Pregnancy in women with type 1 diabetes (T1D) involves greater risks as compared to non-diabetic women, but less information is available about blood glucose and weight control after delivery. Our aim was to evaluate the postpartum metabolic profile (blood glucose and weight control) of women with T1D and the factors related to those metabolic outcomes.
MethodsA retrospective, observational study of 36 women with T1D during pregnancy and for up to one year after delivery.
ResultsFifty percent of patients attended a preconceptional planning program (PPP), and 44.4% of women were treated with continuous subcutaneous insulin infusion. Mean preconceptional HbA1c and body mass index (BMI) were 7.2±1.2% and 23.8±5.0 respectively. In the total cohort, blood glucose control significantly worsened one year after delivery (HbA1c: 7.2±1.2 vs 7.6±1.2%, P<0.001). Lower preconceptional HbA1c values were found in patients who attended PPP (6.6±0.5 vs. 7.8±1.4%; P=0.02), and were maintained for one year after delivery. No differences were found in body mass index (BMI) from the pregestational period to one year after delivery in any of two groups (No PPP 22.5±4.6 vs 23.2±4.8, P=0.078; PPP 25.4±3.4 vs 25.5±3.4kg/m2, P=0.947). Preconceptional HbA1c was shown to be the most important determinant of metabolic control (β=0.962, p<0.001) and weight one year after delivery (β=0.524, p=0.025) and weight gain during pregnancy (β=0.633, p=0.004).
ConclusionsPregnant women with T1D return to prepregnancy body weight one year after delivery, especially those with lower HbA1c levels and BMI before pregnancy. However, blood glucose control deteriorates after delivery, suggesting the need for changes in clinical practice after delivery.
La gestación en mujeres con diabetes tipo 1 (T1D) conlleva mayor riesgo que en las mujeres sanas; sin embargo, existe menos información acerca del control glucémico y del peso tras la gestación. Nuestro objetivo ha sido evaluar el perfil metabólico posparto (control glucémico y peso) en mujeres con T1D y qué factores están relacionados con dichos resultados metabólicos.
MétodosEstudio observacional retrospectivo de 36 mujeres con T1D durante el embarazo y hasta un año posparto.
ResultadosEl 50% de las pacientes realizaron un programa de planificación pregestacional (PPP) y el 44,4% realizaban tratamiento con infusor subcutáneo de insulina. La HbA1c y el índice de masa corporal (IMC) fueron de 7,2±1,2% y 23,8±5,0 respectivamente. En la cohorte total se observó un empeoramiento significativo del control glucémico al año posparto (7,2±1,2 vs 7,6±1,2%, p<0,001). Las pacientes que acudieron al PPP (6,6±0,5 vs. 7,8±1,4%; p=0,02) presentaban una menor HbA1c pregestacional, y esto se mantuvo un año tras el parto. No se objetivaron diferencias en el índice de masa corporal (IMC) entre el periodo pregestacional y al año posparto en ninguno de los 2 grupos (no PPP 22,5±4,6 vs 23,2±4,8, p=0,078; PPP 25,4±3,4 vs 25,5±3,4kg/m2, p=0,947). La HbA1c fue el mayor determinante del grado de control metabólico (β=0,962, p<0,001) y del peso un año posparto (β=0,524 p=0,025) y de la ganancia ponderal durante la gestación (β=0,633, p=0,004).
ConclusionesLas mujeres embarazadas con T1D recuperan el peso preconcepcional al año posparto, especialmente aquellas con menor HbA1c e IMC pregestación. Sin embargo, el control glucémico se deteriora tras el parto, sugiriendo que es necesario modificar nuestra práctica clínica en este periodo.
It is widely known that the risk of both maternal and fetal adverse effects is higher in patients with pre-gestational diabetes (type 1 and type 2) compared to the general population.1 A poor metabolic control prior to pregnancy and in the first weeks of gestation has been associated with an increased risk of spontaneous miscarriages2 and congenital malformations.3–5 Similarly, it has been observed that poor glycemic control during the second and third trimesters of pregnancy is associated with an increased rate of preterm delivery, pre-eclampsia, macrosomia and maternal and perinatal morbidity and mortality.6,7
For these reasons, guidelines and consensus documents currently recommend optimizing glycemic control in the pre-gestational period and maintaining glucose levels as close as possible to normoglycemia throughout pregnancy.8,9 Most of the studies performed in the last decades in type 1 diabetes (T1D) pregnant women have been conducted to clarify what kind of therapy (continuous subcutaneous insulin infusion – CSII vs multiple doses of insulin – MDI) is most appropriate to achieve these glycemic control objectives and to reduce maternal and perinatal morbidity and mortality, although no differences were found between both therapies.10–12 However, a better glycemic control and also a reduction in adverse events on both maternal and fetal outcomes in patients included in a prepregnancy planning program (PPP) has been shown in non-randomized studies.13
Nevertheless, there is little information about what happens with metabolic control after delivery. A recent study14 confirmed prior existing data15 indicating that after birth there is a marked worsening of glycemic control in such patients. This study observed similar weight gain in pregnant patients with diabetes with respect to a nondiabetic population16 but less weight lost during the first year after delivery.
