Aspartame is a non-nutritive sweetener particularly used in ‘diet’ and ‘low calorie’ products and also in a variety of foods, drugs and hygiene products. Aspartame is metabolized by gut esterases and peptidases to three common chemicals: the amino acids, aspartic acid and phenylalanine, and small amounts of methanol. The aim of the present study was to assess potential changes in molecular mediators of aspartame as a chemical stressor in rats.
Materials and methodsThe effects of long-term administration of aspartame (40mg/kg body weight/day) were tested in Wistar Albino rats. The treatment effects were assessed in different conditions, including control groups. After 90 days of treatment, circulating concentrations of different parameters were assessed: corticosterone, lipid peroxidation, antioxidant activity, nitric oxide, reduced glutathione and cytokines (interleukin 2, interleukin 4, tumor necrosis factor-α and interferon-γ).
ResultsThe results show that there was a significant increase in plasma corticosterone, serum lipid peroxidation and nitric oxide level along with a decrease in enzymatic and non-enzymatic antioxidant as well as significant decrease in interleukin 2, tumor necrosis factor-α and interferon-γ. There was also a significant increase in interleukin 4 irrespective of whether the animals were immunized or not.
ConclusionThe findings clearly point out that aspartame acts as a chemical stressor because of increased corticosterone level and increased lipid peroxidation and nitric oxide level induce generation of free radicals in serum which may be the reason for variation of cytokine level and finally results in alteration of immune function. Aspartame metabolite methanol or formaldehyde may be the causative factors behind the changes observed.
El aspartamo es un edulcorante no nutritivo que se utiliza especialmente en productos «dietéticos» y «bajos en calorías», así como en gran diversidad de alimentos, fármacos y productos de higiene. Las esterasas y peptidadas intestinales metabolizan el aspartamo a 3 compuestos químicos comunes: los aminoácidos, el ácido aspártico y la fenilalanina, y cantidades pequeñas de metanol. El objetivo de este estudio fue valorar los posibles cambios de los mediadores moleculares del aspartamo como estresante químico en ratas.
Material y métodosSe estudiaron los efectos de la administración crónica de aspartamo (40mg/kg de peso/día) en ratas albinas Wistar. Los efectos del tratamiento se valoraron en distintas condiciones, incluyendo grupos de control. Tras 90 días de tratamiento se determinaron las concentraciones circulantes de distintos parámetros: corticosterona, peroxidación lipídica, actividad antioxidante, óxido nítrico, glutatión reducido y citocinas (interleucina 2, interleucina 4, factor de necrosis tumoral α e interferón γ).
ResultadosLos resultados mostraron aumentos importantes de la corticosterona plasmática, la peroxidación lipídica y el nivel de óxido nítrico, junto con un descenso de antioxidante enzimático y no enzimático y una reducción importante de la interleucina 2, el factor de necrosis tumoral α y el interferón γ. Había también un aumento importante de la interleucina 4 con independencia de si los animales estaban o no inmunizados.
ConclusiónLos hallazgos indican claramente que el aspartamo actúa como un estresante químico debido al aumento del nivel de corticosterona, y el aumento de los niveles de peroxidación lípidica y óxido nítrico induce la generación de radicales libres en el suero, lo que puede ser la causa de la variación del nivel de citocinas y origina finalmente la alteración de la función inmunológica. Los metabolitos del aspartamo metanol o formaldehído pueden ser los factores causales de los cambios observados.
Nowadays consumers are increasingly concerned about the quality and safety of many products of industrialized countries, in particular the use of artificial sweeteners, flavorings, colorings, preservatives and dietary supplements. General apprehension also exists regarding the possible long-term health effects of the raw materials and technologies used for the packaging, sterilization and distribution of foods. Many non-nutritive sweeteners have been used in foods and beverages to allow people to enjoy the sweet taste without consuming sugar-associated calories. One of these sweeteners is aspartame. This sweetener is incorporated into a number of foodstuffs (drinks, desserts, sweets, etc.) and in table sweeteners, under different brand names and into some 600 medicines.1 Its sweetening power is 180–200 times greater than that of sucrose.2 Because it contains no calories, aspartame is considered a boon to health-conscious individuals everywhere; a recent observation indicated that aspartame is slowly making its way into ordinary products used every day, which do not carry any indication as being for people on diets or diabetic patients. Aspartame in dry products is fairly stable even at high temperatures. However, in solution, its stability is a function of time, temperature, pH and available moisture. Aspartame is most stable between pH values of 3 and 5, even with increasing temperature.3 However, it breaks down and loses its sweetness in normal cooking or baking. Thus its use is limited to a table-top sweetener (Equal®TM – The NutraSweet Co., Deerfield, IL) and as NutraSweet in dry foods, soft drinks, and frozen foods like ice cream. It is slightly soluble in water (about 1.0% at 25°C), sparingly soluble in alcohol and insoluble in fats and oils. Being a peptide, it is amphoteric and is metabolized extensively to release its constituent amino acids and methanol.3 Upon ingestion, this artificial sweetener is immediately absorbed from the lumen and metabolized by gut esterases and