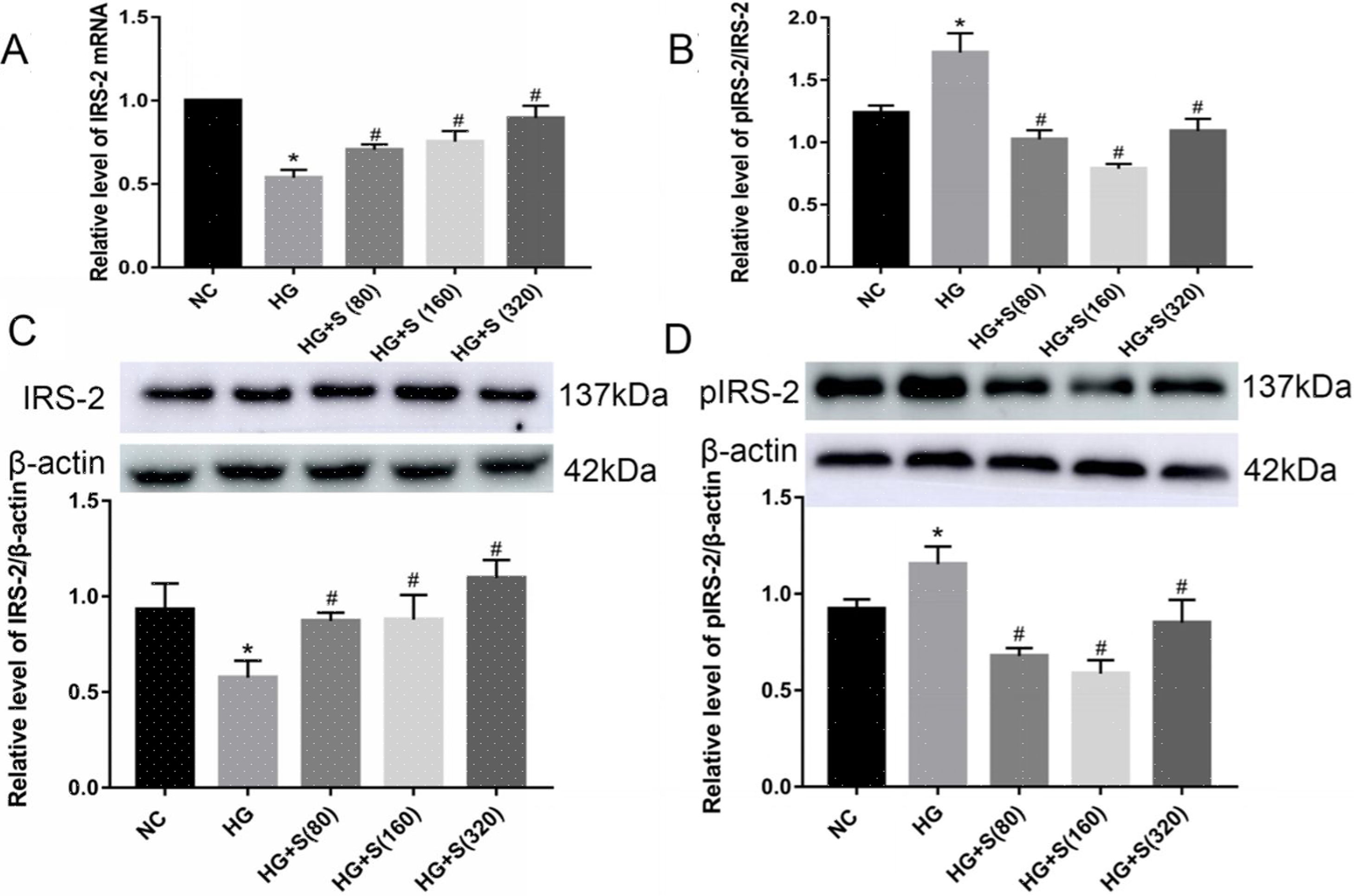
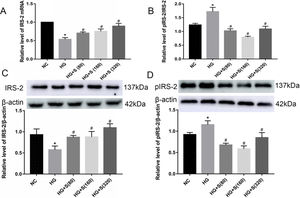
Effects of saponins on IRS-2 mRNA and protein levelsCompared with those in the normal control group, both the mRNA and protein levels of IRS-2 in the high glucose group were significantly decreased. In contrast, the serine 731 phosphorylation level of IRS-2 was increased in high glucose-treated cells (Fig. 2A–D). This indicated that high glucose could suppress the mRNA and protein expression levels of IRS-2 while promoting the serine 731 phosphorylation of IRS-2 protein.
