Few effective therapeutic tools are currently available to fight the increasing prevalence of obesity and its associated comorbidities. Bariatric surgery is the only treatment with proven long-term effectiveness, but is associated to a high surgical risk and significant economic costs because of its technical complexity and the characteristics of patients. This is leading to development of new endoscopic procedures with less clinical risks and economic costs, while maintaining the benefits in terms of morbidity and mortality, which could even serve as a bridging element before surgery in cases where this is unavoidable, allowing for preoperative weight loss and control of comorbidities in order to improve anesthetic risks and possible complications. The purpose of this review was to analyze the most relevant and promising endoscopic techniques currently available.
En la actualidad contamos con escasas herramientas terapéuticas eficaces para combatir la creciente prevalencia de obesidad y comorbilidades asociadas. La cirugía bariátrica es el único tratamiento que ha demostrado su efectividad a largo plazo. Sin embargo, la alta complejidad técnica de la misma, junto a las características de los propios pacientes, implican un elevado riesgo quirúrgico e importante coste económico. Esto está llevando al desarrollo de nuevos procedimientos por vía endoscópica con un menor riesgo clínico y coste económico, manteniendo los beneficios en cuanto a morbimortalidad, incluso pudiendo servir de elemento «puente» previamente a la cirugía en los casos en que esta sea inevitable, pero permitiendo una pérdida de peso y control de comorbilidades que mejoren el riesgo prequirúrgico. El objeto de nuestra revisión es el análisis de las técnicas endoscópicas más relevantes en estos momentos.
Obesity is one of the greatest epidemics of our time. In Spain, 22.9% (24.4% of males, 21.4% of females) of the population has obesity, and its prevalence increases with age, as shown by the ENRICA study.1 In addition, the trends suggest a progressive, constant increase in incidence, which will result in a marked increase in morbidity and mortality in the long term (the Di@bet.es study2).
Obesity management is currently based on hygienic and dietary measures, and relatively few drug alternatives are available. Various compounds, such as fenfluramine and dexfenfluramine, sibutramine and rimonabant, have been withdrawn from the market in recent years, and although the Food and Drug Administration (FDA) recently approved the association of phentermine and topiramate (Qnexa®) and lorcaserin (Belviq®),3 the only pharmacological alternative indicated as an antiobesity drug available in Europe is orlistat.4,5
In this clinical context, surgery for obesity, usually reserved for severe cases such as patients with a body mass index (BMI) >40kg/m2 or >35kg/m2 and associated comorbidities, especially diabetes mellitus, is becoming increasingly important. It is not uncommon to find a high, sometimes an unacceptably high, surgical risk, in these patients, just because of these associated complications. In addition, the increased prevalence of grade II to IV obesity, and the limited resources available oblige us to look for new solutions. There are several alternatives to the traditional surgical methods, the so-called minimally invasive procedures, which involve less surgical risk, improve patient quality of life, and may even decrease the risks, so allowing over time for definitive invasive surgical procedures to be performed, if needed.
Endoscopic methods for the treatment of obesityObesity is one of the main preventable causes of death, and increases mortality by 79–270% in the population aged 20–49 years with BMI values higher than 30kg/m2.6 Obesity is an epidemic which, far from decreasing, appears to be increasing, like the complications and the morbidity and mortality associated with it.7 The current management of obesity is based on dietary and lifestyle changes and, in people with grade III or grade II obesity, on surgery, with a limited role for drugs. Recently, the so-called minimally invasive procedures8–14 have become an alternative to surgery for obesity. Both these procedures and bariatric surgery are based on the same concepts: the restriction of gastric volume to decrease the capacity of the stomach to store food, therefore inducing early satiety, partial nutrient malabsorption (altering the physiology of digestion and enzyme–substrate interaction and/or directly reducing the effective absorption surface), and mixed mechanisms. Endoscopic procedures for the treatment of obesity may be summarized as follows: intragastric balloons, gastric suture systems, endoscopic implants, and other devices, as shown in Table 1.
Endoscopic methods for the treatment of obesity.