peptidases to phenylalanine, aspartic acid and methanol. Orally ingested aspartame components are immediately absorbed from the lumen and reach the portal blood in a manner similar to that of amino acids arising from dietary protein or polysaccharides.4,5 Their concentrations are found increased in the blood stream.6 This sweetener and its metabolic breakdown products (phenylalanine, aspartic acid and methanol) have been a matter of extensive investigation for more than 20 years, including experimental animal studies. Ten per cent of aspartame consists of methanol. Methanol is a toxicant that causes systemic toxicity.7 The primary metabolic fate of methanol is the direct oxidation to formaldehyde and then into formate. The severity of clinical findings in methanol intoxication correlated better with formate levels.8 Methanol is gradually released in the small intestine when the methyl group of aspartame encounters the enzyme chymotrypsin,4 but methanol is more readily generated by the body (thus becoming even more dangerous) when it is heated above 30°C before being ingested. This occurs when soft drinks are left out in the sun or foods containing aspartame are heated. Methanol breaks down into formaldehyde and formic acid in the body. Formaldehyde is an embalming fluid, as a preservative in vaccines and a deadly neurotoxin. Formic acid causes cells to become too acidic, thereby producing metabolic acidosis. Acidosis damages cellular health by causing enzymes to stop functioning. Oxidative stress arises from the imbalance between pro-oxidants and antioxidants in favor of the former, leading to the generation of oxidative damage.9 Generation of free radicals is an integral feature of normal cellular functions, in contrast, excessive generation and/or inadequate removal of free radical results in destructive and irreversible damage to the cell.10 A stressor is a stimulus that is either internal or external, which activates the hypothalamic pituitary adrenal axis and the sympathetic nervous system resulting in a physiological change11 Corticotropin-releasing hormone is released during stress and stimulates the release of adrenocorticotropic hormone,12 which in turn releases corticosterone from the adrenal cortex. Elevation in the corticosterone level accelerates the generation of free radicals13 and alters the normal homeostasis of organ.14
Cytokines belong to the family of signaling molecules. They are released by specific cells of the immune system. Cytokines are small glycoprotein chemical structures that act in a paracrine and endocrine fashion as soluble signals between cells and play a pivotal role in the immune response. They are the hormonal messengers responsible for most of the biological effects in the immune system, such as cell-mediated immunity and humoral immunity. T lymphocytes are a major source of cytokines. Immune cells produce mediators of inflammatory and immune reactions called cytokines. These low-molecular weight glycoproteins in small concentrations are indispensable for normal functioning of the immune system. Their excessive secretion, however, leads to immune cell dysfunctions. Inflammatory cytokines may be produced by mononuclear cells of the immune system in response to numerous agents, such as microorganisms and their products (e.g., lipopolysaccharide – LPS)15,16 as well as some xenobiotics.17 It is postulated that xenobiotics can influence the concentrations of inflammatory cytokines through oxidative stress mechanisms.18–20 The experimental and epidemiological data currently available to evaluate the above toxigenic risks of aspartame are insufficient and often unreliable, due to the inadequate planning and conduct of previous experiments. Recently from previous studies on aspartame and its metabolite, altering the oxidative status of the cells was investigated. Oral aspartame (75mg/kgbody weight/day) consumption causes oxidative stress in brain21 and its (40mg/kgbw/day) consumption caused oxidative stress in brain,22 liver and kidney,23 and also in immune organs.24 This inadequacy, combined with the general limited knowledge about the safety/potential toxigenic effects of substances widely present in the industrialized diet, motivated the design of the current study. However, little is known about the effects of aspartame on cytokine expression. The detailed mechanisms of the effects of aspartame on cytokines are still unclear; therefore, the present study aimed at clarifying whether longer time of aspartame consumption has any effect on cytokine expression in serum of Wistar albino rats.
Materials and methodsChemicalsPure aspartame powder and methotrexate were purchased from Sigma Aldrich chemical, (St. Louis, USA) and all other chemicals used were of analytical grade obtained from Sisco research laboratory (Mumbai, India). ELISA kits for cytokine estimations were obtained from Ray biotech system (USA).
Animal modelAnimal experiments were carried out after approval from the Institutional Animal Ethical Committee (IAEC No: 01/21/14) and the Committee for the Purpose of Control and Supervision of Experiments on Animals (India). The experimental animals were healthy, inbreed adult male Wistar albino rats, weighing approximately 200–220g. The animals were maintained under standard laboratory conditions and were allowed free access to food and water ad libitum (standard rat feed pellets supplied by M/s. Hindustan Lever Ltd., India). Animals of aspartame-treated groups were administered aspartame daily (40mg/kg/day) dissolved in normal saline orally (by means of gavage needle) for 90 days.25 The LD50 of aspartame in mice and rats is >5g/kg.26 All the rats were housed under condition of controlled temperature (26±2°C) with 12h light and 12h dark exposure.
