The prevalence of type 2 diabetes mellitus (T2DM) is increasing among young people worldwide. The American Diabetes Association has defined the risk factors that are associated with this increased risk for developing T2DM in youths.
ObjectiveTo explore school children at high-risk for T2DM in Jordan.
Materials and methodsA descriptive cross-sectional study was conducted. The children were 10–14.9 years old. Weight, height, and waist circumference were measured, and the body mass index Z score (BMI) was determined. The waist to height ratio (WHtR) was calculated. Blood pressure was measured and three child́s risk factors were identified.
ResultsEight hundred and seventy-one schoolchildren were screened; 26.0% were overweight and 19.3% were obese. The most common risk factor among overweight and obese children, accounting for 80.4%, was a positive family history of type 2 diabetes mellitus in first- or second-degree relatives. Children born to mothers with diabetes or gestational diabetes mellitus during the child's gestation represented 17.3%, and around 26.8% were found to have hypertension (stage 1 or 2). In relation to the risk factors, 12.6% presented no risk factors; 54.0% had at least one factor; 29.1% two risk factors and 4.3% had three risk factors.
ConclusionRisk factors for T2DM are very common. Around 54% had one risk factor. Strategies aimed at reducing risk factors for T2DM, especially obesity, among Jordanian school children are urgently needed.
La prevalencia de diabetes mellitus tipo 2 (DM2) está aumentando en los jóvenes de todo el mundo. La American Diabetes Association ha definido los factores de riesgo que se asocian con un mayor riesgo de desarrollar DM2 en los jóvenes.
ObjetivoExplorar en busca de DM2 a los escolares de alto riesgo de Jordania.
Pacientes y métodosSe realizó un estudio transversal descriptivo. Niños de 10-14,9 años. Se midieron el peso, la estatura y el perímetro de la cintura, y se determinó la puntuación Z del índice de masa corporal; también se calculó el cociente entre cintura y talla. Se midió la presión arterial y se identificaron los 3 factores de riesgo de los niños.
ResultadosSe sometió a cribado a 871, de los que el 26,0% tenía sobrepeso y el 19,3% obesidad. El factor de riesgo más frecuente en los niños con sobrepeso y obesos eran los antecedentes familiares de DM2 en parientes de primer o segundo grado, presentes en el 80,4%; los niños nacidos de madres con diabetes o diabetes mellitus gestacional durante la gestación del niño suponían el 17,3% y alrededor del 26,8% tenía hipertensión (estadio 1 o 2). No había factores de riesgo en el 12,6%, el 54,0% tenía al menos un factor, el 29,1% 2 factores de riesgo y el 4,3% 3 factores de riesgo.
ConclusiónLos factores de riesgo de DM2 son muy frecuentes. Alrededor del 54% tenía un factor de riesgo. Las estrategias dirigidas a reducir los factores de riesgo de DM2, especialmente la obesidad, en los escolares jordanos son una necesidad urgente.
Diabetes is a complex, chronic illness requiring continuous medical care. The prevalence of diabetes mellitus (DM) is growing worldwide and has reached epidemic proportions in many developing and most developed countries.1 Globally, type 2 diabetes mellitus (T2DM) accounts for more than 90% of the cases of diabetes; the prevalence of T2DM has increased, impacting on health and economies worldwide. Approximately 1 in 11 adults worldwide now has diabetes mellitus.1,2 Generally, T2DM was considered to be exclusively an adult's chronic medical condition. The first cases of T2DM in youth were reported in the mid of 1990s3 with an increasing incidence of T2DM in children and adolescents noted in both developed and developing countries in recent decades.4 Type 2 diabetes mellitus occurs in youth more often during the second decade of life, coinciding with the physiological occurrence of pubertal insulin resistance.5
Type 2 diabetes mellitus has been expanding as a global public health problem in children and adolescents. The disease is emerging among children all around the world. Epidemiologic studies have shown that the incidence of type 2 diabetes in children and adolescents ranges between 1 and 51 per 1000.6 In the Arab world, the overall prevalence of T2DM was 34.9 per 100,000 persons in Kuwait, and 2.9 per 100,000 per year in Qatar.7 It was 9.04% in Saudi Arabia,8 and the prevalence of prediabetes and T2DM was 5.4% and 0.87%, respectively, in the United Arab Emirates.9 In Sudan 4% of the children were reported to have T2DM.10
The alarming increase of T2DM in youths is mostly attributable to a high prevalence of childhood overweight and obesity. Overweight and obesity are major contributors to the development of insulin resistance, which is considered a feature of T2DM.9,11 As overweight and obesity are the main factors in the development T2DM and Jordan experienced increases in the prevalence of overweight and obesity among children and adolescents. Recent research reported that the prevalence of overweight and obesity in children aged 6–17 years was 17.3%, and 15.7%, respectively.12 In addition, the overall prevalence of malnutrition characterized by overweight and obesity together among children aged 10–17 years old was 46.1%.13
