Neuregulin 1 (NRG-1) is one of the members of the epidermal growth factors proteins. The present study provides novel insights into the relationship between serum levels of NRG-1 and insulin resistance, subclinical atherosclerosis and cardiac dysfunction that occur in type 2 diabetes (T2D).
MethodsThe study included 50 patients with T2D and 40 healthy age- and gender-matched controls. Serum NRG-1 was measured using ELISA. Glycemic parameters, lipid profile and insulin resistance were assessed. Trans-thoracic echocardiography and carotid intima media thickness (CIMT) were studied for all study subjects.
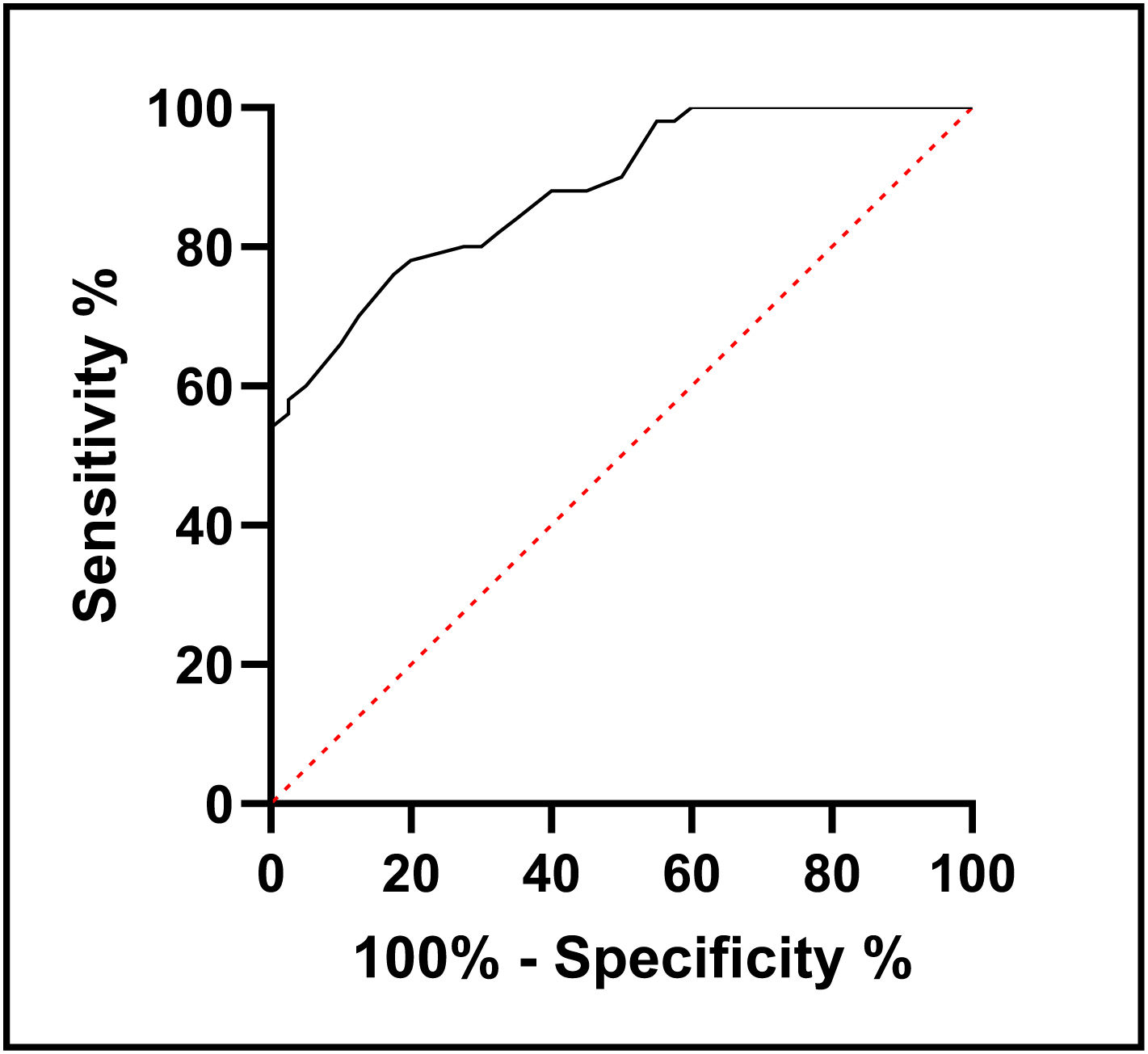
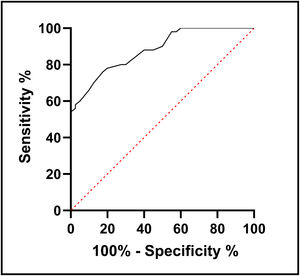
ResultsT2D patients had significantly lower serum NRG-1 levels than controls. Serum NRG-1 was negatively correlated with age, fasting blood glucose, HbA1c, insulin resistance, blood urea, serum creatinine and LDL-C, and positively correlated with HDL-C, eGFR and CIMT. Regarding echocardiographic variables, serum NRG-1 was found to correlate positively with left ventricular global longitudinal strain and negatively with E/Ea ratio. NRG-1 was found to predict subclinical atherosclerosis in type 2 diabetes patients at a cut-off value<108.5pg/ml with 78% sensitivity and 80% specificity.