Experimental designAnimals were randomly divided into 8 groups and each group consisted of 6 animals. Group-1 was control animals which were administered normal saline orally throughout the experimental protocol. Group-3 was control animals treated with aspartame orally for 90 days (40mg/kgbody weight/day). Human beings have very low hepatic folate content.27 In methanol metabolism conversion of formate to carbon dioxide is folate dependent. Hence in the deficiency of folic acid, methanol metabolism could take the alternate pathway (microsomal pathway).28 Conversion of methanol to formaldehyde (HCHO) in humans is catalyzed primarily by alcohol dehydrogenase 1 (ADH1).29 Alternative pathways of metabolism include catalase-mediated reactions, yielding formaldehyde and hydrogen peroxide.30 Formaldehyde may be the ultimate toxicant from methanol exposure. Formaldehyde in turn is converted to formic acid (HCOOH) by the nicotinamide adenine dinucleotide (NAD) dependent alcohol dehydrogenase 3 (ADH3). The metabolism of formate to CO2 is mediated through tetrahydrofolate dependent pathway.31 To simulate this, rats were made folate deficient by feeding them on a special dietary regime for 37 days and after that methotrexate (MTX) in sterile saline was administered for every other day for two weeks32 before euthanasia. MTX folate deficiency was confirmed by estimating the urinary excretion of formaminoglutamic acid (FIGLU)33 in the experiment. Rats on a folate-deficient diet excreted an average of 90–100mg FIGLU/kg body weight/day (range 25–125), while animals on the control diet excreted an average of 0.29mg/kg body weight/day (range 0.15–0.55). These folate-deficient animals showed a significant increase in FIGLU excretion when compared to the control animals (P<0.05). The folate-deficient animals were further divided into 2 groups. Group-2 was folate-deficient diet-fed control, and Group-4 was folate-deficient diet-fed animals treated with aspartame orally for 90 days (40mg/kg body weight/day).
For Groups 5–8, all was immunized with sheep red blood cells (5×109/ml) intraperitoneally, where Group-5 was immunized control animals, Group-6 was immunized folate-deficient diet-fed animals, Group-7 was immunized control animals treated with aspartame orally for 90 days (40mg/kg.body weight/day), and Group-8 was immunized folate-deficient diet-fed animals treated with aspartame orally for 90 days (40mg/kg.body weight/day). The day of immunization was considered as day “0”. On the 5th day, the experimental parameters were measured.
Sample collectionStress-free blood samples were collected between 8 and 10 a.m. to avoid circadian rhythm induced changes as per the technique described previously.34 At the end of the experimental period all the animals were exposed to mild anesthesia by using Pentothal sodium (40mg/kg), and blood was collected from internal jugular vein; plasma and serum were separated respectively by centrifugation at 3000rpm at 4°C for 15min and later used for further biochemical assays.
Biochemical determinationsEstimation of plasma corticosterone levelThe corticosterone level in plasma was determined as described by the procedure of Clark.35 Plasma, sulphuric acid (4N, 2ml) and iron (III) chloride (0.5% (v/v), 0.5ml) mixture was heated in a water-bath maintained at 70°C for 30min with occasional shaking. The absorbance was measured at 780nm against reagent blank.
Estimation of serum protein levelProtein estimations were carried out according to the method of Lowry et al.36 Under alkaline condition the divalent copper ion form a complex with peptide bond in which it is reduced to a monovalent ion. The reduction of folin's phenol reagent by copper-treated protein was measured at 660nm after 30min of incubation in dark.
Assay of lipid peroxidationLipid peroxidation (LPO) was determined as described by Ohkawa et al.37 Malondialdehyde (MDA) forms as an intermediate product of the peroxidation of lipids and serves as an index of the intensity of oxidative stress. MDA reacts with thiobarbituric acid to generate a colored product which absorbs light at 532nm. The level of LPO was expressed as milimoles of malondialdehyde (an intermediary product of lipid peroxidation, using thiobarbituric acid)/L.
Nitric oxide analysisSerum nitric oxide (NO) level was measured as total nitrite+nitrate levels with the use of the Griess reagent by the method of Green et al.38 The Griess reagent consists of sulfanilamide and N-(1-napthyl)-ethylenediamine. The method is based on a two-step process. The first step is the conversion of nitrate into nitrite using nitrate reductase. The second step is the addition of the Griess reagent, which converts nitrite into a deep purple azo compound; photometric measurement of absorbance at 540nm is due to the fact that this azochromophore accurately determines nitrite concentration. NO levels were expressed as μmol/L.
Determination of the activities of enzymatic antioxidantsSuperoxide dismutase (SOD) was assayed according to the method of Marklund et al.39 Pyrogallol autooxidizes rapidly in an aqueous solution at a fast rate with higher pH (pH=8.0) to produce several intermediate products. The inhibition of pyrogallol autooxidation by the enzyme present in the sample is employed in the quantification of activity of SOD. The inhibition of autooxidation is brought about by the addition of enzyme, which is evaluated at the early stage as an increase in absorbance at 420nm in 0, 1, 2 and 3min intervals. The unit of enzyme activity is defined as the enzyme required for 50% inhibition of pyrogallol autooxidation. And the enzyme activity was expressed as (units/ml).