| A. Balloons and stents |
| 1. BioEnterics® Gastric Balloon-Allergan. Spherical silicone balloon 600–800mL in volume, endoscopic placement |
| 2. Ullorex intragastric balloon®. Polyurethane balloon inside a capsule which is ingested by mouth without endoscopy. In contact with gastric acid, it releases CO2 and expands to a volume of 300mL. Remains 25–30 days in the stomach |
| 3. Spatz adjustable intragastric balloon. Silicone balloon mounted on an inflation catheter on one of its sides, which may be removed through the mouth and allows for volume adjustment based on patient symptoms while the balloon is in the stomach. It is usually filled with 500mL of saline |
| 4. Heliosphere Bag®. Air-filled balloon with a volume of 550mL and 10cm in diameter. Its low weight (<30g) limits its side effects |
| 5. Semi-stationary antral balloon. Pear-shaped device filled with 100–150mL of saline and with an antral anchor (with the cone-shaped pole oriented to the pylorus). It has a 30cm-long duodenal appendix with a metallic counterweight |
| 6. Silimed® gastric ballonon. Silicone-coated spherical balloon with a self-adhesive valve that is filled with 650mL of physiological saline. |
| 7. Endogast® (adjustable totally implantable intragastric prosthesis). Oval polyurethane prosthesis inflated with air (210–300mL) inserted similarly to percutaneous endoscopic gastrostomy; connected to a totally implantable subcutaneous system that allows for volume adjustment |
| B. Suture system |
| 1. Transoral gastroplasty |
| 2. Endoluminal vertical gastroplasty and variants |
| 3. Primary obesity surgery endoluminal (POSE system®) |
| 4. Other |
| C. Implants |
| 1. Transoral endoscopic restrictive implant |
| D. Malabsorptive procedures |
| 1. EndoBarrier® |
| 2. ValenTx® |
| E. Injection of substances |
| 1. Botulin toxin A |
| F. Other: electroneurostimulation, butterfly system |
Intragastric balloon (IGB) is a device intended to cause a sensation of satiety and to restrict food intake by occupying space. The IGB is left in place for 6–8 months and is intended to serve as a support for lifestyle and dietary behavior changes. The first description of an IGB dates back to 19829; early models were polyurethane cylinders with a valvular inflation mechanism with 220mL of air and with no endoscopic anchoring and removal system (Garren-Edwards® Gastric Bubble, approved by the FDA and marketed in the US in 1985).13,15,16 Its most common complications included bowel obstruction, balloon deflation, gastric erosion, and esophageal tears and lacerations on placement. These, combined with the poor results found in efficacy studies, attributable to patient selection bias and to a lower volume than initially expected (400mL), led to the withdrawal of the device.
It was only in 1987 that, after an meeting of experts held in Tarpon Springs (Florida), the basic requirements that an IGB should meet were established13,15–17 (Table 2).
Subsequently, new devices appeared, including those from Ballobes (Denmark), Mill-Rose (United Kingdom), Willmen (Germany), and Dow-Corning (Canada), with volumes ranging from 400 to 550mL and filled with air and fluid.16 The IGB most widely used today, with slight modifications, is the BioEnterics® model, introduced in 1991 (BioEnterics® Gastric Balloon, Inamed), but the Heliosphere® device (air-filled, bag-shaped) from the French company Helioscopie Medical Implants is also available. They are both approved for use in Europe, South America and Asia, including Japan, but not by the FDA.9,13 The Endogast® device (ATIIP, Districlass Medical, France), with mixed endoscopic and surgical insertion because of its inflation and deflation port attached to the subcutaneous implantation balloon, is also available.10,13,14,18
The current version of the BioEnterics® Gastric Balloon, available since 1999, consists of a balloon of clear silicone elastomer resistant to gastric acid. It has a radiopaque self-sealing valve system, and is filled with saline, with an adjustable volume of 400–800mL. It is placed under general anesthesia or deep sedation, and should be removed under general anesthesia. Some authors recommend otrotracheal intubation to protect the airway.9 Complications are similar to those of the early devices, but are less common15 (Table 3).
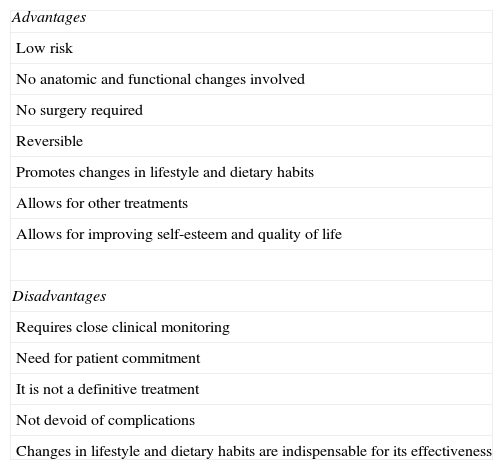
Advantages and disadvantages of the intragastric balloon.
| Advantages |
| Low risk |
| No anatomic and functional changes involved |
| No surgery required |
| Reversible |
| Promotes changes in lifestyle and dietary habits |
| Allows for other treatments |
| Allows for improving self-esteem and quality of life |
| Disadvantages |
| Requires close clinical monitoring |
| Need for patient commitment |
| It is not a definitive treatment |
| Not devoid of complications |
| Changes in lifestyle and dietary habits are indispensable for its effectiveness |
The mechanism of action is based on the inducing of a delay in gastric emptying by physical mechanisms, thus promoting a sensation of filling and early satiety mediated by the secretion of cholecystokinin,9 and so decreasing intake. It also appears to decrease leptin levels and to increase adiponectin levels.19 A potential role of ghrelin19 has been suggested based on the experimental finding of decreased ghrelin levels attributed to the mechanical distention of gastric fundus, although this has not been confirmed in subsequent studies.20 The IGB also allows for integral behavioral treatment, by helping to modify attitudes and promoting patient involvement. Ultimately, an effect similar to that of restrictive low-calorie diets is achieved, but with improved compliance.