ConclusionsA robust relationship was found between serum NRG-1 levels and hyperglycemia, insulin resistance, subclinical atherosclerosis, and cardiac dysfunction in patients with type 2 diabetes. These results shed light on a possible role of NRG-1 as a potential noninvasive biomarker for detection of cardiometabolic risk in T2D.
La neuregulin 1 (NRG-1) es uno de los miembros de las proteínas de los factores de crecimiento epidérmico. El presente estudio proporciona nuevos conocimientos sobre la relación entre los niveles séricos de NRG-1 y la resistencia a la insulina, la aterosclerosis subclínica y la ocurrencia en la diabetes mellitus tipo 2 (T2D).
Material y métodosEl estudio incluyó a 50 pacientes con T2D y 40 controles sanos emparejados por edad y género. Se midió NRG-1 en suero usando análisis de inmunoabsorción ligada a enzimas (ELISA). Se evaluaron parámetros glucémicos, perfil lipídico y resistencia a la insulina. Se estudiaron la ecocardiografía transtorácica y el espesor de la íntima media carotídea (CIMT) para todos los sujetos del estudio.
ResultadosLos pacientes con T2D tenían niveles séricos de NRG-1 significativamente más bajos que los controles. El NRG-1 sérico se correlacionó negativamente con la edad, la glucemia en ayunas, la HbA1c, la resistencia a la insulina, la urea en sangre, la creatinina sérica y el colesterol de las lipoproteínas de baja densidad (cLDL), y lo hizo positivamente con el colesterol de las lipoproteínas de alta densidad (cHDL), la tasa de filtración glomerular estimada (TFGe) y el CIMT. En cuanto a las variables ecocardiográficas, se encontró que el NRG-1 sérico se correlacionó positivamente con el strain longitudinal global del ventrículo izquierdo y negativamente con la relación E\Ea. Se descubrió que NRG-1 predice la aterosclerosis subclínica en pacientes con T2D a un valor de corte < 108,5 pg/mL con una sensibilidad de 78% y una especificidad de 80%.
ConclusionesSe encontró una relación sólida entre los niveles séricos de NRG-1 y la hiperglucemia, la resistencia a la insulina, la aterosclerosis subclínica y la disfunción cardiaca en pacientes con T2D. Estos resultados arrojan luz sobre un posible papel de NRG-1 como un posible biomarcador no invasivo para la detección del riesgo cardiometabólico en T2D.
Diabetes is a grave chronic disease which has major dysmetabolic aberrations in carbohydrate, protein and fat metabolism secondary to insulin insufficiency or tissue resistance to its function.1 Macrovascular complications of type 2 diabetes (T2D) include coronary heart disease, cardiomyopathy, arrhythmias, cerebrovascular disease and peripheral artery disease.2 These complications occur through different pathogenetic pathways including hyperglycemia and insulin resistance. Cardiovascular disease is the main reaper of the lives of diabetic patients.3
T2D carries a high risk of atherosclerosis in large vessels. Atheromatous plaques cause arteries to narrow and disrupt blood flow. These plaques may rupture, causing the formation of blood clots, which eventually lead to cardiovascular events.4 Coexistent hypertension, hyperlipidemia and obesity are further reinforcing risk factors. Diabetes has been associated with two- to four fold higher risks of atherosclerotic cardiovascular disease, such as coronary heart disease, ischemic stroke, and peripheral arterial disease.5
Neuregulin 1 (NRG-1) belongs to the epidermal growth factors (EGF) family of proteins. This family comprises four closely related genes, known as NRG1-4, which produce various isoforms that are characterized by the presence of an EGF-like domain. This domain is responsible for the biological activity of the isoforms and their ability to bind to two specific proteins called ErbB3 and ErbB4. NRG-1 is secreted by cells originating from the endothelial, mesenchymal, and neuronal lineages, while ErbB receptors are found in close proximity to the ligand. This allows for local autocrine, paracrine, or even juxtracrine actions to occur.6
NRG-1 was found to be released from the heart endothelial cells. It has regenerative, and antifibrotic effects on the myocardium.7 NRG-1 and the ErbB receptors are expressed in the central nervous system, skeletal muscle, liver, pulmonary cells, enterocytes, and kidney.8
An animal study in rats showed that NRG-1 injection promoted glucose tolerance following an oral glucose load.9 Another study showed that NRG-1 in vitro reduced cholesterol ester accumulation in monocyte-derived macrophages, and administration of recombinant NRG-1 in Apo E deficient mice shrunk the surface area of aortic atheromatous plaques.10
There is a significant unmet clinical need for the identification of the role of NRG-1 in diabetes in humans and its relation to insulin resistance, subclinical atherosclerosis and cardiovascular disease.
Materials and methodsThis work is a case–control study, including 90 participants. They included 50 patients with T2D and 40 healthy subjects who served as controls.
Patients were chosen from the outpatient clinics of both departments of Endocrinology and Metabolism and Cardiology, Faculty of Medicine, Al-Azhar University, Cairo. Before enrollment, the research objectives and plan were clarified to all study participants and both verbal and written consents were submitted. The study was ethically approved by the Research Ethics committee of Faculty of Medicine (Girls), Al-Azhar University, Cairo, Egypt with approval number 202104/802 and was in line with the principles of the Declaration of Helsinki.
The following exclusion criteria were set including a cutoff for body mass index>33kg/m2, history of smoking, type 1 diabetes, acute complications of diabetes, known micro- or macrovascular diabetic complications, infection, history of impairment of liver function, chronic liver disorders or advanced diabetic kidney disease, history of metabolic or endocrine diseases or chronic intake of medications that could affect the metabolic state. Patients with history of cardiovascular events, ischemic heart disease, heart failure, or cardiac valve problems were also excluded.