The activity of catalase (CAT) was assayed by the method of Sinha.40 Dichromate in acetic acid is reduced to chromic acetate and when heated in the presence of hydrogen peroxide (H2O2) perchloric acid formed as an unstable intermediate. The catalase preparation is allowed to split H2O2 for different periods of time and the reaction was arrested by the addition of dichromate/acetic acid mixture at a particular interval of 0, 15, 30 and 60s. The remaining H2O2 is determined by measuring chromic acetate at 610nm. The activity was expressed as μmols of H2O2 utilized/ml.
Glutathione peroxidase (GPX) activity was estimated by the method of Rotruck et al.,41 which was based on the reaction between glutathione remaining after the action of GPx and 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) to give a compound that absorbs light at 412nm. The GPx activity was expressed as μg of GSH consumed/ml.
Estimation of non-enzymatic antioxidantsReduced glutathione (GSH) in serum was estimated by the method of Moron et al.,42 based on the reaction of GSH with DTNB that gives a compound which absorbs light at 412nm. The level of GSH was expressed as μg of GSH/ml.
Vitamin-C (ascorbic acid) content was determined by the method of Omaye et al.43 Ascorbic acid was oxidized by copper to form dehydroascorbic acid and diketoglutaric acid, which was then treated with 2,4-dinitrophenyl hydrazine to form the derivative of bis-2,4-dinitrophenyl hydrazine. This compound in sulphuric acid undergoes a rearrangement to form a product which absorbs light at 520nm. The level of ascorbic acid was expressed as μg of ascorbic acid/ml.
Estimation of cytokinesSerum cytokines like interleukin 2, interleukin 4, tumor necrosis factor-α and interferon-γ (IL-2, TNF-α, IFN-γ and IL-4) were analyzed according to manufacturer's protocols using standard Rat ELISA immunoassay kit (Ray biotech system, USA).
Statistical analysisData are expressed as mean±standard deviation (SD). All data were analyzed with the SPSS for windows statistical package (version 20.0, SPSS Institute Inc., Cary, North Carolina, USA). Statistical significance between the different groups was determined by one-way analysis of variance (ANOVA). When the groups showed significant difference, then Tukey's multiple comparison tests were followed and the significance level was fixed at p<0.05.
