Polycystic ovary syndrome (PCOS) is one of the most common endocrine diseases in women of reproductive age. PCOS typically develops during adolescence and is a heterogeneous syndrome classically characterized by features of anovulation combined with signs of androgen excess (hirsutism, acne). Increasing obesity in adolescents probably exacerbates signs of PCOS, contributing to its earlier recognition. Recognizing the features of this syndrome can be very challenging in adolescence. Although adolescents’ concerns are often cosmetic, if left untreated these girls are at risk for diabetes, metabolic syndrome, and infertility as they mature. Efforts should be made to diagnose and treat PCOS to minimize the development of symptoms and prevent the onset of cardiovascular and metabolic disturbances.
El síndrome del ovario poliquístico (SOP) es una de las endocrinopatías más comunes en mujeres en edad reproductiva. SOP se presenta típicamente en la adolescencia y es un síndrome heterogéneo que clásicamente se caracteriza por rasgos de la anovulación en combinación con síntomas de exceso de andrógenos (hirsutismo, acné). El aumento de la obesidad en los adolescentes probablemente exacerba señales del SOP, contribuyendo a un reconocimiento más precoz. Reconocer las características de este síndrome puede ser muy difícil en la adolescencia. Aunque las preocupaciones a los adolescentes sean a menudo cosméticas, si no se tratan estas niñas corren el riesgo de desarrollar diabetes, síndrome metabólico, e infertilidad a medida que crecen. Se deben hacer esfuerzos para diagnosticar y tratar el SOP a fin de minimizar el desarrollo de los síntomas y prevenir la aparición de problemas cardiovasculares y metabólicos.
Polycystic ovary syndrome (PCOS) is the leading cause of menstrual irregularities and hyperandrogenism in adolescents.1 In addition, it is the most common hormonal disorder in obesity and one of the most common causes of infertility in women.2 Although it was traditionally thought to be a problem of adulthood, it is now known that its onset takes place in childhood. It should be considered in any adolescent with hirsutism, persistent acne, dysfunctional uterine bleeding and/or obesity. The identification of each of these complaints is a challenge in adolescence. On the other hand, diagnosis of PCOS has long term implications with increased risk of infertility, metabolic syndrome, type 2 diabetes (T2DM) and cardiovascular disease.3
Increasing prevalence of obesity in adolescence has generated a growing concern with the increase of PCOS at this age. Although obesity is not a feature of the syndrome, it affects the degree of insulin resistance, worsening metabolic and reproductive characteristics.4 There is strong evidence that obesity increases severity of clinical manifestations of PCOS and the risk of metabolic dysfunction. In adolescents, as in adult women, there is a positive association between body mass index (BMI) and androgen levels. On the other hand, weight loss is associated with a decrease in testosterone.1 Increase in childhood obesity worsens the symptoms of PCOS in adolescence, although it is unlikely that it increases the prevalence of this syndrome. In other words, obesity seems to unmask young people who were otherwise asymptomatic and are now presenting with symptoms of androgen excess and anovulation.5–7
Long-term monitoring of women with PCOS revealed that 40% of those affected develop type 2 diabetes or impaired glucose tolerance at the age of 408. The risk of developing type 2 diabetes is 10 times higher in patients with PCOS, which represents a public health problem and an opportunity for early intervention.3,9,10
Definition of PCOS in adolescenceUnder the criteria of the National Institutes of Health (NIH), PCOS was defined as the presence of oligoovulation/anovulation and clinical or biochemical hyperandrogenism, not explained by other causes.11 In 2003, a new consensus between the European Society of Human Reproduction and Embryology and the American Society of Reproductive Medicine established the Rotterdam criteria, defining PCOS when at least 2 of these 3 criteria are present, after exclusion of other diseases as with similar phenotypes12:
- •
polycystic ovaries,
- •
oligo-anovulation,
- •
hyperandrogenism (clinical or biochemical).
There are no diagnostic criteria specifically defined for adolescence, when physiological and anatomical changes make this diagnosis a challenge.13
- •
First, it is difficult to distinguish between physiological anovulation often found in puberty and anovulation of PCOS. In healthy adolescents, half of the cycles are anovulatory in the first year after menarche.14
- •
Secondly, the definition of polycystic ovary in adolescence is difficult. The frequent occurrence of multiple follicles during adolescence may be misleading with the appearance of multicystic ovaries, a normal adolescent variant.15 Furthermore, the use of abdominal instead of transvaginal route in teenage virgins decreases sensitivity of ultrasound.