Clinical and biochemical evaluationAn extensive clinical examination, anthropometric measurements, and blood pressure checks were all performed on study participants.
All subjects had fasting blood samples taken aseptically for laboratory testing. The glucose oxidase method was used to assess fasting blood glucose (FBG). Routine enzymatic techniques were used to evaluate the detailed lipid profile, serum creatinine, blood urea, aspartate transaminase (AST), and alanine transaminase (ALT). EDTA-treated blood was used to measure glycated hemoglobin (HbA1c) calorimetrically using cation-exchange resin.
Both serum NRG-1 and C-peptide were measured using ELISA kits supplied by Innova Biotech Co., Beijing, China and Monocent Inc., California, USA respectively.
Some of study patients were on insulin therapy, thus it was not possible to use homeostasis model assessment of insulin resistance (HOMA-IR) to assess insulin resistance. Alternatively, estimated glucose disposal rate (eGDR)11 and insulin resistance index (IR index) were used to assess and define the insulin resistance status in our study participants.12
Estimated glomerular filtration rate (eGFR) was obtained for all participants using Modified Diet in Renal Disease formula.
Measurement of carotid intima media thickness (CIMT)CIMT was measured using a high-resolution B-mode ultrasound system (Philips affiniti by Singha's medical systems India) using phased array linear probe with a high frequency (5 –12MHz). All measurements were performed by one operator. On gray scale image, the intima-media thickness of the common carotid artery was manually measured bilaterally from its far wall at the portion of maximum thickening.13
EchocardiographyTransthoracic echocardiography was conducted to all study participants using ultrasound machine GE-Vivid 6 system (GE-Ultrasound, Horten, Norway). M3S (2.5MHz) matrix probe, and echo Pac version 110-1.3 were utilized. Conventional echo-Doppler was conducted applying the criteria of the American Society of Echocardiography and the European Association of Cardiovascular Imaging for Cardiac Chamber Quantification by Echocardiography in Adults.14
M-mode, Pulsed wave Doppler flow measures, two dimensional (2D) measures and Tissue Doppler imaging (TDI) were all performed by a single operator. Also 2D speckle tracking echo was conducted to measure global longitudinal strain (GLS) which measures subclinical affection of the LV or RV function.
Statistical analysisThe collected data were analyzed using the Statistical Package for Social Sciences (SPSS) version 24. Qualitative data was presented as number (percentage) while quantitative data was expressed as mean±standard of deviations. Test for data normality was done using Shapiro–Wilk test. Both Chi square test (X2) and independent t-test were used in comparing the variables in study groups.
Pearson correlation coefficient and Spearman's rank correlation coefficient were used to study the correlations between NRG-1 and other studied parameters. Multivariate logistic regression analysis was performed to investigate the significant predictors of serum NRG-1 concentrations. Receiver operating characteristic curve was plotted for levels of serum NRG-1 as a classifier tool for diagnosis of subclinical atherosclerosis in patients’ group. Statistical significance was defined as a P-value<0.05.
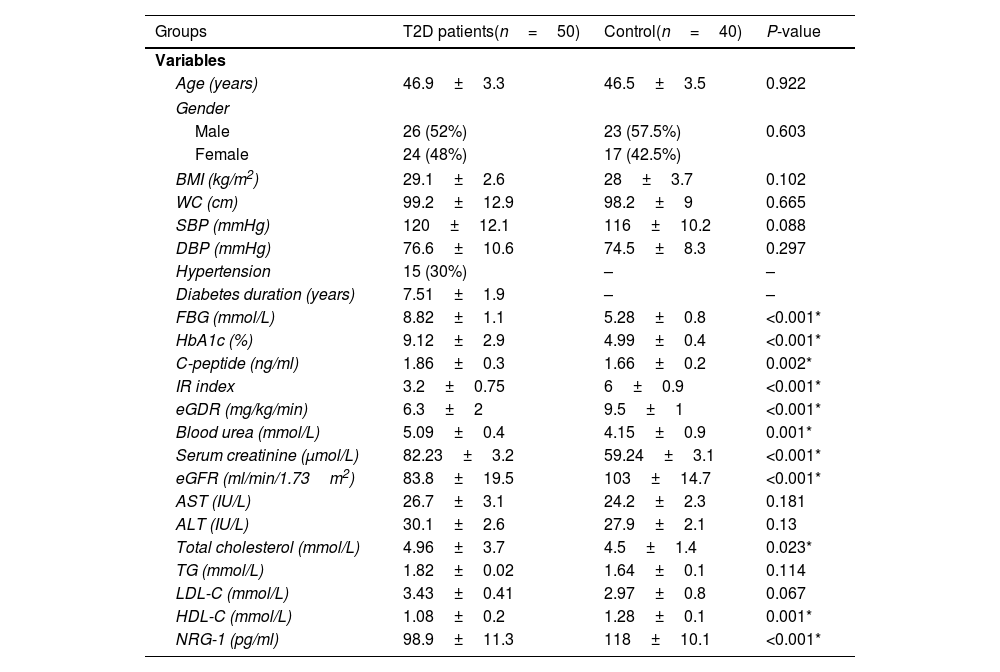
ResultsThe clinical, anthropometric, and biochemical features of the groups under study are shown in Table 1. A significant decrease in serum NRG-1 was found in T2D patients compared to controls. Regarding glycemic indices, FBG, HbA1c and C-peptide values were significantly increased in T2D patients, while IR Index and eGDR were significantly lower, indicating an insulin resistant status among T2D patients compared to controls. All diabetic patients had significantly higher values as regard blood urea, serum creatinine and total cholesterol compared to control group. However, both HDL-C and eGFR were significantly lower in the patients.
