Infertility is a public health disorder affecting 10% of the population worldwide. Research on the impact of body mass index (BMI) on male fertility is very limited as compared to the multiple studies evaluating the impact of overweight in women's fertility. Although 25–30% of the cases of couples consulting for infertility are attributable to male factors, studies evaluating the association between semen parameters and BMI are controversial.
ObjectiveTo assess the impact of BMI on semen parameters in a selected group of men with unexplained infertility.
MethodA retrospective analysis of 168 patients during the 2008–2010 period. They all had at least one semen analysis and related studies to rule out known causes of infertility. Median age of patients was 35 years (22–55), and they were divided into three groups: normal weight (BMI: 20–24.9kg/m2), overweight (BMI 25–29.9kg/m2), and obese (BMI≥30kg/m2).
ResultsThere were no significant differences in semen parameters evaluated between the three groups, and no significant correlation was found between the same parameters and BMI.
ConclusionsThere was no significant association between BMI and conventional semen parameters, but we cannot exclude an impairment in other semen parameters that are not routinely assessed, which could result in a lower potential fertility in these individuals.
La infertilidad es un problema de salud que afecta aproximadamente al 10% de la población mundial. La investigación del impacto del índice de masa corporal (IMC) sobre la fertilidad masculina es limitada comparada con los estudios realizados en mujeres. A pesar de que en el 25-30% de los casos de infertilidad de pareja el factor es exclusivamente masculino, los estudios sobre parámetros seminales e IMC son controvertidos.
ObjetivoEvaluar el impacto del IMC sobre los parámetros seminales en una población seleccionada de hombres que consultaron por infertilidad sin causa aparente.
MétodoAnálisis retrospectivo sobre 168 pacientes durante el período 2008-2010. Todos contaban al menos con un espermiograma y estudios correspondientes para descartar causas conocidas de infertilidad. La mediana de edad de los pacientes fue de 35 años (22–55). Fueron divididos en 3 grupos: normopeso (IMC: 20-24.9kg/m2) sobrepeso (IMC25-29.9kg/m2) y obesos (IMC≥30kg/m2).
ResultadosNo se encontraron diferencias significativas de los diversos parámetros evaluados entre los distintos grupos de IMC. Tampoco se halló correlación significativa entre esos mismos parámetros y el IMC.
ConclusionesNo existió una asociación entre el IMC y los parámetros convencionales del semen, pero no podemos descartar que exista una afectación de otros parámetros seminales, no evaluados rutinariamente, que podrían provocar menor potencial de fertilidad en estos individuos.
Because of sedentary lifestyles and poor dietary habits, obesity is emerging as a rapidly progressing epidemic worldwide, causing significant adverse effects on human health, including infertility.1 In most developed countries, an increase has been reported in the proportion of men and women of reproductive age with overweight and obesity.2 In women, overweight has been shown to be associated with insulin resistance, increased frequency of polycystic ovary syndrome, menstrual cycle changes, miscarriage, and infertility.3–6 According to the World Health Organization (WHO), infertility is defined as the inability to achieve clinical pregnancy after 12 or more months of unprotected sexual relations. This affects approximately 10% of the worldwide population, and has become a true public health disorder.
While 25–30% of cases of infertility are due to male factors only,7 there is no clear evidence of the impact of overweight and obesity on male fertility, in contrast to studies conducted in women.
Male obesity is known to be potentially associated with the development of erectile sexual dysfunction, vascular disease, and all other components of metabolic syndrome. However, results from publications attempting to relate male obesity to semen quality changes have been both conflicting and controversial. In this regard, Kort et al.8 showed that subjects with a body mass index (BMI)>25kg/m2 have a significant decrease in total mobile-normal sperm count as compared to subjects with normal weight. By contrast, Qin et al.9 reported a reduced semen quality in subjects with a low BMI (<18.5kg/m2) as compared to those with a normal (18, 5–25kg/m2) or high BMI (25–29.9kg/m2), reflected in a reduction in sperm count and percentage of normal forms.
Finally, other reports10–12 did not show a correlation between the BMI and some semen quality parameters, such as motility percentages, normal forms, and sperm concentration. Because of the great disparity in the results found and patient selection criteria, the purpose of this study was to assess the impact of the BMI on some semen quality parameters in a selected population of men who consulted for infertility of no apparent cause.