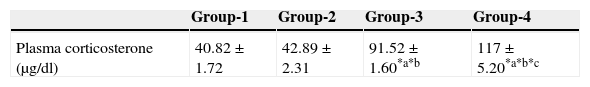
ResultsEffect of aspartame on plasma corticosterone levelThe results are summarized in Table 1. The control, as well as the folate-deficient rats, did not show any significant variation among themselves in the plasma corticosteroid levels. However, both control animals as well as folate-deficient animals when treated with aspartame for 90 days, whether they were un-immunized or immunized, showed a marked increase in corticosteroid levels when compared to control as well as the folate-deficient animals. Among the aspartame-treated animals, the folate-deficient animals showed a significant increase compared to control animals, indicating that aspartame acts as a chemical stressor and methanol may be one of the possible reasons behind change observed.
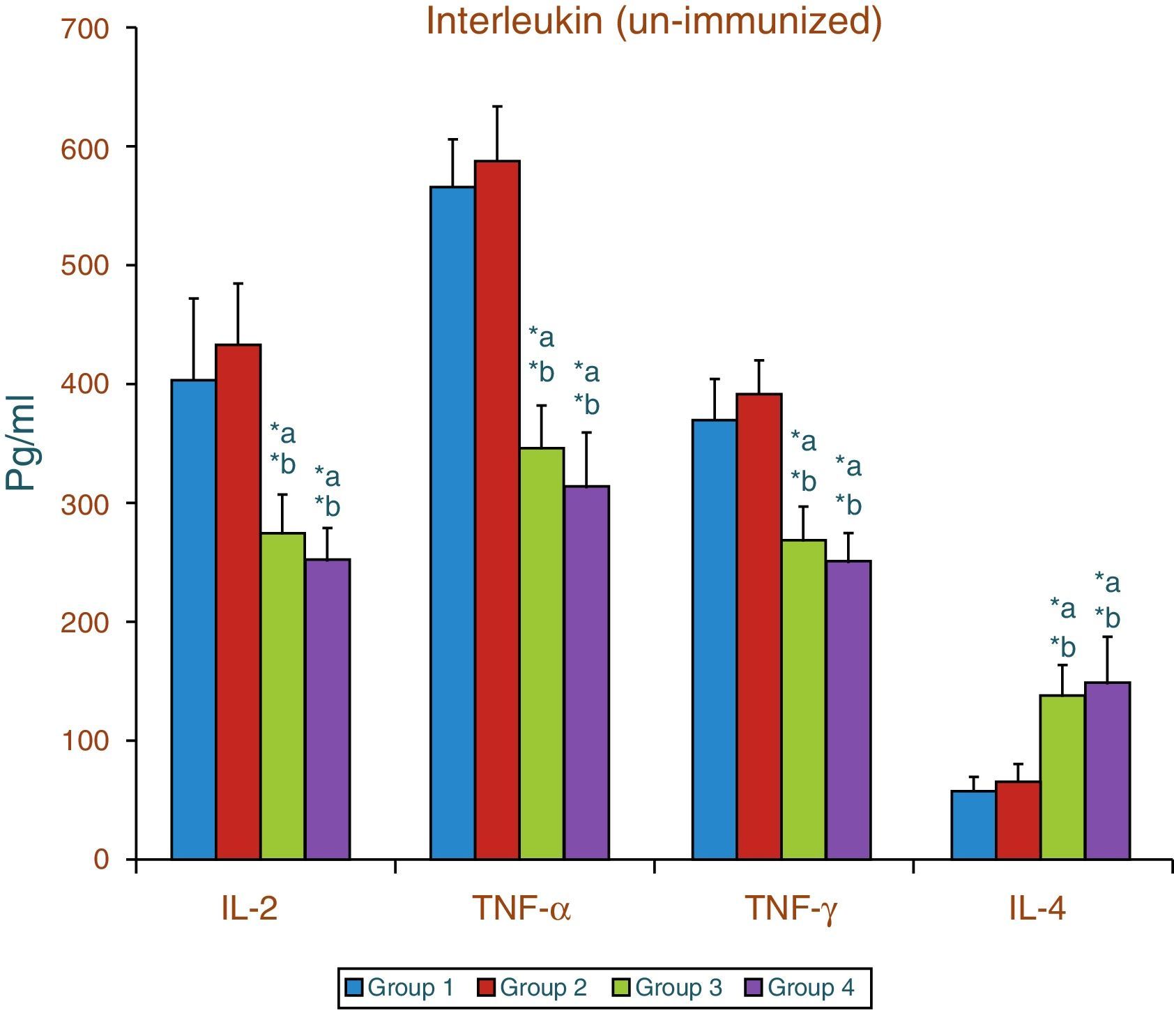
Effect of aspartame on corticosterone level in un-immunized animals and immunized animals.
| Group-1 | Group-2 | Group-3 | Group-4 | |
|---|---|---|---|---|
| Plasma corticosterone (μg/dl) | 40.82±1.72 | 42.89±2.31 | 91.52±1.60*a*b | 117±5.20*a*b*c |
| Group-5 | Group-6 | Group-7 | Group-8 | |
|---|---|---|---|---|
| Plasma corticosterone (μg/dl) | 41.96±1.76 | 42.58±1.64 | 93.61±2.55*p*q | 120±7.22*p*q*r |
Group 1 – Control, Group 2 – Folate deficient, Group 3 – Control + aspartame, Group 4 – Folate deficient + aspartame. Group 5 – Immunized control, Group 6 – Immunized folate deficient, Group 7 – Immunized control + aspartame, Group 8 – Immunized folate deficient + aspartame. Significance at *p<0.05, *a – compared with Group-1, *b – compared with Group-2. *c – compared with Group-3; *p – compared with Group-5, *q – compared with Group-6. *r – compared with Group-7.
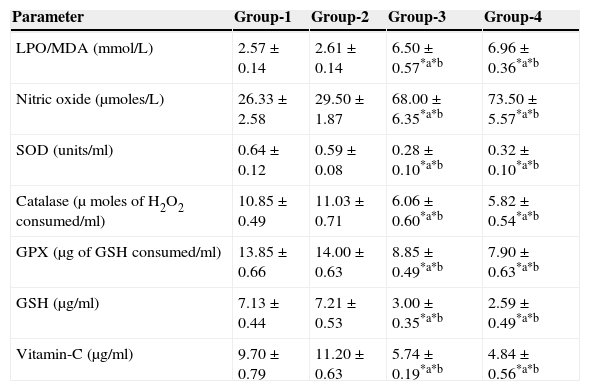
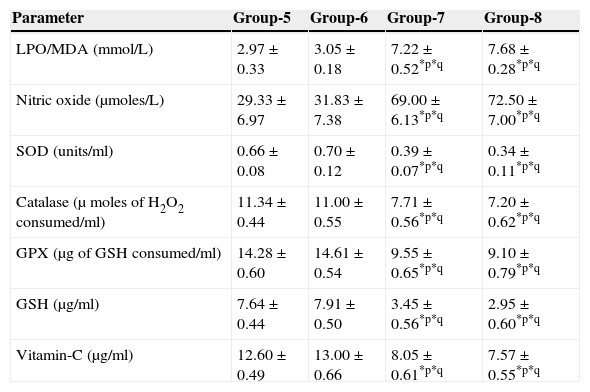
The results are summarized in Tables 2 and 3. The LPO level of folate-deficient diet-fed animals was similar to control animals. However, both in the control animals as well as in folate-deficient diet-fed animals treated with aspartame for 90 days, the LPO levels were increased when compared to controls and folate-deficient animals, irrespective of whether un-immunized or immunized. This clearly indicates the generation of free radicals by aspartame.