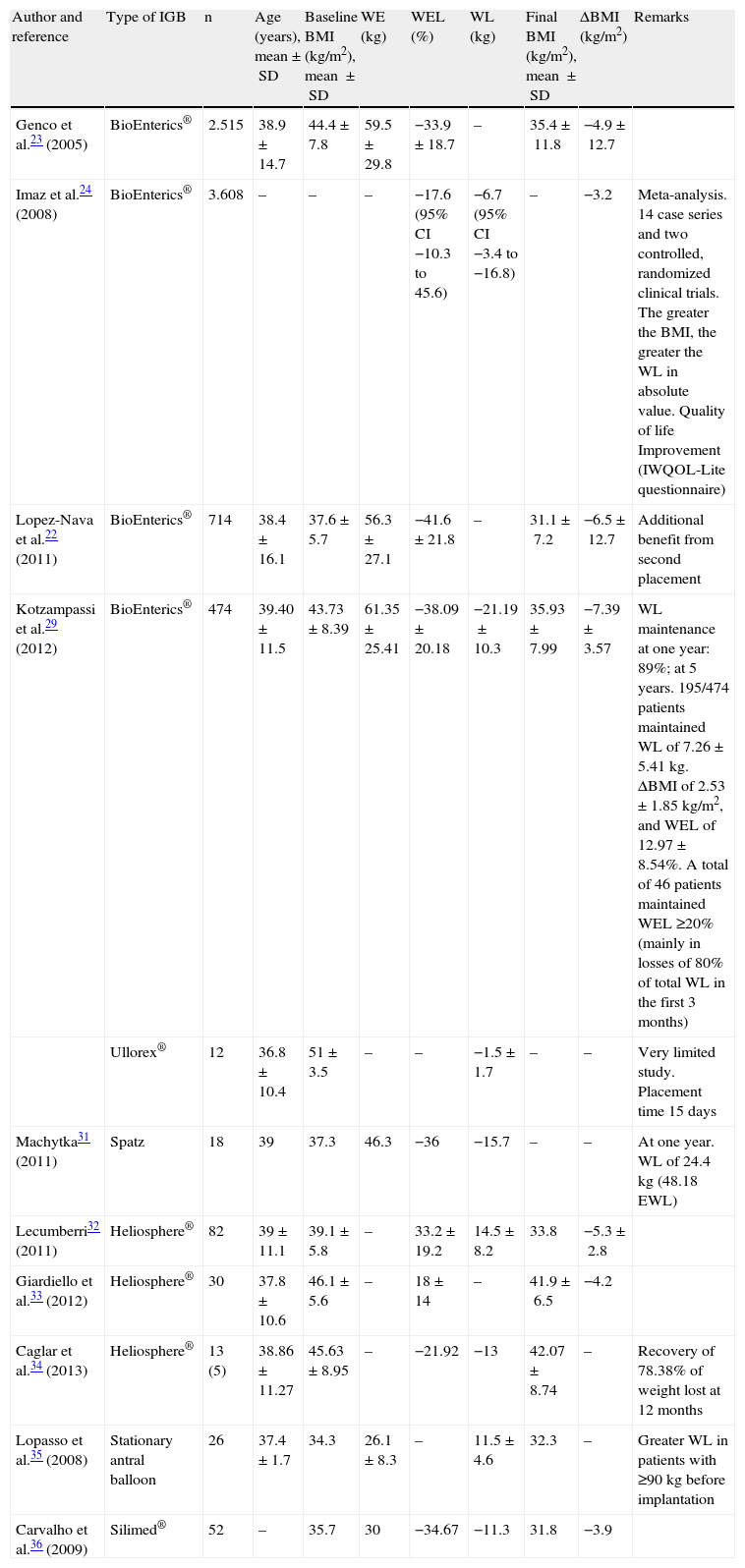
As regards the evidence of its effectiveness, the main studies have focused on the intragastric balloon BioEnterics® (BIB®)-Allergan, the most widely used. BIB® is effective in the short term in 66.6% of patients, with a mean weight loss (WL) of 17.8kg and a significant short-term improvement in comorbidities,21–24 achieving excellent results with modest WLs and improving quality of life (IWQOL-Lite questionnaire).25–28Table 4 shows the results of different studies with various balloons.22–36
Efficacy of intragastric balloon.