Demographic, clinical, and biochemical characteristics of the studied groups.
| Groups | T2D patients(n=50) | Control(n=40) | P-value |
|---|---|---|---|
| Variables | |||
| Age (years) | 46.9±3.3 | 46.5±3.5 | 0.922 |
| Gender | |||
| Male | 26 (52%) | 23 (57.5%) | 0.603 |
| Female | 24 (48%) | 17 (42.5%) | |
| BMI (kg/m2) | 29.1±2.6 | 28±3.7 | 0.102 |
| WC (cm) | 99.2±12.9 | 98.2±9 | 0.665 |
| SBP (mmHg) | 120±12.1 | 116±10.2 | 0.088 |
| DBP (mmHg) | 76.6±10.6 | 74.5±8.3 | 0.297 |
| Hypertension | 15 (30%) | – | – |
| Diabetes duration (years) | 7.51±1.9 | – | – |
| FBG (mmol/L) | 8.82±1.1 | 5.28±0.8 | <0.001* |
| HbA1c (%) | 9.12±2.9 | 4.99±0.4 | <0.001* |
| C-peptide (ng/ml) | 1.86±0.3 | 1.66±0.2 | 0.002* |
| IR index | 3.2±0.75 | 6±0.9 | <0.001* |
| eGDR (mg/kg/min) | 6.3±2 | 9.5±1 | <0.001* |
| Blood urea (mmol/L) | 5.09±0.4 | 4.15±0.9 | 0.001* |
| Serum creatinine (μmol/L) | 82.23±3.2 | 59.24±3.1 | <0.001* |
| eGFR (ml/min/1.73m2) | 83.8±19.5 | 103±14.7 | <0.001* |
| AST (IU/L) | 26.7±3.1 | 24.2±2.3 | 0.181 |
| ALT (IU/L) | 30.1±2.6 | 27.9±2.1 | 0.13 |
| Total cholesterol (mmol/L) | 4.96±3.7 | 4.5±1.4 | 0.023* |
| TG (mmol/L) | 1.82±0.02 | 1.64±0.1 | 0.114 |
| LDL-C (mmol/L) | 3.43±0.41 | 2.97±0.8 | 0.067 |
| HDL-C (mmol/L) | 1.08±0.2 | 1.28±0.1 | 0.001* |
| NRG-1 (pg/ml) | 98.9±11.3 | 118±10.1 | <0.001* |
T2D: type 2 diabetes, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, FBG: fasting blood glucose, HbA1c: glycated hemoglobin, IR index: insulin resistance index, eGDR: estimated glucose disposal rate, eGFR: estimated glomerular filtration rate, AST: aspartate transaminase, ALT: alanine transaminase, TG: triglycerides, LDL-C: low density lipoprotein cholesterol, HDL-C: high density lipoprotein cholesterol, NRG-1: neuregulin 1.
Independent t-test and Chi-square tests were performed.
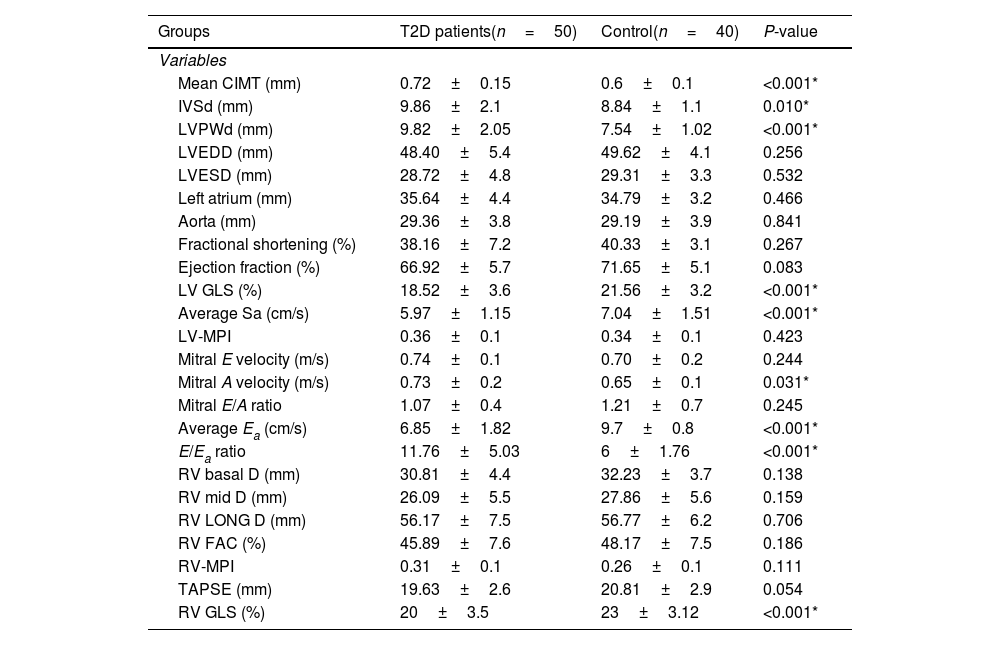
Table 2 shows analysis of CIMT and echocardiographic measurements. Mean CIMT was observed to be significantly higher in T2D patients compared to controls. Left ventricular internal dimensions, interventricular septum thickness at end diastole (IVSd) and left ventricular posterior wall thickness at end diastole (LVPWd) were significantly higher in T2D patients. They also had significantly lower left ventricle global longitudinal strain (LV GLS), an indicator of subclinical LV systolic dysfunction. However no significant differences were found between the studied groups as regards other LV systolic function parameters. Regarding diastolic function parameters, mitral A velocity, average Ea and E/Ea ratio were found to be significantly higher in T2D patients. Meanwhile, no significant differences were observed between both groups in mitral E velocity and E/A ratio.