Type 2 diabetes is often asymptomatic in its early stages. This makes diagnosis difficult without an awareness of the risk factors. Consequently, the American Diabetes Association (ADA) recommends screening for type 2 diabetes beginning at 10 years of age or the onset of puberty in children who are overweight or obese and who have one or more additional risk factors including signs of insulin resistance or conditions associated with insulin resistance such as hypertension, race/ethnicity, a family history of type 2 diabetes in a first- or second-degree relative, or maternal history of diabetes or gestational diabetes mellitus (GDM) during the child's gestation.14
In Jordan, most of the available studies reported the prevalence of type1 and 2 diabetes mellitus in the general population. However, there have not been any studies on the prevalence of T2DM and its related risk factors among overweight or obese children and adolescents. Therefore, the principal aim of this study is to identify the prevalence of three noninvasive risk factors of T2DM in overweight and obese children and adolescent's population in school settings.
Materials and methodsThis school based descriptive cross-sectional study was conducted in the city of Amman between December 2018 and March 2019. The City of Amman has five directorates of education in public schools and one for private ones. The selected schools from those directorates were upon agreeing of the headmasters take part in this study. A total 1400 questionnaires were distributed to parents of children aged 10–14.9 years old, attending 5th–8th grades. A total of 871 students were effectively enrolled in this study from eight public and five private schools. with a response rate of 62.2%.
Data were collected through a questionnaire and direct measurements (i.e., students’ weight, height, waist circumference and blood pressure). A self-administered questionnaire was sent to parents through their children. The questionnaire asked for the child's date of birth, birth weight, maternal history of diabetes or GDM during the child's gestation, and family history of type 2 diabetes in first- or second-degree relatives.
School directors were informed of the purpose and contents of the data collection tool, and permission was obtained to conduct the study. An explanation of the aim of the study was provided to students to obtain their initial approval. An informed consent document including a simple explanation of the aim of the study was sent with each student home to be signed by their parents.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration. This study was approved by The Hashemite University (IRB number: 9/6/2017/2018) and Ministry of Education (3/10/51911).
Height, weight, and waist circumference (WC) were measured by a trained nutritionist. Height was measured to the nearest 0.1cm in standing position at Frankfurt plane with the occipital, shoulder and the buttock touching the vertical stand using a stadiometer seca (Germany). Weight was measured to the nearest 0.1kg using an electronic scale with participants wearing light clothes and no shoes. Waist circumference was measured midway between the lower rib margin and the iliac crest with plastic tape to the nearest 1mm. Weight and height were entered into WHO Anthro-Plus software (v1.0.4, WHO, Geneva, Switzerland). Body mass index-for-age Z-score (BAZ) was calculated. For the association of the BMI-for-age with overweight and obesity, values >+1SD represent overweight (equivalent to BMI 25kg/m2 at 19 years) and >+2SD represent obesity (equivalent to BMI 30kg/m2 at 19 years) according to the WHO reference curves (2007). Whereas, values between +1 SD and −2 SD was considered normal and values >−2 and −>3 were considered thinness and severe thinness respectively.15 Waist to height ratio (WHtR) was calculated. A WHtR cutoff of ≥0.5 is generally accepted as a universal cutoff for obesity in children (aged ≥6 years) and adults.16
Blood pressure (BP) was measured by a trained nurse using a calibrated MicroLife automated blood pressure A3 Plus (CH-9443 Widnau/Switzerland) monitor with an appropriate cuff size. All students were instructed to rest for at least 5min. Blood pressure was measured in the right arm for all participants. Measurements were taken two times with short intervals between readings, and the average of BP readings was calculated. Around 13% of the students refused to measure their blood pressure as they scared. Blood pressure was categorized for children between 1 and 13 years of age as follows: normal BP – both systolic BP (SBP) and diastolic BP (DBP) <90th percentile; elevated BP – SBP and/or DBP ≥90th percentile but <95th percentile; stage 1 hypertension – SBP and/or DBP ≥95th percentile; stage 2 hypertension – SBP and/or DBP ≥95th percentile+12mmHg for age, sex, and height. For children ≥13 years of age: normal BP – BP <120/80mmHg; elevated BP – SBP between 120 and 129 with a DBP <80mmHg; stage 1 hypertension – BP between 130/80 to 139/89mmHg; stage 2 hypertension – BP ≥140/90mmHg.17
Analyses were carried out using Statistical Package for Social Sciences (SPSS) software (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp). Descriptive statistics are presented in percentages for categorical variables. The chi-square test was used to compare percentages. A P-value of <0.05 was considered statistically significant.