Population and methodsThis retrospective study was based on data collection from 1654 clinical histories of patients who attended the andrology section of the endocrinology division of Hospital Durand during the 2008–2010 period for infertility. In order to disregard any other variable with a potential impact on semen parameters and to use body weight as the only factor that could be related to sperm morphology, motility, and count, patients with varicocele (clinical or subclinical) diagnosed by spermatic cord examination using Valsalva maneuver or bilateral funicular Doppler, as well as those with gynecomastia, leukocytospermia in semen examination and those reporting a history of conditions which could compromise testicular function such as trauma, postpubertal mumps orchitis, or maldescended testes were excluded from the study. Patients who showed hypogonadotropic or hypergonadotropic hypogonadism, thyroid changes, and hyperprolactinemia after the relevant hormone evaluation were also excluded. Those who had received medical treatment or taken drugs of abuse in the prior 90 days and those with a history of radiotherapy or chemotherapy due to oncological conditions were also excluded. Patients with azoospermia on seminal examination, regardless of etiology, as well as those with chromosomal abnormalities were also excluded.
A total of 168 patients, representing approximately 10% of the initial population and with a median age of 35 years (range, 22–55 years), were enrolled into the study. As this was a retrospective study, approval from the ethics committee and a signature of informed consent by the patients were not required. Taking as the patient enrollment model the study conducted by Kort et al.8, all subjects had at least one spermiogram and a routine immunological screening (Mar Test) according to 1999 WHO guidelines.13 Patients underwent a clinical examination which included weight and height to calculate their BMI (kg/height2), the degree of androgenization, the measurement of testicular volume using a Prader orchidometer, and a breast examination to rule out gynecomastia. Patients were divided into three groups based on BMI: patients with normal weight with BMI 20–24.9kg/m2 (n=34), overweight patients with BMI 25–29.9kg/m2 (n=100), and obese patients with BMI greater than 30kg/m2 (n=34). The group of patients with BMI<20kg/m2 was very small, and was therefore not included in this study.
Since semen variables showed a non-Gaussian distribution, semen parameter variables were normalized using logarithmic transformation. For statistical evaluation, a Kolmogorov–Smirnov, a Pearson's correlation analysis, and an ANOVA were used with a significance of p<0.05. Statistical software SPSS 7.5 was used.
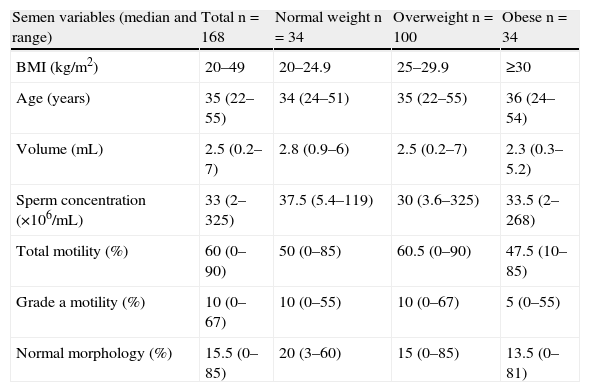
ResultsTable 1 shows the values of sperm concentration, percentages of sperm with total motility and grade a sperm motility (rapid), and the proportion of normal forms for the different groups assessed. Data are given as mean and standard deviation. In the overall patients, no correlation was found between the BMI and sperm concentration (r: −0.04; p=0.581), total motility (r: −0.06; p=0.474), grade a motility (r: −0.06; p=0.474), and normal morphology (r: −0.03; p=0.73).
Semen variables in the 168 patients, grouped by body mass index.
| Semen variables (median and range) | Total n=168 | Normal weight n=34 | Overweight n=100 | Obese n=34 |
| BMI (kg/m2) | 20–49 | 20–24.9 | 25–29.9 | ≥30 |
| Age (years) | 35 (22–55) | 34 (24–51) | 35 (22–55) | 36 (24–54) |
| Volume (mL) | 2.5 (0.2–7) | 2.8 (0.9–6) | 2.5 (0.2–7) | 2.3 (0.3–5.2) |
| Sperm concentration (×106/mL) | 33 (2–325) | 37.5 (5.4–119) | 30 (3.6–325) | 33.5 (2–268) |
| Total motility (%) | 60 (0–90) | 50 (0–85) | 60.5 (0–90) | 47.5 (10–85) |
| Grade a motility (%) | 10 (0–67) | 10 (0–55) | 10 (0–67) | 5 (0–55) |
| Normal morphology (%) | 15.5 (0–85) | 20 (3–60) | 15 (0–85) | 13.5 (0–81) |
BMI: body mass index.
Statistical analysis of all four semen parameters showed no significant differences between the means of the three patient groups assessed: sperm concentration (p=0.985), total motility (p=0.268), grade a motility (p=0.268), and normal forms (p=0.891).
DiscussionBecause of the high number of publications reporting conflicting results about the impact of the BMI and its relationship to semen quality, it was decided to assess the association of the most relevant parameters of the conventional spermiogram–count, motility, and morphology of male gametes–to the BMI in a population of patients who attended our center because they wanted to conceive.