Carotid intima media thickness and echocardiographic findings among the studied groups.
| Groups | T2D patients(n=50) | Control(n=40) | P-value |
|---|---|---|---|
| Variables | |||
| Mean CIMT (mm) | 0.72±0.15 | 0.6±0.1 | <0.001* |
| IVSd (mm) | 9.86±2.1 | 8.84±1.1 | 0.010* |
| LVPWd (mm) | 9.82±2.05 | 7.54±1.02 | <0.001* |
| LVEDD (mm) | 48.40±5.4 | 49.62±4.1 | 0.256 |
| LVESD (mm) | 28.72±4.8 | 29.31±3.3 | 0.532 |
| Left atrium (mm) | 35.64±4.4 | 34.79±3.2 | 0.466 |
| Aorta (mm) | 29.36±3.8 | 29.19±3.9 | 0.841 |
| Fractional shortening (%) | 38.16±7.2 | 40.33±3.1 | 0.267 |
| Ejection fraction (%) | 66.92±5.7 | 71.65±5.1 | 0.083 |
| LV GLS (%) | 18.52±3.6 | 21.56±3.2 | <0.001* |
| Average Sa (cm/s) | 5.97±1.15 | 7.04±1.51 | <0.001* |
| LV-MPI | 0.36±0.1 | 0.34±0.1 | 0.423 |
| Mitral E velocity (m/s) | 0.74±0.1 | 0.70±0.2 | 0.244 |
| Mitral A velocity (m/s) | 0.73±0.2 | 0.65±0.1 | 0.031* |
| Mitral E/A ratio | 1.07±0.4 | 1.21±0.7 | 0.245 |
| Average Ea (cm/s) | 6.85±1.82 | 9.7±0.8 | <0.001* |
| E/Ea ratio | 11.76±5.03 | 6±1.76 | <0.001* |
| RV basal D (mm) | 30.81±4.4 | 32.23±3.7 | 0.138 |
| RV mid D (mm) | 26.09±5.5 | 27.86±5.6 | 0.159 |
| RV LONG D (mm) | 56.17±7.5 | 56.77±6.2 | 0.706 |
| RV FAC (%) | 45.89±7.6 | 48.17±7.5 | 0.186 |
| RV-MPI | 0.31±0.1 | 0.26±0.1 | 0.111 |
| TAPSE (mm) | 19.63±2.6 | 20.81±2.9 | 0.054 |
| RV GLS (%) | 20±3.5 | 23±3.12 | <0.001* |
CIMT: carotid intima media thickness, IVSd: interventricular septum thickness at end diastole, LVPWd: left ventricular posterior wall thickness at end diastole, LVEDD: left ventricle end-diastolic dimension, LVESD: left ventricle end-systolic dimension, LV GLS: light ventricle global longitudinal strain, LV-MPI: left ventricular myocardial performance index, Average Ea: average early diastolic velocity of lateral mitral annulus, Average Sa: average early diastolic velocity of MV annulus, Mitral E velocity: early transmitral diastolic velocity, Mitral A velocity: late transmitral diastolic velocity, E/Ea ratio: peak early transmitral filling wave velocity (E) to early diastolic velocity of lateral mitral annulus (Ea), RV basal D: right ventricular basal dimension, RV mid D: right ventricle middle dimension, RV LONG D: right longitudinal dimension, RV FAC; right ventricle fractional area change, RV-MPI: right ventricular myocardial performance index, TAPSE: tricuspid annular plane systolic excursion, RV GLS: right ventricle global longitudinal strain.
Independent t-test was performed.
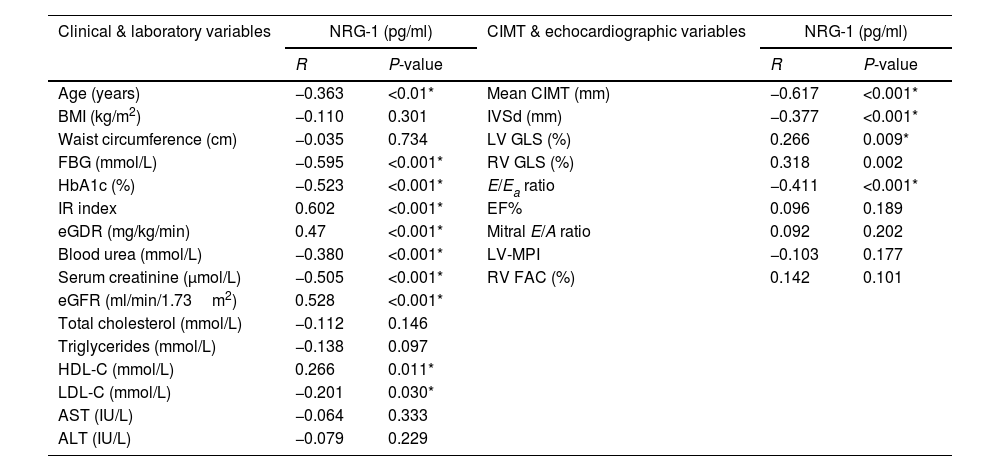
Correlations between serum NRG-1 and studied parameters are shown in Table 3. Serum NRG-1 had statistically significant negative correlations with age, FBG, HbA1c, blood urea, serum creatinine and low density lipoprotein cholesterol (LDL-C). Meanwhile, serum NRG-1 correlated positively with high density lipoprotein cholesterol (HDL-C), and eGFR. Regarding insulin resistance, a positive correlation was observed between serum NRG-1 and both IR index and eGDR, denoting the negative association between NRG-1 and insulin resistance.