Effect of aspartame on serum antioxidant level in un-immunized animals.
| Parameter | Group-1 | Group-2 | Group-3 | Group-4 |
|---|---|---|---|---|
| LPO/MDA (mmol/L) | 2.57±0.14 | 2.61±0.14 | 6.50±0.57*a*b | 6.96±0.36*a*b |
| Nitric oxide (μmoles/L) | 26.33±2.58 | 29.50±1.87 | 68.00±6.35*a*b | 73.50±5.57*a*b |
| SOD (units/ml) | 0.64±0.12 | 0.59±0.08 | 0.28±0.10*a*b | 0.32±0.10*a*b |
| Catalase (μ moles of H2O2 consumed/ml) | 10.85±0.49 | 11.03±0.71 | 6.06±0.60*a*b | 5.82±0.54*a*b |
| GPX (μg of GSH consumed/ml) | 13.85±0.66 | 14.00±0.63 | 8.85±0.49*a*b | 7.90±0.63*a*b |
| GSH (μg/ml) | 7.13±0.44 | 7.21±0.53 | 3.00±0.35*a*b | 2.59±0.49*a*b |
| Vitamin-C (μg/ml) | 9.70±0.79 | 11.20±0.63 | 5.74±0.19*a*b | 4.84±0.56*a*b |
Group 1 – Control, Group 2 – Folate deficient, Group 3 – Control + aspartame, Group 4 – Folate deficient + aspartame. Significance at *p<0.05, *a – compared with Group-1, *b – compared with Group-2.
Effect of aspartame on serum antioxidant level in immunized animals.
| Parameter | Group-5 | Group-6 | Group-7 | Group-8 |
|---|---|---|---|---|
| LPO/MDA (mmol/L) | 2.97±0.33 | 3.05±0.18 | 7.22±0.52*p*q | 7.68±0.28*p*q |
| Nitric oxide (μmoles/L) | 29.33±6.97 | 31.83±7.38 | 69.00±6.13*p*q | 72.50±7.00*p*q |
| SOD (units/ml) | 0.66±0.08 | 0.70±0.12 | 0.39±0.07*p*q | 0.34±0.11*p*q |
| Catalase (μ moles of H2O2 consumed/ml) | 11.34±0.44 | 11.00±0.55 | 7.71±0.56*p*q | 7.20±0.62*p*q |
| GPX (μg of GSH consumed/ml) | 14.28±0.60 | 14.61±0.54 | 9.55±0.65*p*q | 9.10±0.79*p*q |
| GSH (μg/ml) | 7.64±0.44 | 7.91±0.50 | 3.45±0.56*p*q | 2.95±0.60*p*q |
| Vitamin-C (μg/ml) | 12.60±0.49 | 13.00±0.66 | 8.05±0.61*p*q | 7.57±0.55*p*q |
Significance at *p<0.05, *p – compared with Group-5, *q – compared with Group-6. Group 5 – Immunized control, Group 6 – Immunized folate deficient, Group 7 – Immunized control+aspartame, Group 8 – Immunized folate deficient+aspartame.
The results are summarized in Tables 2 and 3. The nitric oxide level of folate-deficient diet-fed animals was similar to control animals. However, both in the control animals as well as in folate-deficient diet-fed animals treated with aspartame for 90 days, the nitric oxide levels were significantly increased when compared to controls and folate-deficient animals irrespective of whether un-immunized or immunized. This also supports clearly generation of free radicals by aspartame.
Effect of aspartame on serum superoxide dismutase (SOD) levelThe results are summarized in Tables 2 and 3. Compared to control animals, folate-deficient animals did not show any significant variation in serum SOD level. However, both aspartame-treated control as well as folate-deficient animals showed significant decrease in SOD level whether un-immunized or immunized.
Effect of aspartame on serum catalase (CAT) levelThe results are also summarized in Tables 2 and 3. The folate-deficient animals did not show any significant variation in serum catalase level compared to control animals. However, both aspartame-treated control as well as folate-deficient animals showed a significant decrease in catalase level irrespective of un-immunized or immunized.
Effect of aspartame on serum glutathione peroxidase (GPX) levelThe results are summarized in Tables 2 and 3. The glutathione peroxidase level of folate-deficient animals was similar to control animals. However, both in the control animals as well as in folate-deficient animals treated with aspartame for 90 days, glutathione peroxidase level was significantly decreased when compared to controls and folate-deficient animals irrespective of whether un-immunized or immunized. This clearly supports that aspartame alters serum enzymatic level due to excess free radical production.
Effect of aspartame on serum GSH levelThe results are summarized in Tables 2 and 3. Compared to control animals, folate-deficient animals did not show any significant variation in serum GSH level. However, at 90 days both aspartame-treated control as well as folate-deficient animals showed a significant decrease in serum GSH level irrespective of whether un-immunized or immunized.
Effect of aspartame on serum Vitamin-C levelThe results are summarized in Tables 2 and 3. The serum Vitamin-C level of folate-deficient animals was similar to control animals. However, both in the control animals as well as folate-deficient animals treated with aspartame for 90 days, serum Vitamin-C level was significantly decreased when compared to controls and folate-deficient animals irrespective of whether un-immunized or immunized. This clearly indicate that aspartame alters serum non-enzymatic level due to excess free radical production.