Effects of saponins on IRS-2 mRNA and protein levels. INS-1 cells were treated with or without saponins for 24h. (A) Effect of saponins on IRS-2 mRNA. The level of IRS-2 mRNA was detected by RT-qPCR. (B–D) Effects of saponins on the protein levels of IRS-2, pIRS-2 and the pIRS-2/IRS-2 ratio. Cell lysates were subjected to western blotting and incubated with antibodies against IRS-2 and pIRS-2. Data are shown as the mean±SD of at least three independent experiments. Differences between two groups were compared using t-tests or ANOVA. *P<0.05 compared with the control group; #P<0.05 compared with the HG group.
Moreover, the mRNA and protein levels of IRS-2 in the saponin intervention groups were significantly increased compared with those in the high glucose group. However, the serine 731 phosphorylation level of IRS-2 protein in the saponins group was significantly reduced when compared to that in the high glucose group (Fig. 2A–D). This indicated that saponins could increase the mRNA and protein levels of IRS-2 while inhibiting the serine 731 phosphorylation level of IRS-2 protein induced by high glucose.
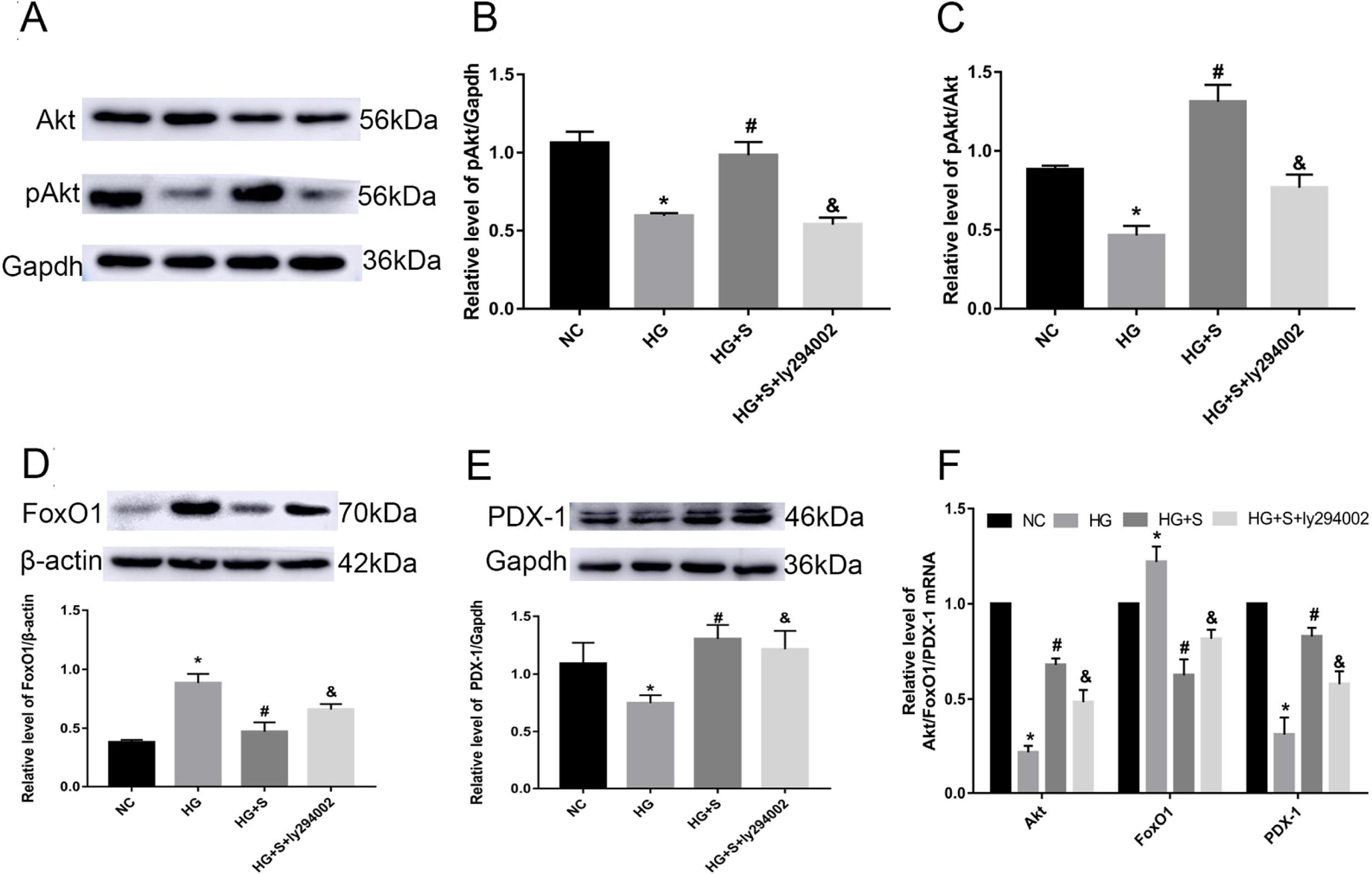
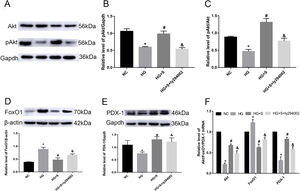
Effects of saponins on the PI3K/Akt/FoxO1 signaling pathway and downstream molecule PDX-1Compared with those in the normal control group, the phosphorylation level of Akt, pAkt/Akt ratio and Akt mRNA in the high glucose group were obviously decreased. Higher mRNA and protein levels of pAkt and the pAkt/Akt ratio were detected in the saponin intervention group compared with the high glucose group. When the PI3K inhibitor ly294002 was added, it caused a significant decrease compared to the saponin intervention group (Fig. 3A–D).