With this information available, our study aimed to evaluate the postpartum metabolic profile, including glycemic control and body weight, after pregnancy in women with T1D. In addition, we sought to find which factors before and during pregnancy were related with those metabolic outcomes. For this reason, we analyzed differences between patients who did or did not attend PPP and patients treated with CSII versus MDI.
Subjects and methodsThis is a retrospective observational study of 36 pregnant women with type 1 diabetes followed at the Diabetes and Pregnancy Unit of the Hospital Clínic i Universitari of Barcelona during 2011 and 2012. The study was approved by the ethics committee of the hospital. All women were included regardless of whether they had performed the specific pregnancy planning program of our unit. Only the women who did not complete one year of postpartum follow-up in our service were excluded.
During routine follow-up, type 1 diabetic women of fertile age are informed about the importance of planning their gestation and about the availability of the pregestational planning program (PPP) in our service. Thus, when those patients show gestational desire they are referred to this program. Patients attending the PPP were evaluated by the physician intended for that purpose with the aim of assessing the patient's obstetric history, diabetes evolution, the status of microangiopathic complications, the presence of other cardiovascular risk factors and current metabolic control with the treatment pattern adjustment if necessary. Thereafter, monthly visits were performed in order to achieve HbA1c <6.5% (48mmol/mol) in two separate consecutive determinations and then, in that moment, pregnancy was recommended.
During the pregnancy period, patients were followed according to the protocol for pregnant patients with type 1 diabetes at our service, which consisted of joint visits of the diabetologist and obstetrician every 1–3 weeks. The methods and control objectives during pregnancy are those recommended by the Guidelines of the Spanish Group of Diabetes and Pregnancy.8 In the postpartum period, patients were visited six weeks postpartum and three times more during the first year postpartum.
Anthropometrical (weight and BMI), analytical (HbA1c determined through Automated analyzer Tosoh's G8 HPLC analyzer calibrated values DCCT – benchmarks 4–6%) and clinical characteristics of patients, as well as type of insulin treatment and insulin doses, were collected during pre-conceptional period, in each of the trimesters of pregnancy and one year after delivery. Data collection about delivery and newborn (NB) (kind of delivery, gestational age, percentage of preterm deliveries – defined as those of less than 37 weeks gestation, birth weight, percentage of large-for-gestational age NB – >90th percentile, major and minor malformations – as stated in Chapter XVII, ICD-10 WHO, neonatal hypoglycaemia – glycaemia <40mg/dL, neonatal hyperbilirubinemia – bilirubin >10.5mg/dL, Apgar score, arterial and venous pH) was also performed.
Further comparisons of the results were made by dividing the cohort by two criteria: patients who received pre-gestational control versus those who did not and patients treated with CSII or MDI during pregnancy.
Data are shown as mean and standard deviation, median and interquartile range or proportions, as appropriate. Comparison of means was performed using Student's t-test for paired or unpaired data (as necessary) for normally distributed variables and by Mann–Whitney U-test for non-normal variables. For the comparison of two qualitative variables we used a Chi-square test. A p≤0.05 was considered statistically significant. Finally, a standard multiple regression analysis was performed, including covariates that show some relationship with dependent variable and excluding variables that were highly correlated to avoid multicollinearity; as a result, the variables included were: age, diabetes duration, preconceptional HbA1c, preconceptional BMI, preconceptional insulin dose and third trimester HbA1c. Statistical analysis was performed using SPSS Statistics 17.0 (SPSS Inc. Chicago, IL).