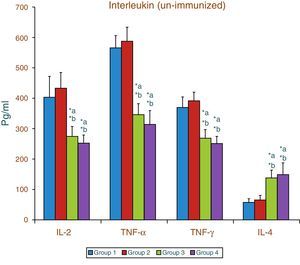
Effect of aspartame on serum cytokines (IL-2, TNF-α, IFN-γ and IL-4) level of un-immunized animalsThe results of interleukin expression are summarized in Fig. 1. The IL-2, TNF-α, IFN-γ and IL-4 level did not get significantly altered in folate-deficient animals when compared to control animals. However, both control animals as well as folate-deficient animals treated with aspartame for 90 days showed significant decrease in IL-2, TNF-α, and IFN-γ, and an increase in IL-4 level, when compared to control as well as folate-deficient fed animals.
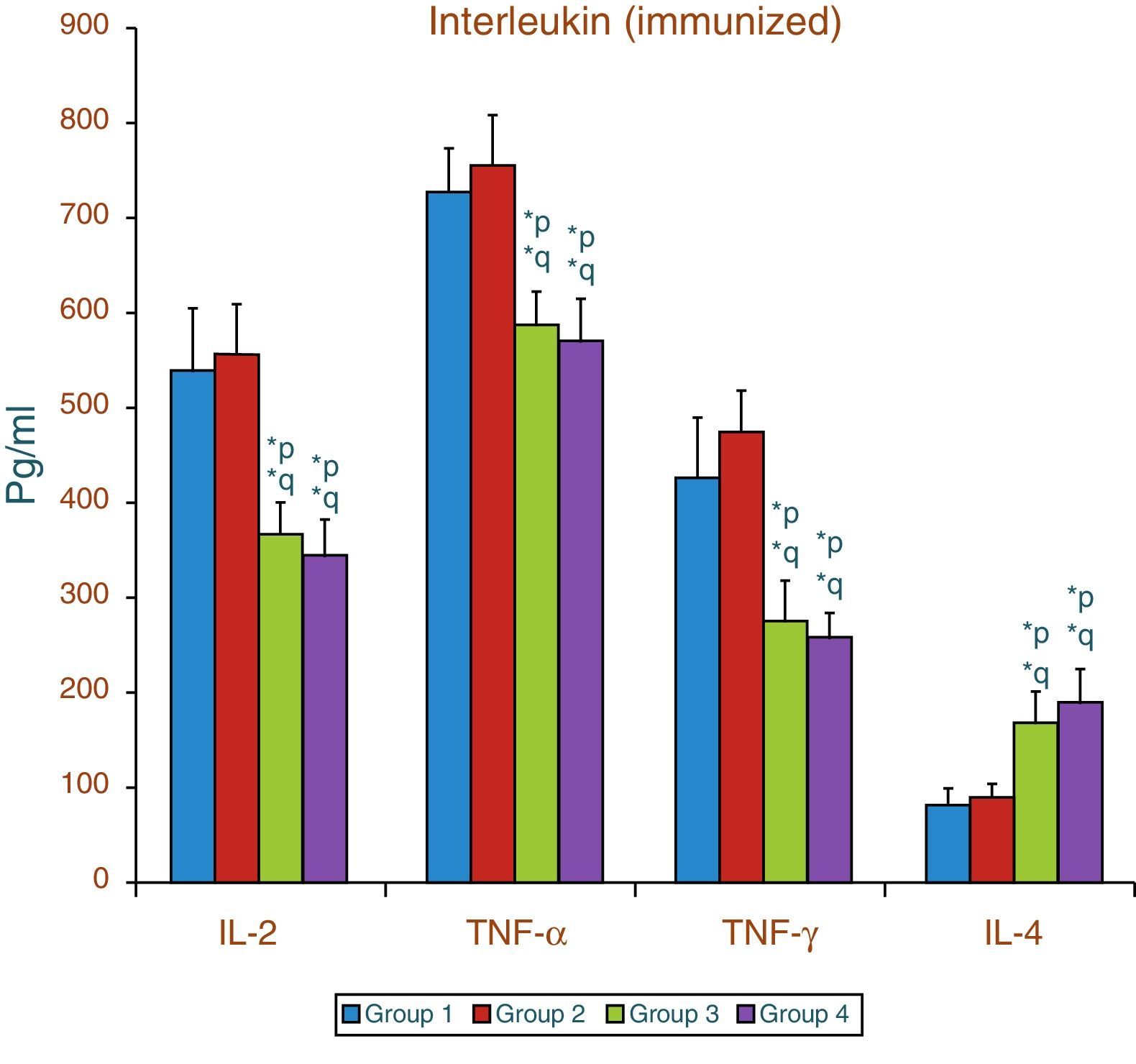
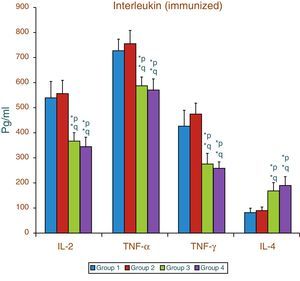
Effect of aspartame on serum cytokines (IL-2, TNF-α, IFN-γ and IL-4) levels of immunized animalsThe results of interleukin expression are summarized in Fig. 2. Compared to control animal, The IL-2, TNF-α, IFN-γ and IL-4 level did not get significantly altered in folate-deficient animal. However, both control animals as well as folate-deficient animals treated with aspartame for 90 days showed significant decrease in IL-2, TNF-α, and IFN-γ, and an increase in IL-4 level, when compared to control as well as folate-deficient fed animals. The alteration observed in these cytokines after aspartame administration clearly indicates that its action affects not only at cellular level but also affects cytokine expressions.