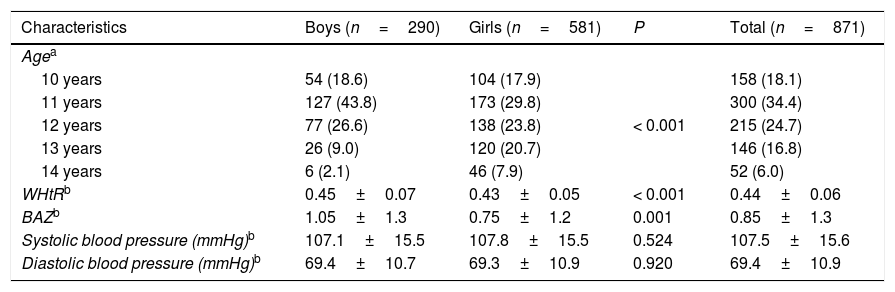
ResultsA total of 871 school children aged 10–14.9 years were enrolled, of which 290 (33.3%) were boys and 581 (66.7%) were girls; the mean age was 11.9± 1.1 years with a predominance of children aged 11 years old, with 34.4%. As shown in Table 1, the mean WHtR and BAZ were significantly higher in boys compared to girls (0.45 vs. 0.43. P<0.001; 1.05 vs. 0.75. P=0.001) respectively.
Characteristics of participants.
| Characteristics | Boys (n=290) | Girls (n=581) | P | Total (n=871) |
|---|---|---|---|---|
| Agea | ||||
| 10 years | 54 (18.6) | 104 (17.9) | < 0.001 | 158 (18.1) |
| 11 years | 127 (43.8) | 173 (29.8) | 300 (34.4) | |
| 12 years | 77 (26.6) | 138 (23.8) | 215 (24.7) | |
| 13 years | 26 (9.0) | 120 (20.7) | 146 (16.8) | |
| 14 years | 6 (2.1) | 46 (7.9) | 52 (6.0) | |
| WHtRb | 0.45±0.07 | 0.43±0.05 | < 0.001 | 0.44±0.06 |
| BAZb | 1.05±1.3 | 0.75±1.2 | 0.001 | 0.85±1.3 |
| Systolic blood pressure (mmHg)b | 107.1±15.5 | 107.8±15.5 | 0.524 | 107.5±15.6 |
| Diastolic blood pressure (mmHg)b | 69.4±10.7 | 69.3±10.9 | 0.920 | 69.4±10.9 |
WHtR: waist to height ratio. BAZ: body mass index-for-age Z-score.
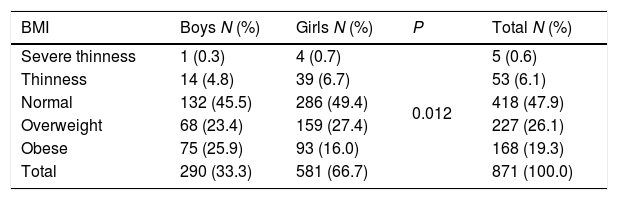
The risk-based screening for type 2 diabetes should be considered in children and adolescents with overweight or obesity. Table 2 showed that nearly 50% of the participants were of normal BMI. The overall prevalence of overweight was 26%. A higher prevalence of overweight was found among girls, with 27.2% being overweight compared to 23.4% of boys. Obesity was verified among 19.3% of the total sample. Here, on the contrary, a higher prevalence of obesity was found among boys, with 25.9% being obese compared to 16.0% of girls. Additionally, significant association (P=0.012) was found between gender and BMI status among children.