Effects of saponins on the PI3K/Akt/FoxO1 signaling pathway and downstream molecule PDX-1. INS-1 cells were treated with or without saponins or the PI3K inhibitor ly294002 for 24h. (A–E) Effects of saponins on the protein level of pAkt, FoxO1, and PDX-1 and the pAkt/Akt ratio. Cell lysates were subjected to western blotting and incubated with antibodies against anti-Akt, anti-pAkt, anti-FoxO1, and anti-PDX-1. (F) Effect of saponins on Akt, FoxO1 and PDX-1 mRNA. The mRNA levels were detected by RT-qPCR. Data are shown as the mean±SD of at least three independent experiments. Differences between two groups were compared using t-tests or ANOVA. *P<0.05 compared with the control group; #P<0.05 compared with the HG group; &P<0.05 compared with the HG+S group.
Compared with those in the normal control group, the mRNA and protein levels of FoxO1 in the high glucose group were obviously increased. Lower levels of FoxO1 mRNA and protein were detected in the saponin intervention group compared with the high glucose group. When ly294002 was added, it resulted in a significant increase compared to the saponin intervention group (Fig. 3D and E).
The results for the downstream molecule and insulin initiating factor PDX-1 showed that high glucose significantly downregulated the mRNA and protein levels of PDX-1. However, saponins increased the mRNA and protein levels of PDX-1, which were also reversed by ly294002 (Fig. 3D and F).
These results suggested that cotreatment with saponins regulated the PI3K/Akt/FoxO1 signaling pathway and downstream molecule PDX-1.