- •
Finally, a slight acne and hirsutism are common and there might be a transient functional hyperandrogenism during adolescence.16–18
PCOS seems to be a malfunction with ovarian overproduction of androgens in genetically determined women19–22 and the heterogeneity of clinical manifestations may be explained by interaction of the disease with environment, mainly diet.23,24
The genesis of PCOS appears to begin much earlier than in adolescence, possibly even in intrauterine life. One of the early manifestations of PCOS can be premature pubarche (appearance of pubic hair before 8 years old).4,25–27 A low weight at birth and a rapid recovery(catch-up) of weight in early life are associated with an increased risk of premature pubarche and precocious puberty. Among girls with premature pubarche, those with a history of low birth weight, even if not obese, have the greatest hyperinsulinism and a greater risk of developing a variant of PCOS with hyperinsulinemic hyperandrogenism, dyslipidemia and central obesity.28–32
The complete clinical syndrome is manifested with maturation of the hypothalamic–pituitary–ovary axis that occurs in puberty The increase of LH circulating levels, characteristic of puberty, is exaggerated in girls with predisposition for PCOS, increasing the production of androgens by the ovary.26 The presentation of PCOS in adolescence is also affected by metabolic changes associated with body fat distribution. Normal puberty and adolescence are associated with increased insulin resistance and increased fasting insulin concentrations.5,33 Women with PCOS, whether lean or obese,34,35 have higher insulin resistance than women without PCOS of similar weight. Furthermore, studies show worsening of insulin resistance with overweight and obesity associated with PCOS.36,37 The hyperinsulinism leads to suppression of hepatic production of SHBG, which amplifies the effects of steroid hormones, with higher free testosterone concentrations.5 In addition, insulin increases steroidogenic response of thecal cells to luteinizing hormone (LH),38 stimulating the production of ovarian androgens.
The main source of hyperandrogenemia in PCOS is the ovary.39 Abnormally high levels of insulin, either by genetic predisposition or by excessive weight gain, or both, will increase these potential adverse effects.40 Despite a state of insulin resistance in other tissues, ovarian steroidogenic response to insulin is maintained.41
Clinical featuresPCOS in adolescence can manifest in different forms, including hirsutism, menstrual irregularity, acanthosis nigricans and precocious pubarche and/or puberty. Moreover, persistent acne or hair loss can be the main complaints of these adolescents. In PCOS, symptoms start slowly and progressively. Sudden appearance and rapid progression of hirsutism or virilization point to other diagnosis, such as an androgen producing tumor.
ObesityTables of percentiles (P) for BMI should be used as they are the only correct way to identify obesity and overweight in this age group. Obesity is defined when BMI is above the 95th percentile and overweight when BMI is between the 85th and 95th percentiles for age and sex.42
Hyperandrogenism- •
Hirsutism
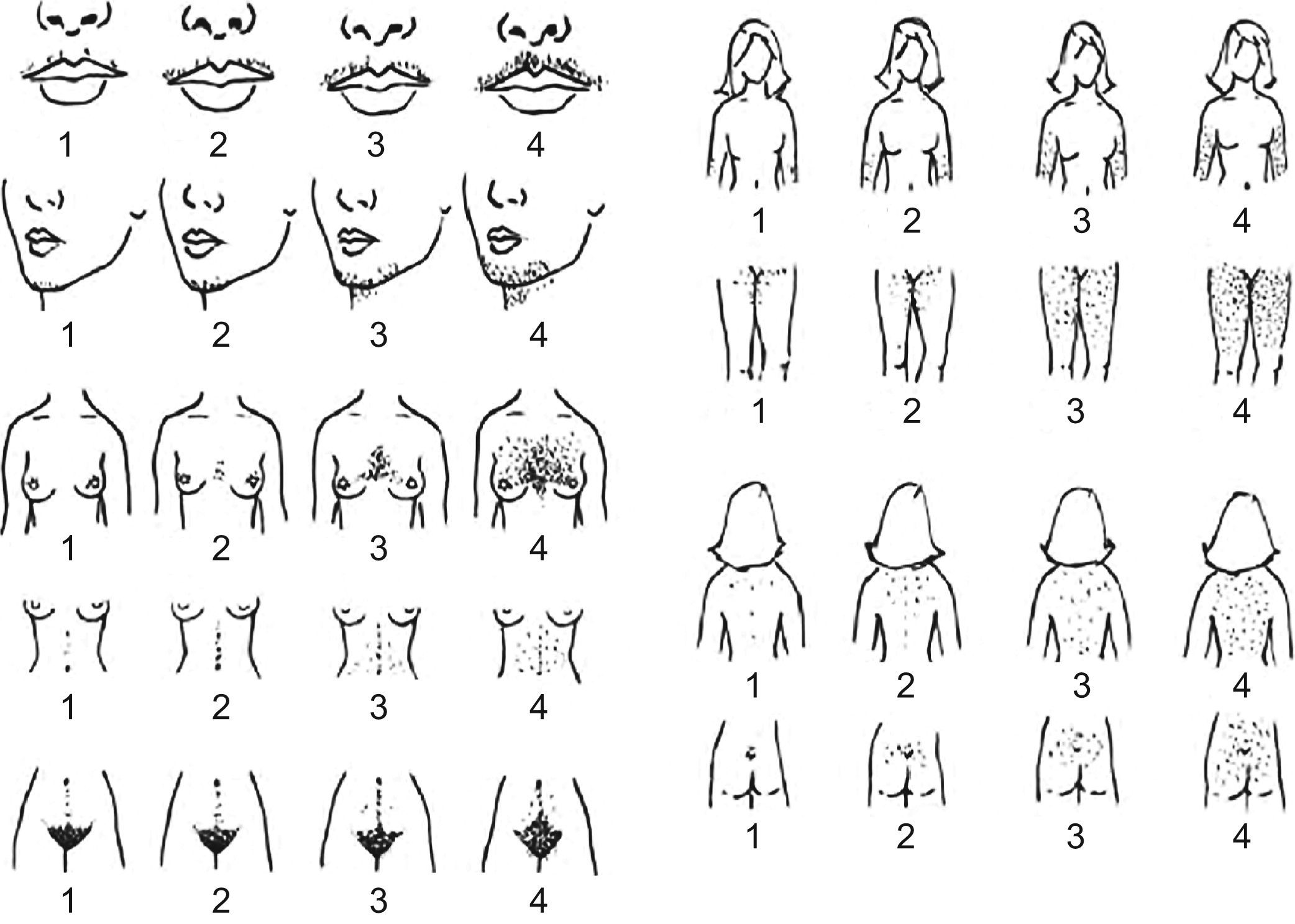