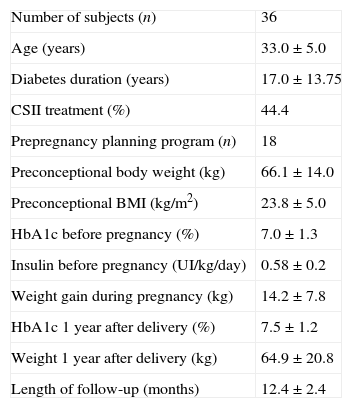
ResultsA total of 36 patients were included in the study. They had a mean age of 32.4±3.8 years and 17.2±9.2 years of diabetes duration. In the total cohort, preconceptional HbA1c, considering the latest available prior to gestation, was 7.2±1.2% (55mmol/mol), 30% of the patients had a HbA1c lower than 6.5% (48mmol/mol) and 52.8% lower than 7% (53mmol/mol). Preconceptional BMI and weight was 24.2±4.0kg/m2 and 66.1±14.0kg, respectively. One year after childbirth HbA1c increased to 7.6±1.2% (60mmol/mol) (p<0.001), but BMI and weight returned to prepregnancy values (24.3±4.2kg/m2 with p=0.261, and 66.5±14.0kg with p=0.417) (Table 1).
Patient characteristics before pregnancy and one year after delivery.
| Number of subjects (n) | 36 |
| Age (years) | 33.0±5.0 |
| Diabetes duration (years) | 17.0±13.75 |
| CSII treatment (%) | 44.4 |
| Prepregnancy planning program (n) | 18 |
| Preconceptional body weight (kg) | 66.1±14.0 |
| Preconceptional BMI (kg/m2) | 23.8±5.0 |
| HbA1c before pregnancy (%) | 7.0±1.3 |
| Insulin before pregnancy (UI/kg/day) | 0.58±0.2 |
| Weight gain during pregnancy (kg) | 14.2±7.8 |
| HbA1c 1 year after delivery (%) | 7.5±1.2 |
| Weight 1 year after delivery (kg) | 64.9±20.8 |
| Length of follow-up (months) | 12.4±2.4 |
Fifty percent of patients (18) attended the pre-pregnancy program while the other half did not. Patients who underwent PPP achieved lower HbA1c levels [6.6±0.5% (49mmol/mol) vs 7.8±1.4% (62mmol/mol), P=0.02] with no significant differences in weight or units of insulin/day administered before pregnancy, in comparison to the women who did not attend the PPP. Regarding the evolution of HbA1c during pregnancy, it should be noted that HbA1c during first (6.5±0.6 (48mmol/mol) vs 7.2±1.1% (55mmol/mol), P<0.05) and third trimester of gestation (6.2±0.7% (44mmol/mol) vs 6.8±0.9% (51mmol/mol), P=0.030) was lower in the group that had performed pregnancy planning. In the evaluation one year after childbirth, patients who had conducted the PPP continued to have significantly lower HbA1c respect to those who had not performed the program [7.2±0.9% (55mmol/mol) vs 8.1±1.3% (65mmol/mol), P=0.018]. Although worsening of HbA1c from preconceptional to one year after delivery was higher in patients who had conducted PPP that difference was not statistically different (0.3±1.3% vs 0.6±0.9%, P=0.456). No statistically significant differences were observed between the two groups in weight gain during pregnancy neither in weight one year after childbirth. Likewise, there were no significant differences between groups in the results of data delivery and NB.
A comparative analysis of patients treated with MDI and CSII during pregnancy was also performed. 44.4% of women were treated with CSII. There were no differences in preconceptional HbA1c between these two groups. Instead, in the third trimester HbA1c was higher in pregnant women treated with CSII [6.9±0.8% (52mmol/mol) vs 6.1±0.8% (43mmol/mol), P=0.008]. There were also no differences in HbA1c increase during the first year after childbirth (1.2±1.0 vs 1.2±1.2%, P=0.372). Prepregnancy weight and weight gain during pregnancy were similar in both groups, but with a tendency to be higher in the group treated with CSII. One year postpartum, both groups of patients reduced the weight to almost pregestational levels although significant greater weight loss with CSII was found (14.9±3.8 vs 11.6±5.5kg, P=0.046). Data concerning delivery and NB are summarized in Table 2. All patients under treatment with CSII during pregnancy maintained the same therapy one year after delivery.
A multiple regression analysis was performed in order to assess the factors that determined a higher weight gain during pregnancy. A lower preconceptional HbA1c was the most important determinant of the higher weight gain (β=0.633, p=0.004), while a higher HbA1c in the third trimester (β=0.524, p=0.006) and a lower maternal age (β=0.257, p=0.003) were also independently associated. We also performed a multiple regression analysis to assess the factors that allowed greater postpartum weight loss. We found that lower preconceptional HbA1c (β=0.524, p=0.025) and lower prepregnancy BMI (β=0.925, p=0.008) were independently correlated with it. Finally, we performed another multiple regression analysis to evaluate factors that lead to a greater deterioration of HbA1c after delivery, but only an association with higher preconceptional HbA1c (β=0.962, p<0.001) was found.