| Author and reference | Type of IGB | n | Age (years), mean±SD | Baseline BMI (kg/m2), mean ±SD | WE (kg) | WEL (%) | WL (kg) | Final BMI (kg/m2), mean ±SD | ΔBMI (kg/m2) | Remarks |
| Genco et al.23 (2005) | BioEnterics® | 2.515 | 38.9±14.7 | 44.4±7.8 | 59.5±29.8 | −33.9±18.7 | – | 35.4±11.8 | −4.9±12.7 | |
| Imaz et al.24 (2008) | BioEnterics® | 3.608 | – | – | – | −17.6 (95% CI −10.3 to 45.6) | −6.7 (95% CI −3.4 to −16.8) | – | −3.2 | Meta-analysis. 14 case series and two controlled, randomized clinical trials. The greater the BMI, the greater the WL in absolute value. Quality of life Improvement (IWQOL-Lite questionnaire) |
| Lopez-Nava et al.22 (2011) | BioEnterics® | 714 | 38.4±16.1 | 37.6±5.7 | 56.3±27.1 | −41.6±21.8 | – | 31.1±7.2 | −6.5±12.7 | Additional benefit from second placement |
| Kotzampassi et al.29 (2012) | BioEnterics® | 474 | 39.40±11.5 | 43.73±8.39 | 61.35±25.41 | −38.09±20.18 | −21.19±10.3 | 35.93±7.99 | −7.39±3.57 | WL maintenance at one year: 89%; at 5 years. 195/474 patients maintained WL of 7.26±5.41kg. ΔBMI of 2.53±1.85kg/m2, and WEL of 12.97±8.54%. A total of 46 patients maintained WEL ≥20% (mainly in losses of 80% of total WL in the first 3 months) |
| Ullorex® | 12 | 36.8±10.4 | 51±3.5 | – | – | −1.5±1.7 | – | – | Very limited study. Placement time 15 days | |
| Machytka31 (2011) | Spatz | 18 | 39 | 37.3 | 46.3 | −36 | −15.7 | – | – | At one year. WL of 24.4kg (48.18 EWL) |
| Lecumberri32 (2011) | Heliosphere® | 82 | 39±11.1 | 39.1±5.8 | – | 33.2±19.2 | 14.5±8.2 | 33.8 | −5.3±2.8 | |
| Giardiello et al.33 (2012) | Heliosphere® | 30 | 37.8±10.6 | 46.1±5.6 | – | 18±14 | – | 41.9±6.5 | −4.2 | |
| Caglar et al.34 (2013) | Heliosphere® | 13 (5) | 38.86±11.27 | 45.63±8.95 | – | −21.92 | −13 | 42.07±8.74 | – | Recovery of 78.38% of weight lost at 12 months |
| Lopasso et al.35 (2008) | Stationary antral balloon | 26 | 37.4±1.7 | 34.3 | 26.1±8.3 | – | 11.5±4.6 | 32.3 | – | Greater WL in patients with ≥90kg before implantation |
| Carvalho et al.36 (2009) | Silimed® | 52 | – | 35.7 | 30 | −34.67 | −11.3 | 31.8 | −3.9 |
ΔBMI: change in body mass index. WEL: weight excess loss.
In diabetes mellitus, Genco et al. found with BIB® remission (32.8%) or improvement (54.4%) at explantation,23 with significant decreases in glycosylated hemoglobin (HbA1c). In another study, Crea et al. found an HbA1c decrease from 7.5±2.1% at baseline to 5.7±1.9% at explantation. The positive effect on blood glucose control remained at 6 (5.6±0.7%) and 12 months (5.5±0.9%).37 However, no data are available for periods longer than one year.
Candidates for IGB placement should be motivated adults, ideally with moderate overweight, who are predisposed to change their lifestyle and to cooperate with the medical team. Indications for IGB9,13,16,38 include:
- 1.
Adjuvant treatment of obesity in cases refractory to conservative measures:
- -
BMI 30–34.99kg/m2 with moderate comorbidities.
- -
BMI 35–39.99kg/m2.
- -
BMI 40–49.99kg/m2 in patients not eligible for bariatric surgery (high surgical risk, comorbidities or refusal of surgery).
- -
- 2.
In patients with BMI >50kg/m2 as an adjuvant to weight loss before bariatric surgery.
- 3.
Exceptionally, in patients with BMI ranging from 27 to 29.9kg/m2 with very severe comorbidities which may improve with weight loss.
IGB contraindications include9,13,16,38:
- 1.
Giant hiatal hernia (>3–5cm).
- 2.
Morphofunctional pharyngoesophageal changes (relative).
- 3.
Severe grade C-D esophagitis (Los Angeles scale, Lundell, 1999) (relative).
- 4.
Esophageal varices/severe liver disease. Lesions amenable to bleeding.
- 5.
Active peptic ulcer.
- 6.
Prior gastric or abdominal surgery (relative)/gastroparesis.
- 7.
Pregnancy or wish to become pregnant.
- 8.
Active or remitting tumors <5 years.
- 9.
Coagulopathy or use of NSAIDs (relative)/coumarins/corticosteroids.
- 10.
Crohn's disease (relative).
- 11.
Severe psychiatric disorders.
- 12.
Active alcoholism. Drug addictions.
- 13.
Diseases where decompensation secondary to vomiting and/or dehydration may occur.
- 14.
Age >65 years (relative) and <18 years.
- 15.
Non-cooperative patient.
- 16.
Technical impossibility of removal in <48h in the event of emptying (relative).
- 17.
Secondary obesity.