Prevalence of thinness and severe thinness, normal, overweight and obesity among study participants.
| BMI | Boys N (%) | Girls N (%) | P | Total N (%) |
|---|---|---|---|---|
| Severe thinness | 1 (0.3) | 4 (0.7) | 0.012 | 5 (0.6) |
| Thinness | 14 (4.8) | 39 (6.7) | 53 (6.1) | |
| Normal | 132 (45.5) | 286 (49.4) | 418 (47.9) | |
| Overweight | 68 (23.4) | 159 (27.4) | 227 (26.1) | |
| Obese | 75 (25.9) | 93 (16.0) | 168 (19.3) | |
| Total | 290 (33.3) | 581 (66.7) | 871 (100.0) |
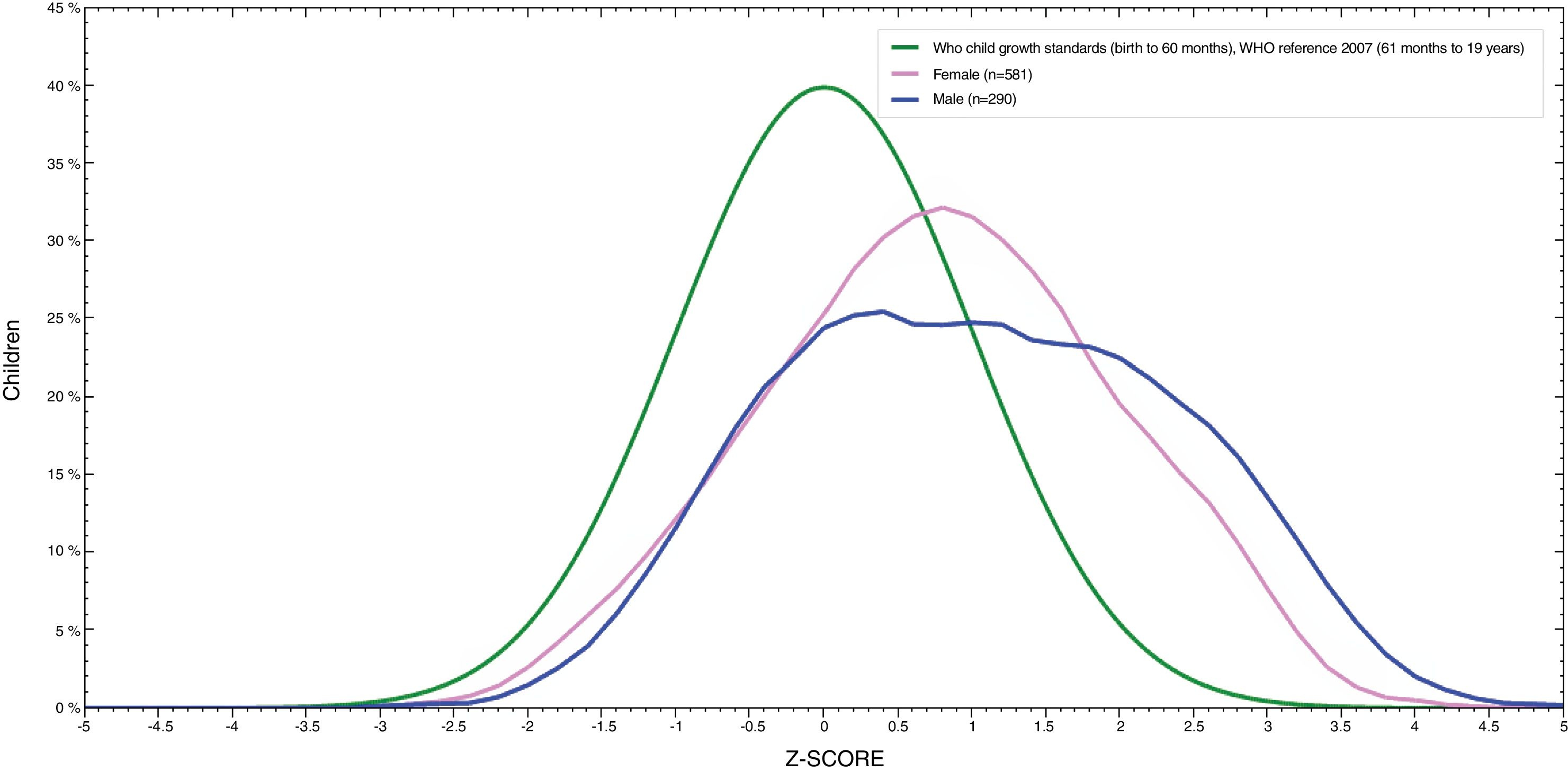
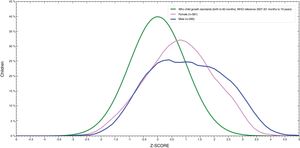
Fig. 1 showed that BMI for age distribution curve shifted to the right when compared to the WHO reference curve in both boys and girls. The mean BAZ (standard deviation) was 0.85 (1.3). The curve of boys was more inclined to the right than for girls and BAZ for boys was higher than for girls (1.05 vs. 0.75).
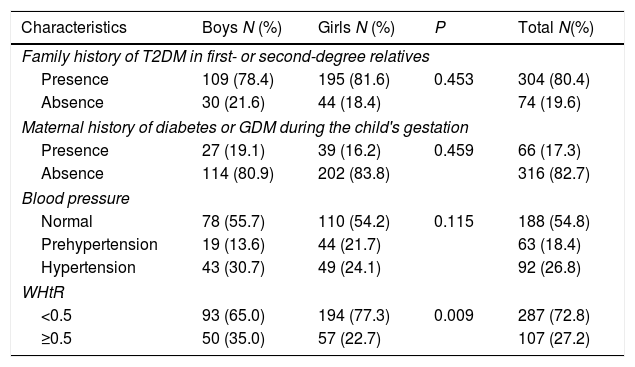
Gender characteristics of overweight and obese school children at high risk for developing T2DM are shown in Table 3. A total of 80.4% of children had a positive family history of T2DM in a first- or second-degree relatives excluding mothers, whereas 17.3% of children had mothers with a maternal history of diabetes or GDM during the child's gestation. Children who had high systolic or diastolic BP at the time of data collection were 26.8%. Children who had abnormal WHtR were 27.2% and significant association (P=0.009) was found between gender and WHtR among children.