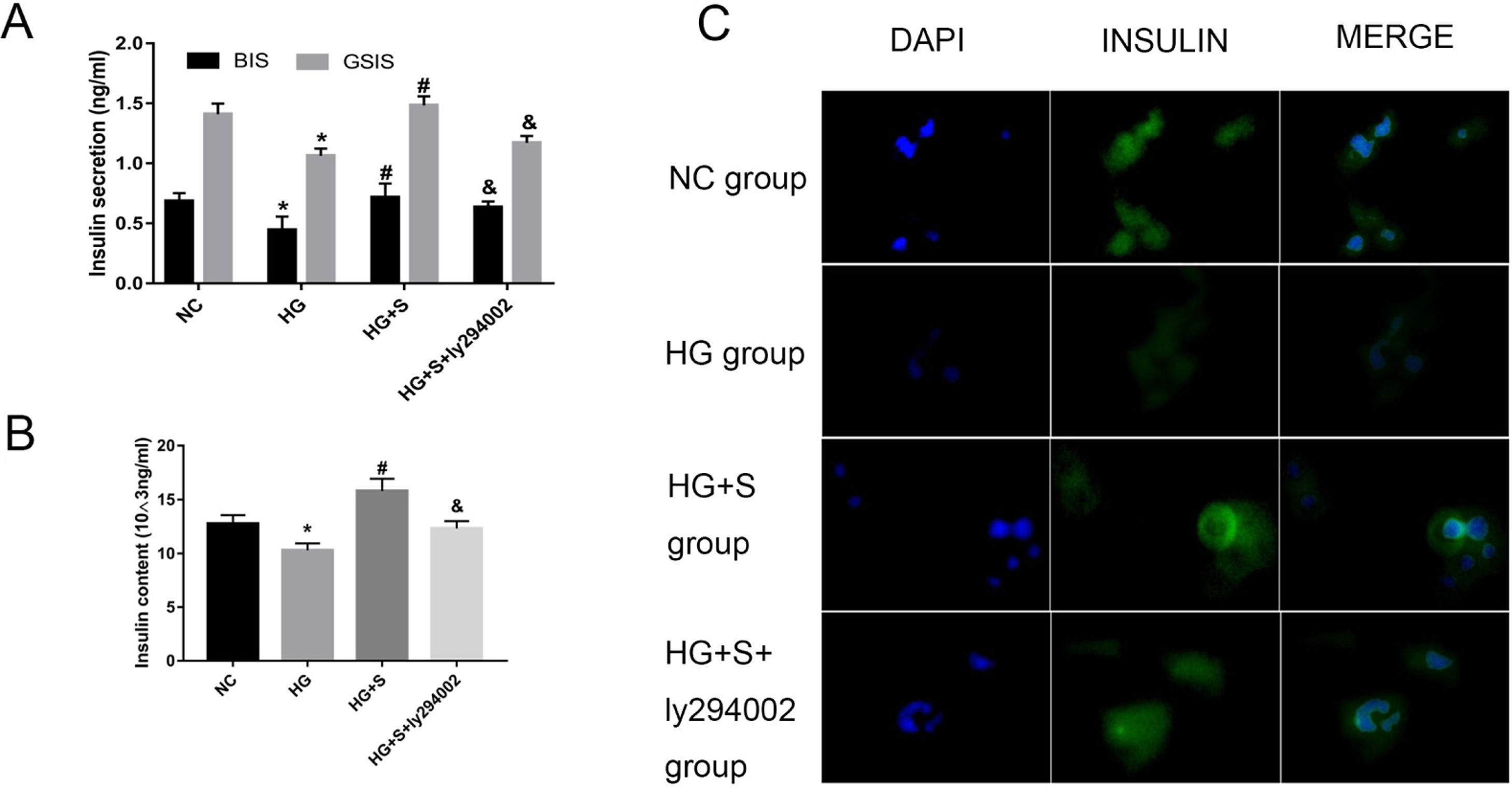
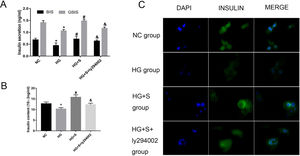
The PI3K/Akt/FoxO1 signaling pathway mediated the effects of saponins on insulin secretionTo determine whether saponins could elevate insulin secretion via the PI3K/Akt/FoxO1 signaling pathway, we measured GSIS and intracellular insulin content to evaluate the effects of saponins on insulin secretion. High glucose treatment decreased insulin secretion compared to the normal control group. However, the injury effect of high glucose was reversed by saponin treatment. Nevertheless, saponin cotreatment with ly294002 appeared to reverse the saponin-induced insulin-stimulating effect. This confirmed that saponins could protect the insulin secretion function via the PI3K/Akt/FoxO1 insulin signaling pathway (Fig. 4A and B).
The PI3K/Akt/FoxO1 signaling pathway mediated the effects of saponins on insulin secretion. INS-1 cells were treated with or without saponins or the PI3K inhibitor ly294002 for 24h. (A) Effects of saponins on GSIS and intracellular insulin content of cells in different groups. (B) Effects of saponins on intracellular insulin content of cells in different groups. (C) Insulin secretion in different groups as determined by immunofluorescence microscopy. Data are shown as the mean±SD of at least three independent experiments. Differences between two groups were compared using t-tests or ANOVA. *P<0.05 compared with the control group; #P<0.05 compared with the HG group; &P<0.05 compared with the HG+S group.