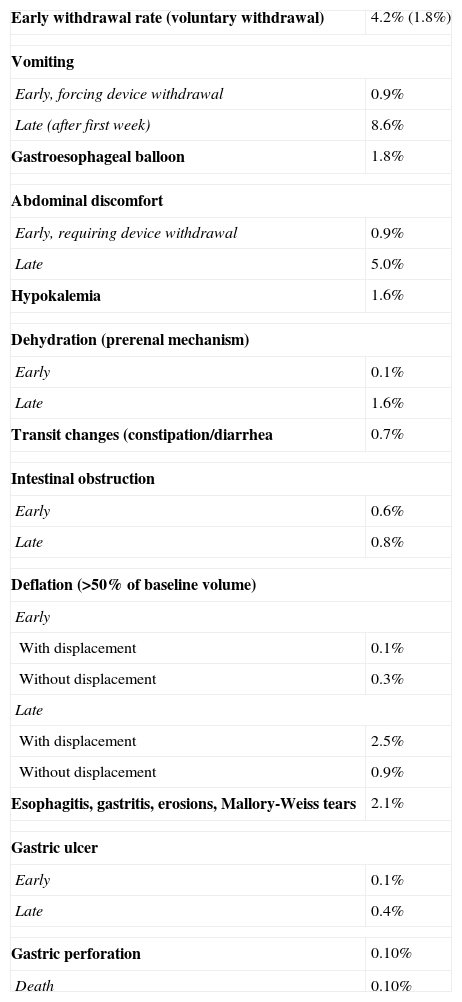
The most common side effects are nausea and vomiting (99%), which start 4–5h after placement and may last for 4–5 days with a poor response to antiemetics. These are associated with epigastric pain in 30% of cases. It is therefore advisable to treat with proton pump inhibitors (PPIs) all patients with an implanted IGB. Thiamine deficiency may also occure13,16,24,39 (Table 5).
Complications of intragastric balloon.
| Early withdrawal rate (voluntary withdrawal) | 4.2% (1.8%) |
| Vomiting | |
| Early, forcing device withdrawal | 0.9% |
| Late (after first week) | 8.6% |
| Gastroesophageal balloon | 1.8% |
| Abdominal discomfort | |
| Early, requiring device withdrawal | 0.9% |
| Late | 5.0% |
| Hypokalemia | 1.6% |
| Dehydration (prerenal mechanism) | |
| Early | 0.1% |
| Late | 1.6% |
| Transit changes (constipation/diarrhea | 0.7% |
| Intestinal obstruction | |
| Early | 0.6% |
| Late | 0.8% |
| Deflation (>50% of baseline volume) | |
| Early | |
| With displacement | 0.1% |
| Without displacement | 0.3% |
| Late | |
| With displacement | 2.5% |
| Without displacement | 0.9% |
| Esophagitis, gastritis, erosions, Mallory-Weiss tears | 2.1% |
| Gastric ulcer | |
| Early | 0.1% |
| Late | 0.4% |
| Gastric perforation | 0.10% |
| Death | 0.10% |
Saline-filled devices usually have 10–20mL of methylene blue added to them in order to early detect deflation because it stains urine a greenish color when it enters the bloodstream.9,13,15
A minimum daily intake of 1.5L should be ensured. Once the device is placed, a qualitative low-calorie, low-fat diet fractionated in five meals and introduced gradually is recommended.20 Control visits should be made at least once every 15–30 days for six months after implantation to check weight and general status, and once monthly after explantation.
Suture methodsTransoral gastroplastyThe TOGA® system (Satiety Inc., Palo Alto, CA, USA) is an endoscopic procedure similar to restrictive surgery but less invasive consisting of a pair of disposable staplers. The sleeve stapler creates a stapling encompassing the whole thickness of the proximal stomach wall and performs a plication, mimicking a vertical sleeve parallel to the lesser curvature. The restriction stapler is then used to reduce the outlet by creating the restriction “pouch” of vertical gastroplasty.9–11,13,14,18,39,40 Patients recover in 24h. Control X-rays and an upper gastrointestinal series are required before discharge. Intake is limited for the first two weeks to thin fluids and protein supplements, and is gradually increased in consistency over the following two weeks. Solids are started after the fourth week. PPIs should be taken at least from 2 to 3 weeks before to 4 weeks after the procedure.40 This device allows for shorter hospital stays as compared to surgery, as it is performed in 30–120min and promotes early oral tolerance. The procedure can, therefore, be performed in an outpatient setting without greater complications than with other procedures.11,40 Although this is an irreversible procedure, therapeutic failure does not increase the difficulty or risk of conversion to a laparoscopic gastric bypass, and could therefore be considered in a two-stage approach as a prior step to surgery.11 Its main disadvantage lies in that it is a novel procedure for which evidence is scarce.