Gender characteristics of overweight and obese participants at high risk for developing T2DM.
| Characteristics | Boys N (%) | Girls N (%) | P | Total N(%) |
|---|---|---|---|---|
| Family history of T2DM in first- or second-degree relatives | ||||
| Presence | 109 (78.4) | 195 (81.6) | 0.453 | 304 (80.4) |
| Absence | 30 (21.6) | 44 (18.4) | 74 (19.6) | |
| Maternal history of diabetes or GDM during the child's gestation | ||||
| Presence | 27 (19.1) | 39 (16.2) | 0.459 | 66 (17.3) |
| Absence | 114 (80.9) | 202 (83.8) | 316 (82.7) | |
| Blood pressure | ||||
| Normal | 78 (55.7) | 110 (54.2) | 0.115 | 188 (54.8) |
| Prehypertension | 19 (13.6) | 44 (21.7) | 63 (18.4) | |
| Hypertension | 43 (30.7) | 49 (24.1) | 92 (26.8) | |
| WHtR | ||||
| <0.5 | 93 (65.0) | 194 (77.3) | 0.009 | 287 (72.8) |
| ≥0.5 | 50 (35.0) | 57 (22.7) | 107 (27.2) | |
In normal weight children, a separate analysis showed that 79.0% of children had a positive family history of T2DM in their first- or second-degree relatives excluding mothers, while 11.0% of children had mothers with a maternal history of diabetes or GDM during the child's gestation, 9.2% had high systolic or diastolic BP, and none of normal weight children had abnormal WHtR.