The insulin secretion level was semiquantitatively analyzed by the average optical density of the region showing as green fluorescence under a microscope. The results showed that cells in the high glucose group had lower fluorescence intensity compared to the normal control group. However, fluorescence intensity increased significantly in the presence of saponins, indicating that treatment with saponins could increase insulin secretion. We also found that saponin cotreatment with ly294002 resulted in lower fluorescence intensity, which likewise indicated that saponins protect the insulin secretion function via the PI3K/Akt/FoxO1 signaling pathway (Fig. 4C).
DiscussionCurrently, many studies have proven that M. charantia saponins can reduce blood glucose and even promote insulin secretion. Han et al.23 found that the saponins extracted from M. charantia in an aqueous two-phase extraction system induced significant hypoglycaemic activity in hyperglycaemic and normal mice through experiments. Wang et al.24 showed that oral administration of saponins of M. charantia could significantly reduce fasting blood glucose levels and ameliorate insulin resistance by animal experiments. In the present study, we investigated the effects of saponins on insulin secretion and the underlying mechanisms.
Based on the results of cell morphology and viability, saponins protected cells from injury caused by high glucose in a concentration-dependent manner. To determine the insulin secretory function, we assessed the effects of saponins by an ELISA kit. Both GSIS and intracellular insulin content were increased in a concentration-dependent manner in INS-1 cells. Studies have shown that saponins from the traditional medicinal plant M. charantia stimulate insulin secretion in vitro,25 which is consistent with our results.
In this study, we found that high glucose increased the serine 731 phosphorylation level of IRS-2 and reduced the mRNA and protein levels of IRS-2. However, saponin treatment reversed these effects, indicating that saponins could inhibit the serine 731 phosphorylation of IRS-2 induced by high glucose. Studies have shown that the abnormal increase in the phosphorylation level of the serine site of IRS-2 blocks the transmission of insulin signaling and causes cell dysfunction,26 which indicates that saponins could improve pancreatic β-cell function.
Researchers have found that suppression of the signaling pathway is implicated in high glucose-induced β-cell dysfunction. We hypothesized that the protection by saponins against high glucose might be mediated by the PI3K/Akt/FoxO1 pathway and the downstream molecule PDX-1. The core molecule of the biological effect of insulin through the PI3K/Akt/FoxO1 signaling pathway is Akt. Phosphorylation is the main mechanism of Akt activation. FoxO1 leads to the inhibition of pancreatic β-cell growth and decrease in PDX-1 transcription.27 Studies have shown that one of the mechanisms of pancreatic β-cell injury by high glucose is the inhibition of insulin gene transcription factor PDX-1 activity and its expression level.28 In addition, PDX-1 also regulates the expression of other genes related to insulin secretion, thus affecting the glucose-stimulated insulin secretion function of β-cells (GSIS) and the changes in the insulin secretion phase.29–31 Our data indicated that high glucose inhibited the mRNA and phosphorylation levels of Akt, increased the mRNA and protein level of FoxO1, and decreased the mRNA and protein level of PDX-1, while cotreatment with saponins reversed these effects. In addition, coadministration of ly294002 with saponins abolished these changes. Moreover, saponins increased insulin secretion, which was reversed by ly294002 as demonstrated by ELISA and immunofluorescence. Accordingly, the data suggested that saponins reversed the inhibitory effects of high glucose on insulin secretion in INS-1 cells via the PI3K/Akt/FoxO1 signaling pathway.
ConclusionIn conclusion, we demonstrated that saponins of M. charantia can elevate insulin secretion in INS-1 pancreatic β-cells and that their function is mediated by the PI3K/Akt/FoxO1 signaling pathway. A few studies have proven that M. charantia saponins can increase insulin secretion in vivo. Therefore, we plan to conduct animal experiments and clinical trials on the effects of saponins in insulin secretion. Besides, the effects of saponins on insulin secretion deserve a more in-depth investigation, and the protective mechanism of saponins in pancreatic β-cells still needs to be verified by follow-up experiments to provide a more comprehensive experimental basis for the prevention and treatment of T2DM.
FundingThe paper is derived from the project “Effect of saponins of M. charantia on function of pancreatic β-cells and expression of PDX-1 in high glucose environment”, whose funding is from Shandong Provincial Administration of Traditional Chinese Medicine.
Conflict of interestThe authors declare they have no conflict of interest.