The first series of patients in whom TOGA® was used included 21 patients with grade III or grade II obesity with comorbidities and a mean BMI of 43.3kg/m2. Mean weight excess loss (WEL) was 16%, 23%, and 25% one, three, and six months after treatment, with an overall mean of 24.4% and a mean BMI of 37.8kg/m2, with no severe complications and a significant improvement in quality of life and, in some cases, also in comorbidities (hypertension and diabetes).41 However, a control endoscopy performed at six months showed multiple dehiscences between the staples and gaps in 13 patients, which required modifications.41,42 After this first evaluation, once the failures of the first examination were resolved, a second pilot study was conducted on 11 patients, who were administered NSAIDs before surgery to minimize the ischemic process secondary to stapling and to try and decrease dehiscence. In this study, mean WELs of 19.2%, 33.7%, and 46% were achieved at one, three, and six months respectively.42 Familiari et al. reported a multicenter (Italy–Belgium), prospective, controlled, one-arm clinical trial with TOGA® in which 67 patients (47 female) aged 23–59 years (mean, 41 years) who met bariatric surgery and associated comorbidity criteria were enrolled. Mean baseline BMI was 41.5±3.6kg/m2 (n=27 with BMI <40), excluding patients with BMI >55 Fifty-three patients completed the protocol, with BMI excess losses of 33.9%, 42.6%, and 44.8% at three, six, and 12 months, respectively. WL was greater in the subgroup with baseline BMI >40kg/m2 as compared to the subgroup with BMI <40kg/m2 at 12 months (52.2% versus 41.3%, p<0.05). Quality of life significantly increased at six and 12 months (SF-36 and IWQOL-Lite questionnaires, p<0.001 for all items).40 In diabetic patients, HbA1c decreased from 7% to 5.7% (p<0.01). In non-diabetic patients with a mean baseline HbA1c of 5.9%, suggesting prediabetes, a significant improvement occurred at 12 months (HbA1c of 5.4%, p<0.0005), in agreement with the decreased insulin secretion after intervention reported by Chiellini et al.40,43 No significant differences were found at 12 months in total cholesterol, but differences were seen in HDL (+10.5mg/dL, from 47.0 to 57.5mg/dL, p<0.0001) and triglycerides (−449mg/dL, from 142.9 to 98mg/dL, p<0.0001). LDL cholesterol slightly increased (113.7–120mg/dL, p=0.081),32 and a mild, non-significant decrease was seen in both systolic and diastolic BP at 12 months, although the latter showed a trend to statistical significance (p=0.07). As regards dehiscence and the appearance of gaps between sutures, with the new device they only were severe enough to affect effectiveness in 11 patients (Table 6).40
Indications and contraindications of the transoral gastroplasty system.
| Indications |
| Bariatric surgery criteria (BMI >40 or >35kg/m2 plus comorbidities) |
| Obesity lasting >2.5 years |
| Failure of hygienic-dietary measures and/or drugs |
| Age ranging from 18 to 60 years |
| Patient able to understand the procedure and its potential risks and benefits, and willing to follow the instructions of the multidisciplinary team |
| Contraindications |
| BMI >55kg/m2 |
| Active alcoholism/drug addiction |
| Severe mental disease |
| Inflammatory bowel disease |
| Structural/functional/esophageal changes (grade C-D esophagitis, Barrett's esophagus, achalasia, esophageal diverticulum, “nutcracker” esophagus, etc.) |
| Hiatal hernia ≥2cm |
| Active Helicobacter pylori infection |
| Congenital or acquired gastrointestinal tract abnormalities |
| Severe cardiopulmonary or systemic diseases, including HIV or neoplasms |
| Concomitant infectious process at the time of the procedure |
| Personal history of scleroderma |
| Severe coagulopathy, use of oral anticoagulants, or presence of lesions amenable to bleeding |
| Prior abdominal surgery |
| Inadequately controlled thyroid disease |
| Current or desired pregnancy and postpartum |
Common complications13,40–42 include abdominal pain, nausea, vomiting, dysphagia, pharyngitis, temporomandibular dysfunction, and lumbar and neck pain. Respiratory insufficiency was reported in one patient, and another patient experienced asymptomatic pneumoperitoneum which resolved with conservative measures. Monitoring requires a multidisciplinary team and includes visits at one, three, six, and 12 months, and is based on medical judgment thereafter. Frequent control endoscopy and upper GI series with contrast are recommended.42