Effect of aspartame on serum cytokine level in immunized animals, Significance at *p<0.05, *p – compared with Group-5, *q – compared with Group-6. Group 5 – Immunized control, Group 6 – Immunized folate deficient, Group 7 – Immunized control+aspartame, Group 8 – Immunized folate deficient+aspartame.
A homeostatic balance exists between the formation of free oxygen radicals and their removal by endogenous scavenging antioxidants.44 In this study, the folate-deficient diet-fed animals were used to mimic the human methanol metabolism. However, the folate-deficient diet-fed animals did not show any significant changes in the parameters studied and remained similar to controls animals. The increase in corticosterone level indicates that aspartame may act as a chemical stressor. Changes in membrane disruptions, as by increased lipid peroxidation and nitric oxide, indicate free radical production,45 which is a potent source of oxidative injury.46 Increased corticosterone along with free radical production can lead to suppression of immune system and can alter the release of cytokines. Cytokines are glycosylated polypeptides that are secreted and influence the action of immune cells.47 Cytokines, the chemical messengers between immune cells, play crucial roles in immune response.48 Cytokines are released to the location of inflammation induced by pathogens or tissue damage and facilitate the arrival of neutrophils, monocytes and other cells involved in antigen clearance and healing the tissue.49,50 Leukocyte mobilization into the blood stream in response to cytokines is well documented.51,52 The local production of these molecules coordinates the function of innate and adaptive immune cells. T cell population responses to antigens extensively after immunization.53,54
Oxidative stress refers to a disturbance in the balance between the production of reactive oxygen species (ROS) and antioxidant defences;55 abnormally high levels of free radicals and the simultaneous decline of antioxidant defence mechanisms can damage cellular lipids, proteins or DNA inhibiting their normal function. The three primary scavenging enzymes involved in detoxifying the free radicals in mammalian systems are SOD, CAT and GPx.56 SOD dismutases the highly reactive superoxide anion to the less reactive species H2O2.57 CAT and GPx efficiently react with H2O2 to form water and molecular oxygen.58,59 GSH has a multifactorial role in antioxidant defence. It is a direct scavenger of free radicals as well as a co-substrate for peroxide detoxification by glutathione peroxides.60 Ascorbic acid, well known as a potent water-soluble antioxidant, effectively intercept oxidants in the aqueous phase before they attack and cause detectable oxidative damage.61 Cytokines belong to the signaling molecules and have an important task in cellular communication. They are critical for the development and function of innate and adaptive immune responses. On the basis of their pattern of cytokine synthesis, CD4+ Th (helper) cells were originally classified into Th1 and Th2 lymphocytes,62 which are involved in cellular and humoral immune responses, respectively.63 The cytokines produced are known as Th1-type cytokines and Th2-type cytokines. Type 1 helper T cells are characterized by the production of Th1-type cytokines (IL-2, TNF-α and IFN-γ,) Th1 cells are involved in cell-mediated immunity.64 Type 2 helper T cells are characterized by the production of Th2-type cytokines (IL-4). Th2 cells are thought to play a role in humoral immune responses.65 Cytokines like IL-4 generally stimulate the production of antibodies. Improved understanding of Th1 and Th2 differentiation will improve our overall understanding of the immune system. The plasma release of both Th1-type and Th2-type cytokines unbalance the immunity. Leukocyte mobilization into the blood stream in response to cytokines is well documented.51,52 It has been shown that IL-4 is an essential differentiation factor for Th2 cells.66 Oxidative stress might induce IL-4 production by mast cells and basophils and direct the Th2 cell differentiation.67 IL-4 may be considered as a pro-oxidative cytokine that can increase the oxidative potential of target cells.68 IL-4 induced the generation of ROS such as superoxide anion and hydrogen peroxide.69 This imbalance between oxidants and antioxidants favors skewing of the Th2 immune response while suppressing Th1 differentiation.70 The results of present study seem to suggest that the aspartame metabolite methanol or formaldehyde may be involved in inducing oxidative stress in serum and in changes of cytokine secretion where Th1 cytokines are down-regulated and Th2 cytokines are up-regulated, which may alter cell and humoral immunity. This report is strengthened by previous published studies by Parthasarathy et al.,71 where methanol intoxication alters immune function.
The results of the present study clearly point out that increasing corticosterone levels indicate that aspartame acts as a chemical stressor and that aspartame metabolite could accelerate lipid peroxidation leading to an increase in oxidative stress and altered cytokine secretion. It suggests that the dysregulation of cytokines is the result of an imbalance in Th1 and Th2 cells and can have important implications during the development of immune responses which may alter immune function. Aspartame metabolites methanol or formaldehyde may be the causative factors behind the changes observed. More insight is needed to elucidate the precise molecular mechanisms involved in these changes.
The authors gratefully acknowledge the University of Madras for their financial support [UGC No. D.1.(C)/TE/2012/1868]. The authors acknowledge Mr. Sunderaswaran Loganathan for his constant support and help.