Correlation between serum NRG-1 and the studied variables in all study participants.
| Clinical & laboratory variables | NRG-1 (pg/ml) | CIMT & echocardiographic variables | NRG-1 (pg/ml) | ||
|---|---|---|---|---|---|
| R | P-value | R | P-value | ||
| Age (years) | −0.363 | <0.01* | Mean CIMT (mm) | −0.617 | <0.001* |
| BMI (kg/m2) | −0.110 | 0.301 | IVSd (mm) | −0.377 | <0.001* |
| Waist circumference (cm) | −0.035 | 0.734 | LV GLS (%) | 0.266 | 0.009* |
| FBG (mmol/L) | −0.595 | <0.001* | RV GLS (%) | 0.318 | 0.002 |
| HbA1c (%) | −0.523 | <0.001* | E/Ea ratio | −0.411 | <0.001* |
| IR index | 0.602 | <0.001* | EF% | 0.096 | 0.189 |
| eGDR (mg/kg/min) | 0.47 | <0.001* | Mitral E/A ratio | 0.092 | 0.202 |
| Blood urea (mmol/L) | −0.380 | <0.001* | LV-MPI | −0.103 | 0.177 |
| Serum creatinine (μmol/L) | −0.505 | <0.001* | RV FAC (%) | 0.142 | 0.101 |
| eGFR (ml/min/1.73m2) | 0.528 | <0.001* | |||
| Total cholesterol (mmol/L) | −0.112 | 0.146 | |||
| Triglycerides (mmol/L) | −0.138 | 0.097 | |||
| HDL-C (mmol/L) | 0.266 | 0.011* | |||
| LDL-C (mmol/L) | −0.201 | 0.030* | |||
| AST (IU/L) | −0.064 | 0.333 | |||
| ALT (IU/L) | −0.079 | 0.229 | |||
BMI: body mass index, FBG: fasting blood glucose, HbA1c: glycated hemoglobin, IR index: insulin resistance index, eGDR: estimated glucose disposal rate, eGFR: estimated glomerular filtration rate, HDL-C: high density lipoprotein cholesterol, LDL-C: low density lipoprotein cholesterol, AST: aspartate transaminase, ALT: alanine transaminase, CIMT: carotid intima media thickness, IVSd: interventricular septum thickness at end diastole, LV GLS: left ventricle global longitudinal strain, RV GLS: right ventricle global longitudinal strain, E/Ea ratio: peak early transmitral filling wave velocity (E) to early diastolic velocity of lateral mitral annulus (Ea), EF: ejection fraction, LV-MPI: left ventricular myocardial performance index, RV FAC; right ventricle fractional area change.
A significant negative correlation was also found between serum NRG-1 and mean CIMT. Regarding echocardiographic variables assessing left ventricular systolic function, serum NRG-1 was found to correlate positively with left ventricular global longitudinal strain. Also, serum NRG-1 was negatively correlated with E/Ea ratio, a measure of diastolic function. Regarding left ventricular dimensions, NRG-1 was found to correlate negatively with interventricular septal diameter in diastole.
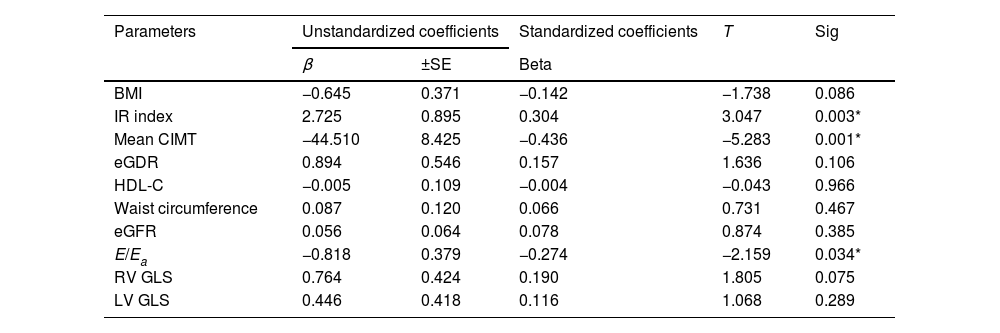
In Table 4, logistic regression analysis shows that predictors of serum NRG-1 level were found to be IR index (P-value 0.003), atherosclerosis (represented by mean CIMT, P-value 0.001) and ventricular diastolic dysfunction (expressed by E/Ea, P-value 0.034).