Transoral endoscopic vertical gastroplasty: EndoCinch™ and RESTORe systems™The EndoCinch™ device (C.R. Bard Inc., NJ, USA), initially approved by the FDA in 2000 for intraluminal treatment of reflux disease, has gained relevance again among restrictive intraluminal bariatric procedures.8,9,11,13,18,39 EndoCinch is a sewing system contained in a capsule attached to the end of a flexible endoscope, prefilled with a needle with polypropylene thread and a vacuum system. Negative pressure is applied in the vacuum system in the target area, by introducing gastric wall mucosa and submucosa inside the sewing system, which allows for several types of suture to be performed. The RESTORe™ system (Bard-Davol, Warwick, RI, USA) is a modification of the previous system which allows for deeper plications encompassing the whole wall thickness without it being necessary to remove the endoscope to reload the suture kit8,13,45 Running suture is performed in 5–7 interwoven stitches from the gastric fundus to the most distal portion of the gastric body. When tightened, the suture acts as a girth, approximating the anterior and posterior gastric aspects to form a gastric sleeve that significantly restricts distention and intake. The procedure takes 45min. Screening for Helicobacter pylori is required in all patients, and those with a positive result are given eradication treatment before the procedure. Patients with symptoms of gastroesophageal reflux should receive treatment with PPIs for at least 14 days before the procedure.44 After the procedure, patients are monitored for at least 1h, and if no contraindications exist, may be discharged on a liquid diet for three days, after which they should return to the office for review. If there are no incidents, a soft solid diet is gradually introduced over one week, after which a healthy, balanced and unrestricted diet is permitted.44
Fogel et al.44 performed the EndoCinch™ procedure on 64 patients aged 16–62 years (mean, 31.5±10.1 years), of whom 59 (94.1%) completed 12 months of follow-up. Baseline BMI was 39.9±5.1 (28.0–60.2kg/m2), and mean presurgical weight was 104.8±18.5kg (74–178kg). The authors reported WELs of 21.1%±6.2%, 39.6%±11.3%, and 58.1±19.9% at one, three, and 12 months of follow-up respectively, with BMI values at one, three, and 12 months of 36.5±4.8, 33.5±4.5, and 30.6±4.7kg/m2 respectively. The authors also performed an analysis with stratification by BMI groups: group I, with BMI >40kg/m2 (n=33, mean presurgical weight 113.3±20.8kg, mean presurgical BMI 43.4±3.8kg/m2); group II, with BMI ranging from 35 to 40kg/m2 (n=19, mean presurgical weight 101.3±64kg, mean presurgical BMI 38.5±1.2kg/m2); and group III, with BMI <35kg/m2 (n=12, mean presurgical weight 86.9±7kg, mean presurgical BMI 32.4±2.4kg/m2). The greatest WELs were recorded in group III (29.5±6.7, 54.0±13.5, and 85.1.±24.0%, BMI 29.5±2.2, 27.3±2.2, and 24.4±2.4kg/m2) at one, three, and 12 months, followed by group II (20.6±4.3, 39.4±7.1, and 56.5±13.9%, BMI 35.3±1.2, 32.4±1.4, and 29.8±2.3kg/m2), and group I (18.6±4.5, 34.6±8.0, and 48.9±10.7%, BMI 39.7±3.8, 36.4±3.7, and 33.5±4.0kg/m2) at the same times. No severe complications were reported, but suture disruption was found in three patients, repeat endoscopy being required in two of them.44 We have found no data on the effect on HbA1c or blood glucose.
As regards RESTORe™, it is relatively safe according to Brethauer et al.45 and achieved gastric volume reduction in all patients in their study. After one year,46 of the 18 patients initially treated, with a mean baseline weight of 105.6kg and a mean BMI of 38.6kg/m2, 14 completed the study (three consent withdrawals and one loss to follow-up), with a WL of −11.0±10.0kg (range −4.2kg to −33.3kg), a mean BMI decrease of −4.0±3.5kg/m2 (range −1.4 to −11.8kg/m2), and a mean decrease in abdominal circumference of −12.6±9.5cm (range −27.0 to 4.5cm). Mean WEL at 12 months was −27.7±21.9% (range −9.4% to −73.6%), with 50% of patients showing WELs ≥30%. The greatest differences were seen three months after the procedure, with no changes at six and 12 months. This could be explained by the results seen in the endoscopy performed one month after the procedure, in which plications remained unchanged in only two patients, while partial dehiscence was seen in 15 patients. At one year, sutures had partially and completely released spontaneously in eight and five patients respectively.46 We have found no evidence on the effect of the device in diabetes.
The indications of the EndoCinch® system include: age ranging from 16 to 62 years, a BMI >28kg/m2, and an express commitment to comply with the follow-up. It is contraindicated in patients with prior vascular events, poorly controlled diabetes mellitus, hiatal hernia >3cm or prior gastric surgery.44 Pharyngeal discomfort is the most common complication reported.44
The indications of the RESTORe® system45 include: age ranging from 18 to 60 years, a BMI ranging from 40 to 45 or 35–40kg/m2 with associated comorbidities, obesity starting ≥5 years before and refractory to conservative measures, and stable weight in the previous two months (defined as weight changes ≤5% in that period). The contraindications include: poorly controlled endocrine disorders, prior gastric or obesity surgery, diabetic gastroparesis or diabetes starting >10 years before, hiatal hernia >2cm, active Helicobacter pylori infection, eating behavior disorder and, overall, any medical or psychological condition that would limit compliance with the follow-up protocol and/or represent an unacceptable risk.45 It appears to have a good safety profile in the short45 and long term,46 with minor complications (abdominal pain, distention, diarrhea, and nausea) after the procedure.