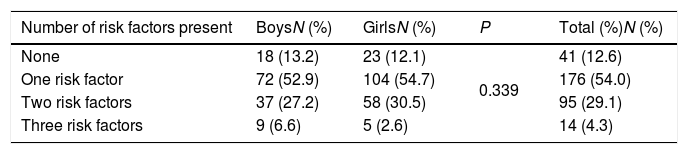
Table 4 shows the distribution of risk factors among the overweight and obese school children who had an assessment for the three studied risk factors. Almost 12.6% of participants did not have any risk factors. Approximately 54% of the participants had one risk factor, and were identified as at
Distribution of participants according to number of risk factors present.
| Number of risk factors present | BoysN (%) | GirlsN (%) | P | Total (%)N (%) |
|---|---|---|---|---|
| None | 18 (13.2) | 23 (12.1) | 0.339 | 41 (12.6) |
| One risk factor | 72 (52.9) | 104 (54.7) | 176 (54.0) | |
| Two risk factors | 37 (27.2) | 58 (30.5) | 95 (29.1) | |
| Three risk factors | 9 (6.6) | 5 (2.6) | 14 (4.3) |
risk for T2DM based on ADA guidelines. A total of 29.1% of the participants had two risk factors, and 4.3% had three risk factors.
DiscussionThe American Diabetes Association established guidelines that define children and adolescents who are overweight/obese and have one or more other T2DM risk factors as being at risk of developing T2DM. To the best of our knowledge, this study was the first to address some of the risk factors for T2DM among school children in Jordan.
Considering overweight/obesity are the main drives in the development of T2DM. This study indicates that the prevalence of overweight and obesity were 26% and 19.3%, respectively. In other words, 45% of the children had increased body weight. This study indicates that the prevalence of obesity (19.3%), was higher than that of other studies also carried out in Jordan, which had percentages of 8%, and 5.4%.13,18 On the other hand, the prevalence of overweight was consistent with Neg et al.,18 which was 25.4%. The other two studies showed a lower prevalence of overweight compared to the results of the current study; they were 17.3% and 17%.12 Another study reported that the prevalence of overweight among 10–17 years old children was 36.2%.13 This discrepancy is not of interest since overweight and obesity might be currently affecting a larger number of individuals earlier in life or is simply related to sampling variation; age group coverage or method of diagnosis. In the current study, the most alarming sign was that BMI for age distribution curve was shifted to the right, which indicates a high rate of overweight and obesity in both males and females.
Abdominal obesity such as waist circumference and WHtR is another measure used in the evaluation of cardio metabolic risk factors including T2DM. Some researchers have suggested the use of abdominal obesity measures for the evaluation of obesity and T2DM in children and adolescents.19 In the current study, 27.2% of overweight and obese participants had WHtR≥0.5, WHtR could be used along with BMI to screen for overweight and obesity among youth. A previous study reported that a WHtR≥0.5 at 7–9 years increased the risk of having three or more cardiometabolic risk factors in adolescence. In addition, central obesity causes a higher risk for developing insulin resistance.20 In Jordan, none of the studies used waist circumference as a measure for abdominal obesity.
Along with overweight and obesity, a family history of diabetes is associated with T2DM in children. Many studies have shown a strong family history among affected youth, with 74–100% having a first- or second-degree relatives with type 2 diabetes.21 Children with a positive family history of T2DM showed reductions in insulin sensitivity at a younger age than children with no family history. Results from this study showed that approximately 80% of children had a positive family history of T2DM. The present findings seem to be consistent with other research, which found that a positive family history of T2DM was present in 81% of cases,22 and 83.3% of cases in Hungarian school children.23
In the Middle Eastern countries, the prevalence of a positive family history of T2DM was 51.1% in Kuwaiti,24 92% in Sudanese,10 71.4% in Egyptian,25 79.4% in Emirati26 and 70.59% in Saudi overweight and obese children and adolescents.27 On the other hand, positive family history was of low prevalence (11.2%) in Nigeria.28
Youth with a positive family history of T2DM may demonstrate impaired balance between insulin sensitivity and insulin secretion. Studies showed that insulin sensitivity declines by 25–30% as youth transition from prepuberty to puberty.29 Normally, this decrease in insulin sensitivity is compensated by increased insulin secretion. In youth who are predisposed to develop prediabetes and/or type 2 diabetes, β cell compensation is inadequate to lower insulin sensitivity, which is associated with lower insulin clearance that could be an early compensation for the insulin resistance.