Multivariate regression analysis using serum neuregulin 1 as dependent variable to identify the most predictor parameter.
| Parameters | Unstandardized coefficients | Standardized coefficients | T | Sig | |
|---|---|---|---|---|---|
| β | ±SE | Beta | |||
| BMI | −0.645 | 0.371 | −0.142 | −1.738 | 0.086 |
| IR index | 2.725 | 0.895 | 0.304 | 3.047 | 0.003* |
| Mean CIMT | −44.510 | 8.425 | −0.436 | −5.283 | 0.001* |
| eGDR | 0.894 | 0.546 | 0.157 | 1.636 | 0.106 |
| HDL-C | −0.005 | 0.109 | −0.004 | −0.043 | 0.966 |
| Waist circumference | 0.087 | 0.120 | 0.066 | 0.731 | 0.467 |
| eGFR | 0.056 | 0.064 | 0.078 | 0.874 | 0.385 |
| E/Ea | −0.818 | 0.379 | −0.274 | −2.159 | 0.034* |
| RV GLS | 0.764 | 0.424 | 0.190 | 1.805 | 0.075 |
| LV GLS | 0.446 | 0.418 | 0.116 | 1.068 | 0.289 |
β: regression coefficient, SE: standard error, BMI: body mass index, CIMT: carotid intima media thickness, eGDR: estimated glucose disposal rate, HDL-C: high density lipoprotein cholesterol, eGFR: estimated glomerular filteration rate, E/Ea: peak early transmitral filling wave velocity (E) to early diastolic velocity of lateral mitral annulus (Ea), RV GLS: right ventricle global longitudinal strain, LV GLS: left ventricle global longitudinal strain.
In order to use serum level of NRG-1 to predict subclinical atherosclerosis in people with type 2 diabetes, receiver operating characteristic analysis was developed. It was found that the cut-off value was <108.5pg/ml, with sensitivity 78% and specificity 80% (Fig. 1).
DiscussionCardiovascular morbidity and mortality pose a significant threat that jeopardizes the longevity and welfare of patients with T2D. Diabetes generates a dysmetabolic milieu which evokes a chronic systemic inflammatory status. This, in turn, provokes the deleterious mechanisms underlying vascular formation of atherosclerosis as well as cardiac dysfunction. Those mechanisms are not fully appreciated and are not handled effectively by the currently available pharmacotherapies.15
The enormous epidermal growth factors (EGF) family of proteins includes NRG-1.6 NRG-1 isoforms play crucial roles in growth, differentiation and survival of nerve, cardiac and skeletal muscle tissues. The NRG-1/ErbB pathway may be regarded as a potential target for treatment of neuromuscular and heart diseases.16
The current work explores the impact of serum NRG-1 levels on subclinical atherosclerosis and cardiac dysfunction in T2D.
Results of the present study showed that T2D patients displayed significantly lower serum levels of NRG-1 when compared to control group. Furthermore, negative correlations were observed between NRG-1 and different glycemic parameters including FBG, HbA1c, as well as with insulin resistance. These findings give insight that serum NRG-1 might have a positive effect in the regulation of glucose metabolism and a possible protective role against type 2 diabetes and insulin resistance.
As far as we reviewed the literature, serum level of NRG-1 was not previously studied in T2D patients. Most research on NRG-1 was performed on experimental animals. It was found that diabetic rats showed significant downregulation of NRG1, ErbB2, ErbB3 and ErbB4 gene expression.17
The current study found a negative correlation between NRG-1 and insulin resistance in T2D patients. Arpaci in 2019 studied the association between serum NRG-1 and insulin resistance in a group of women having polycystic ovary syndrome (PCOS). It was found that women having PCOS had lower NRG-1 levels compared to controls and that circulating NRG-1 was inversely correlated with HOMA-IR.18
Acute administration of NRG-1 was found to reduce plasma glucose in experimental mice and pigs, via enhancing glucose uptake by liver and skeletal muscle.19 Also acute NRG-1 injection promoted glucose utilization after an oral load of glucose in rats.9
Several mechanisms for this effect on glucose disposal have been suggested. NRG-1 was reported to influence glucose transporter translocation in skeletal myoblasts.20 In vitro chronic NRG-1 treatment could regulate muscle glucose metabolism by upregulation of expression of glucose transporter 4 (GLUT4) after treatment with NRG-1 in L6E9 muscle cells for 48h.21
NRG-1 was also found to activate signaling pathway, namely, the ErbB3/protein kinase B in liver cells.9 Furthermore, insulin paucity impairs expression of ErbB3 in hepatocytes, suggesting an interrelation between insulin and the NRG-1/ErbB3 pathway in promoting glucose homeostasis.22 This effect suggests that the NRG-1 signaling pathway may be a promising target that could be therapeutically addressed in states of insulin resistance.
Furthermore, the present study results also showed a negative correlation between NRG-1 and LDL-C and a positive correlation with HDL-C. This further denotes a possible positive effect of NRG-1 on lipid profile, and thence on ameliorating the atherosclerotic process in type 2 diabetes.
As regards serum NRG-1 and renal functions, the present study showed a positive correlation between serum NRG-1 and eGFR. Furthermore, serum NRG-1 was negatively correlated with serum creatinine, denoting its positive effect on renal functions.
Vandekerckhove et al. (2016) reported that NRG-1 attenuated the evolution of diabetic kidney disease in a mouse model of type 1 diabetes with high risk for cardiovascular disease.23 They showed that NRG-1 averted renal hyperfiltration, and glomerular fibrosis.
Common carotid intima-media thickness (CIMT) is a precise marker of early arterial atherosclerotic changes and vascular remodeling which has a strong correlation with cardiovascular risk factors. It has been approved as a marker for atherosclerosis.24
In the present work, T2D patients were found to show significantly increased CIMT measurement compared to controls. Furthermore, a significant negative correlation was found between serum NRG-1 levels and the CIMT. This underscores a likely protective effect of serum NRG-1 against atherosclerosis in T2D.