Primary obesity surgery, endoluminalThe so-called POSE procedure (primary obesity surgery, endoluminal), in which plications are performed with an endoscopic system at gastric antrum and fundus level in order to restrict gastric surface and volume, thus inducing early satiety, is becoming increasingly popular. This appears to be a simple procedure, which may be performed in an outpatient setting and has no severe complications, with WELs up to 45–49.4% and BMI decreases of −5.8kg/m2. However, its actual potential is unknown because of the novelty of the procedure and the scarce efficacy and safety data currently available. No data are also available about its effect on blood glucose and patients with diabetes.13,47
ImplantsTransoral endoscopic restrictive implantThe transoral endoscopic restrictive implant system8,9,11,14,18,39,48,49 is a complex, difficult to insert device. Using a stapler, five plications are made encompassing the whole wall thickness approximately 3cm distal to the cardia. Plication results in two concentric rings of staples reinforced by a plastic ring. This technique involves the stapling of plications, and silicone anchor placement until five plications and their respective anchors have been placed. These act as the basis for the restrictive implant. Antibiotic prophylaxis is required for 6h after the procedure with amoxicillin–clavulanate IV.48,49 Before discharge, an upper gastrointestinal series is performed to check for leaks. A liquid diet is started for two weeks, followed by a mashed diet for an additional 2–4 weeks, after which solid food is reintroduced.48
De Jong et al.,48 in a study on 13 patients with a mean baseline BMI of 42.1kg/m2, reported BMI decreases by −0.9 and −4.2kg/m2 at one and three months respectively, and WELs of 12.3% and 22.2% at the same times. They also found an improved quality of life at three months (SF-36 questionnaire). The side effects were mild, and mainly consisted of odynophagia, nausea, vomiting, fever, epigastric pain, back pain, and reflux. There have been two reports of pneumoperitoneum treated conservatively, and one patient experienced perforation requiring surgery.48
Malabsorptive proceduresEndoBarrier® and ValenTx systems®Duodenal-jejunal bypass sleeve (EndoBarrier® system, GI Dynamics Inc., Watertown, MA, USA) is a reversible malabsorptive method consisting of a nitinol anchor in its proximal end, fixed on the duodenal bulb with an anti-migration system, and a suture for endoscopic withdrawal. From this, a self-expandable sleeve of waterproof fluoropolymer, approximately 60cm in size, extends to prevent chyme from blending with biliopancreatic secretion and, thus, its digestion and absorption.8–11,13,14,18 The system may be implanted in 30min9,11,18,50 and, if plain X-rays show adequate placement and the patient tolerates a liquid diet, he/she may be discharged in 24h on a PPI and multivitamin supplements.50 During the first two weeks, patients receive a liquid diet of 600kcal and, subsequently, a normal diet of 1200kcal in females and 1500kcal in males, plus 1500mL of calorie-free/low-calorie fluids in all phases.50 EndoBarrier® is removed endoscopically at 12–24 weeks under general anesthesia.9,50 Because of its characteristics, this device may serve as a “bridge” to final surgery, allowing for a reduction in presurgical risks.50 A variant of EndoBarrier®, the 120cm ValenTx® system, which includes a restrictive component implanted in the cardia by an endoscopic–laparoscopic technique and endoscopic extraction, mimicking the Roux-en-Y gastric bypass, is currently being tested.13,18
When first used in humans, EndoBarrier® achieved a WEL of 23.6%,9 with a significant decrease in HbA1c.11 In their trial, conducted on 25 patients followed up for 12 weeks, Tarnoff et al. reported a mean WEL of 22.1%.51 Schouten et al.50 found in 26 patients a WEL of 19±10.9% at 12 weeks and 24.3±5.8% at 24 weeks (−5.5 points from baseline BMI), a 1.1% decrease in HbA1c, and decreased requirements of hypoglycemic medication from the first week, which allowed for the withdrawal of the device. Improved control persisted after the end of intervention.13,50 The ValenTx® system has also shown good initial results, with WELs up to 40% at 12 weeks and no severe adverse effects.13,18
EndoBarrier® is indicated for patients aged 18–55 years with BMI ranging from 40 to 60 or >35kg/m2 with associated comorbidities.13,50 Its contraindications include the use of anticoagulants/severe coagulopathy, inflammatory bowel disease, active Helicobacter pylori infection, severe reflux disease, prior abdominal surgery, and eating behavior disorders or major psychiatric disorders.13,50 Potential complications include nausea, vomiting, and abdominal pain, particularly in the first week.13,50 Local inflammatory events with pseudopoly formation may occur at explantation.13,50,51 The most severe side effects reported include upper gastrointestinal bleeding and device obstruction and migration.9,10,13,51
ConclusionsObesity is the epidemic of our time, associated with high morbidity and mortality, but very few therapeutic resources are available to fight it. Surgery for obesity is currently the only definitive treatment able to provide significant weight loss, and even induce the remission of diabetes and other comorbid conditions, but it is invasive and often has a high technical complexity, involving a high risk, plus high costs in material and facilities. Endoscopic procedures may represent a low-risk, low-cost treatment option as compared to surgery while maintaining its benefits in terms of morbidity and mortality. In cases where surgery is inevitable, such procedures may serve as a bridging element which allows the pre-surgical risk to be decreased through WL and improvements in blood glucose control and other factors. However, as these are new methods tested in relatively few studies, the evidence regarding their long-term safety and efficacy is still very limited.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Martínez-Ortega AJ, Aliaga-Verdugo A, Pereira-Cunill JL, Jiménez-Varo I, Romero-Lluch AR, Sobrino-Rodríguez S, et al. Procedimientos endoluminales/endoscópicos en el tratamiento de la obesidad. Endocrinol Nutr. 2014;61:264–273.