Studies on maternal diabetes or GDM and risk of T2DM in youth have suggested that exposure to diabetes during pregnancy has an effect on in utero environment and causes an intrauterine diabetic environment, which increases the risk of developing diabetes in offspring through an epigenetic mechanism.30
Maternal history of diabetes or gestational diabetes mellitus is another non-modifiable risk factor for youth onset T2DM. A smaller number of studies discussed maternal diabetes alone; most of the studies discussed in the context of a family history of T2DM in a first- or second-degree relatives. In UK, Candler et al., reported that 50% of T2DM youth are born to mothers who had diabetes.22 In Kuwaiti children, 20% of T2DM children had mothers with a history of diabetes.24
Insulin resistance is another risk factor for T2DM in youth, Hypertension is considered to be a clinical feature of insulin resistance. Hypertension is a comorbidity of T2DM in youth and thought to be a major risk for nephropathy and atherosclerosis. Hypertension is interrelated to overweight and obesity. In youth it ranges from 3.8% to 24.8%31; the other association observed was between hypertension and increased waist circumference.
In the current study, hypertension was found in 26.8% of the participants. Studies from various parts of the world on hypertension as a risk factor for T2DM in youth revealed that it was present in 32% of Bengali children and adolescents. In Brazil, the prevalence of hypertension was 9.6% among adolescents 12–17 year of age. In Nigeria, the prevalence of prehypertension/hypertension among children identified to be at risk for type 2 DM was 22.8%.28 Hypertension affected 75% of Hungarian overweight and obese school children at risk for T2DM.23 Hypertension is an important risk factor not only for T2DM but also for cardiovascular diseases. It is a modifiable risk factor unlike family history; therefore, it can be prevented with lifestyle modifications.
Given the significance of all mentioned risk factors, one important fact of concern that 54% of this sample presented one risk factor for T2DM while 29.1% presented two factors. Because this problem is a new public health issue, the number of studies addressing the risk factors for T2DM in children and adolescents were few. de Macêdo et al.,32 reported that 53.4% presented no risk factors; 24.3% had at least one factor and 18.8% two risk factors. In Nigeria, 51.9% of students had none of the risk factors while 30.9% had at least one risk factor.28 The results of the above-mentioned studies were inconsistent with the results of the current study. In those studies, the prevalence of overweight and obesity were lower than in the current study, which can be one of the reasons for this considerable difference.
The current study had some important limitations should be noted, First, small sample size. Second, the inability to screen the other conditions associated with insulin resistance such as acanthosis nigricansas, dyslipidemia, polycystic ovary syndrome, or small-for-gestational age birth weight as risk factors of T2DM in schoolchildren. Third, low response rate might be due to the refusal from parents to take part in this study, or to the fact that students, especially boys, forgot giving the questionnaire to their parents or bringing back the study questionnaire sent to their parents. Despite these limitations, this study was unique in its examination of risk factors among schoolchildren. Most previous studies have evaluated adults and there were no data available about the at-risk adolescent population. In addition, among the strengths of this study is that the first research included waist circumference data for this age group, and the findings of this study add advantages for school health screening programs and may provide a base line data for future studies.
ConclusionIn conclusion, risk factors for T2DM are very common. Around 54% had one risk factor. Strategies aimed at reducing risk factors for T2DM, especially obesity, among Jordanian school children are urgently warranted. Early identification by school and health care personnel of adolescents at risk presents an opportunity for early intervention to delay the onset of type 2 diabetes and possibly even to prevent the disease altogether.
FundingThe authors disclosed that they didn’t receive any financial support for this research
Authors’ contributionAll authors were involved in preparing and revision of the paper, and all authors read and approved the final manuscript.
Conflict of interestThe authors stated that they have no conflict of interest
We would like to thank the children and their parents who took part in this study, and the headmasters and teachers for their help and cooperation.