Many studies have been reported showing a relationship between the BMI and semen parameters, mainly sperm concentration. Careful analysis of the study populations showed their heterogeneity as regards participant age. Another factor limiting the analysis of the data collected in other publications was the low number of patients with overweight included as compared to the control group used. In some of these reports, the patient selection criterion was not sufficiently strict, unlike in this study. For example, the Qin et al. study9 showed a lower semen quality in subjects with low weight as compared to those with normal weight or overweight. It should be noted that they included subjects from the general population, excluding those with some gonadal pathologies, but not those with varicocele, hydrocele, or abnormal testis location. It should be stressed that varicocele is one of the most common causes of semen impairment, with prevalence rates of 10% in the general population and approximately 40% in infertile subjects. Other studies, while demonstrating an inverse relationship between the BMI and semen variables, used non-conventional calculation parameters difficult to interpret such as normal-motile sperm count: volume×concentration×% motility×% normality).8 Therefore, differences between studies reported to date and our results may be attributed to the presence of current or preexistent conditions which were not duly excluded and could have been responsible for potential changes in the spermiogram.
Unlike the abovementioned reports, this study agrees with the MacDonald et al.14 meta-analysis of 31 studies conducted in different countries (the US, China, Denmark, Hungary, and Italy) in which no relationship was seen between the BMI and the assessed parameters. However, it should be noted that this study did not only include patients with infertility for no apparent cause, but a strict selection was made to rule out those with any demonstrable cause. This made it possible to exclude patients with different diseases, which could otherwise have biased the results. The results of a retrospective study recently published by Eskandar et al.10 agree with ours. Their study enrolled patients consulting for infertility but, in contrast to our patient selection, those with varicocele, chromosomal diseases, and maldescended testes were not excluded.
The lack of correlation found in our research may be due to the fact that, although multiple metabolic changes are recognized in obese subjects, these may not directly affect the standard semen variables. However, it cannot be ruled out that other sperm parameters not included in the routine basic assessment are affected by body weight increase.
A priori, from a strictly hormonal viewpoint one could imagine that lower serum testosterone levels would have a negative impact on the semen variables tested. However, this was not seen in our study results.
Many reports have related obesity to decreased plasma testosterone levels due to increased peripheral conversion in adipose tissue, with the resultant elevation of estradiol levels and a decreased hepatic synthesis of SHBG.15–18 In this regard, considering the direct action of testosterone on spermatogenesis, it is striking that semen quality parameters are not impaired in subjects with a high BMI. This discrepancy may be explained by the results reported by Strain et al., who found in obese patients decreased circulating levels of testosterone and its free fraction but no clinical signs of hypogonadism. It may therefore be inferred that decreased plasma testosterone levels are not associated with a significant drop in intratesticular testosterone concentration and would thus have a mild effect on spermatogenesis. This would explain the lack of impact on the semen parameters tested.19 The other hypothesis used to explain the lack of impact on semen in these subjects is based on the fact that although overweight and obesity induce an increase in scrotal temperature, this would not be able per se to impact on the tested variables, although it could impair sperm function, behavior that was not assessed in our study.20,21 Increased scrotal temperature could play a negative role in the control of the spermatogenic cycle, with an increase in reactive oxygen species being a potential cause of damage to spermatic DNA ranging from simple base oxidation to total fragmentation of the DNA molecule causing a severe impairment of the fertilizing capacity of sperm. This variable cannot be assessed in the routine spermiogram performed for evaluating male fertility. It is therefore important to note that only standard semen variables were considered in this evaluation and that, as regards the reproductive potential of subjects with overweight and obesity, supplemental functional aspects that may contribute to a more reliable assessment of the true fertilizing capacity of these subjects should be evaluated.
The results obtained in our evaluation of the impact of BMI on semen parameters clearly show that in overweight and obese patients, weight reduction has a greater impact on quality of life than on reproductive potential, at least as regards sperm count, motility, and morphology. Future research will undoubtedly contribute to clarifying this controversial subject. Controversy also exists regarding surgical procedures such as gastric bypass and its impact on spermatogenesis, in particular because no data are yet available on a large population of young men interested in their future fertility and with adequate time available for the relevant postoperative evaluation.
It should be noted that this study has some limitations due to protocol design, such as the performance of a single spermiogram and the lack of a control group of fertile men. It should therefore be interpreted with caution when assessing the potential impact of body weight on human semen quality and its impact on reproductive potential.
A return to normal weight is beneficial for these patients not only from the purely metabolic viewpoint, but also because it contributes to improving their sexual and possibly reproductive performance, with the resultant benefits for overall health.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Mormandi EA, et al. Aumento del peso corporal y calidad seminal: una asociación controvertida. Endocrinol Nutr. 2013;60:303–7.