DiscussionIn view of recent data regarding postpartum metabolic control in patients with T1D, we wanted to evaluate this data in the local patient cohort. One year after delivery, we observed a worsening of HbA1c compared to the values before pregnancy in the entire cohort. This data is consistent with a recent publication.14 Various reasons have been proposed to explain this worsening in glycemic control, such as lesser stringent glycemic goals after birth, fewer self-monitoring blood glucose, decreased frequency of visits and greater dedication to newborn care with less attention to maternal diabetes care. However, in contrast to Cyganek et al.,14 we observed that patients who did not attend the PPP and those who had worse glycemic control prior to gestation, achieved higher HbA1c levels one year after delivery. This data suggest that we should strengthen the follow-up of this group of women after delivery to avoid worsening HbA1c, taking advantage of the degree of control and the habits and knowledge acquired during pregnancy.
However, in terms of weight change, our data were substantially different than the ones identified in the previously cited study,14 as weight gain during pregnancy in the entire cohort was lower (13.6 vs. 14.4kg) and even also lower than that observed in a cohort of 2026 nondiabetic pregnant women.16 It should also be noted that in the analysis of weight one year after delivery, women only weighted 0.5kg more than in the prepregnancy period. This data may be relevant since previous studies have linked the increase of weight after pregnancy with increased weight in the long-term and an increasing rate of obesity in women in the general population.16 As we observed in the multiple regression analysis, pregestational HbA1c was related with both, weight gain during pregnancy and weight one year after delivery; thus, this data confirms the importance of achieving improved glycemic control prior to gestation. Moreover, this regression analysis showed a relationship between lower pregestational BMI and weight loss after delivery, indicating that probably weight control before gestation has to be taken into account in pregnancy planning programs.
On the other hand, 50 percent of the T1D pregnant women followed at our center, performed the pregnancy planning program, a higher proportion than that reported elsewhere.12–15 This is probably due to the fact that 44.4% of the subjects were treated with CSII prior to gestation and this group of patients often have greater adherence to follow-up.7,15 Regarding the assessment of the effectiveness of this program, we found that the T1D women included in the PPP had lower HbA1c before pregnancy, which was maintained during the first and third trimesters of pregnancy and one year after delivery, but we failed to prove statistically significant differences in data regarding delivery and newborn outcomes. The limited number of individuals in our study may explain the inability to demonstrate these differences.
Finally, we evaluated the evolution of pregnancies and the postpartum metabolic control in our cohort of T1D women depending on the therapy (CSII vs MDI). Only one of the 16 patients on treatment with CSII started this therapy in order to prepare pregnancy, while the other 15 T1D women were already treated with CSII before pregnancy. All of them continued with CSII treatment one year postpartum. Pregnant women with T1D in this situation tend to have longer diabetes duration, more labile control, a history of severe or unnoticed hypoglycaemia and achieving optimal objectives of glycemic control is difficult. This might be the reason why the HbA1c before conception and one year after delivery is higher in the CSII group than in the MDI group, although this difference did not reach statistical significance. On the other hand, in our cohort of T1D pregnant women no differences were found in weight gain during pregnancy between groups depending on treatment, which is concordant with the results of Cyganek et al.14 However, in other cohorts the group in CSII treatment gained more weight during pregnancy than the MDI group.12 An interesting finding was the significant higher weight loss after delivery in the CSII group, which does not coincide with the results of a previous publication.14
Limitations of the study are related to the retrospective observational design and its low statistical power due to the small sample size. For a better interpretation of HbA1c values it might also be useful to have an exact counting of hypoglycemic events, as they are very common in this group of patients. However, the evaluation of clinical practice did not allow us to have complete and reliable data on hypoglycemia. Moreover, we did not collect data about type of feeding of newborns nor contraceptive method after delivery and the interpretation of weight changes could be influenced by these data.
In conclusion, we observed that T1D pregnant women return to prepregnancy body weight one year after delivery, especially those with lower HbA1c concentrations and BMI before gestation. However, glycemic control deteriorates after delivery, even in women who attended pregnancy programs with high motivation. This data provides useful information to propose changes in our clinical practice setting after delivery while highlighting the importance of keeping a good glycemic control in the postpartum.
Conflict of interestThe authors declare that they have no conflict of interest.