Xu et al. (2009) showed that NRG-1 was found to reduce accumulation of cholesterol esters in monocyte-derived macrophages in vitro. They also reported that administration of recombinant NRG-1 in ApoE deficient mice produced significant shrinkage in the surface area of aortic atheromatous plaque.10 Clement et al. (2007) also reported that NRG-1 suppressed neointimal atherosclerotic plaque formation and vascular injury in the rat model.25
It was found that circulating levels of NRG-1 were lower in patients having ischemic heart disease, whether with acute coronary syndromes or with stable coronary artery disease compared to controls.26 Additionally, circulating NRG-1 levels were found to be inversely related to the severity of the coronary artery disease lesions.10
The proposed anti-atherosclerotic effect of NRG-1 could be partly explained by the positive effect found on the lipid profile observed in our results, besides a possible anti-inflammatory effect of NRG-1. In agreement, cell culture studies have shown that NRG-1 inhibited foam cell formation in human macrophages. Recombinant NRG-1 was also found to inhibit inflammatory responses by diminishing the expression of interleukin-1β, monocyte chemoattractant protein-1, intercellular adhesion molecule-1, matrix metalloproteinase-9, and cyclooxygenase-2 in monocytes.27
In the present study, serum NRG-1 was found to predict subclinical atherosclerosis in type 2 diabetes patients at a cut-off value<108.5pg/ml with 78% sensitivity and 80% specificity. This suggests that serum NRG-1 is a candidate to be a diagnostic biomarker for subclinical atherosclerosis in T2D.
NRG-1 was found to be released by the heart endothelium. It exerts cell-protective, regenerative, and antifibrotic effects of NRG-1 on the myocardium.7 Treatment of various animal models with rhNRG-1 was found to reverse aspects of heart failure.16
Diabetic cardiomyopathy is associated with impaired systolic and diastolic function. 2 D speckle-tracking Echocardiographic imaging has been proven to be a relevant diagnostic tool in assessment of cardiac function. Left ventricular global longitudinal strain (GLS) robustly reflects the LV myocardial systolic deformation over the longitudinal axis. This parameter was found to mirror left ventricular systolic dysfunction in T2D at its early stages, even when LVEF is still normal.28
In the present study, impairment of LV and RV GLS was observed in T2D patients compared to controls. This indicates subclinical LV and RV systolic function affection. Also serum NRG-1 was found to correlate positively with LV GLS.
The earliest manifestation of diabetic LV dysfunction is the increased LV diastolic stiffness. These related cellular and associated systemic derangements often lead to heart failure. NRG-1 was observed to affect the diastolic features of isolated cardiac muscle cells. It was reported to enhance the reuptake of calcium by the cytosol as well as by the sarcoplasmic reticulum. This eventually ameliorates and reverses stiffness of ventricular cardiomyocytes.29
Regarding echocardiographic assessment in the current study, statistically significant differences were observed between control group and T2D in MV A velocity by conventional echo Doppler, and average Ea and E/Ea ratio by tissue Doppler imaging. The study also showed that serum NRG-1 correlated negatively with E/Ea ratio which measures diastolic function.
Regarding internal ventricular dimensions, our study showed significantly higher IVSd and LVPWd indicating increased in LV wall thickness. This may be expected as about one third of our cases were hypertensive.
Li et al. (2011) found that the NRG-1 administration in rats was found to promote cardiac function and reverse the remodeling of the heart in rats having diabetic cardiomyopathy. NRG-1 was found to regulate cardiomyocytes apoptosis and cardiac fibrosis.30
Logistic regression analysis showed that predictors of serum NRG-1 level were found to be IR index, CIMT and ventricular diastolic dysfunction (indicated by E/Ea).
ConclusionsThe present study findings point to the presence of a robust relationship between serum NRG-1 level and insulin resistance, subclinical atherosclerosis, and cardiac dysfunction in patients with type 2 diabetes. Thus, NRG-1 may be a potential noninvasive biomarker for detection of cardiometabolic risk in T2D. Its positive effects on glucose metabolism and atherosclerosis render it a promising therapeutic target that merits further large-scale future studies.
Study strength and limitationsThe present study is one of the scarce studies that assessed serum NRG-1 in humans and the findings may encourage further research in this direction. The relatively small study population sample size represents a limitation of the present study. Moreover, the study constitution does not allow precise delineation of the causal nexus direction between serum NRG-1 levels and insulin resistance, ventricular function, and subclinical atherosclerosis in T2D. This necessitates consequent larger prospective studies to be able to generalize the study findings.
Authors’ contributionsConception and design: OF and OHA. Investigation: OF, EM and OHA. Methodology: AS, AEAE and EGK. Manuscript writing: AS and EGK. Review & editing: OF, EM, OHA and AEAE. All authors have read and approved the published version of the manuscript.
Ethics approval and consent to participateThe study was approved by the Research Ethics committee of Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt with approval number 202104/802 and was in line with the principles of the Declaration of Helsinki. Before enrollment, the research objectives and plan were clarified to all study participants and both verbal and written consents were submitted.
FundingThis work was totally a self-funded research by the authors, without financial support from any organization.
Conflict of interestsThe authors declared that they have no conflict of interest.
We are really thankful to the managerial and nursing staff of the departments of Endocrinology and Metabolism and Cardiology at Al-Zahraa University Hospital, Faculty of Medicine (Girls), Al-Azhar University, Cairo, who facilitated patient identification and sampling, and helped us to complete this work.