Hirsutism is defined as the presence of terminal hair that appears excessively in a male pattern in women. Terminal hair is the thick, long and pigmented hair that normally exists in the pubic and axilary areas after puberty. One must distinguish between hirsutism and hypertrichosis, a condition of excessive hair growth in a generalized distribution that can be inherited or secondary to the use of certain drugs.43 In the assessment of hirsutism it is important to know the treatments previously carried out to remove hair. Even taking into account its limitations, the Ferriman–Gallwey scale (Figure 1) is useful to confirm and monitor hirsutism. According to the Endocrine Society guidelines published in 200843 hirsutism is indicated by a Ferriman–Gallwey hirsutism score ≥8.
Figure 1.Ferriman–Gallwey hirsutism scoring system. Each of the nine body areas most sensitive to androgen is assigned a score from 0 (no hair) to 4 (frankly virile), and these separate scores are summed to provide a hormonal hirsutism score. [Reproduced from Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society Clinical Practice Guideline.43]
(0.24MB). - •
Hirsutism equivalents
Other clinical manifestations of androgen excess are acne, androgenetic alopecia, seborrhea and hidradenitis suppurativa, which are considered hirsutism equivalents. Hyperandrogenism should be considered in adolescents with inflammatory acne of early onset, or that is severe, persistent or refractory to conventional treatment.44 Hirsutism and its equivalents represent manifestations of excess androgens that are present in 2/3 of women with biochemical hyperandrogenism. Not all hirsutism is caused by hyperandrogenism. About 1/2 of women with mild hirsutism and 1/6 of those with moderate hirsutism do not have hyperandrogenism.44
PCOS is the most common cause of hyperandrogenemia. Other causes are rare.43,45 Non-classic congenital adrenal hyperplasia is present in less than 5% of hyperandrogenic women; affected patients may present with premature pubarche, adolescent or adult-onset hirsutism, symptoms of anovulation and/or family history of hirsutism or infertility. Androgen-secreting tumors are present in 0.2% of women with hyperandrogenism.43 Other causes of hyperandrogenism to consider according to the symptoms include hyperprolactinemia, Cushing's syndrome, acromegaly and thyroid dysfunction.
Menstrual cycles of adolescents, especially in the first year after menarche, may be anovulatory and irregular. However, it is a myth that they are completely chaotic. By the third year after menarche, 95% of menstrual cycles have an expected duration between 21 and 45 days and the bleeding lasts 2 to 7 days.46–49 Menstrual irregularities that remain 2 years after menarche are associated with PCOS in about 70% of cases.50 A menstrual flow greater than 80mL per cycle or a subjective impression of heavier than normal flow (more than 6 full pads or tampons per day) is considered abnormal.48 Abnormal uterine bleeding is among the most frequent complaints of adolescents.51,52 The majority of cases is related to anovulatory cycles in the first 12–18 months after menarche due to immaturity of the hypothalamic–pituitary–ovarian axis,53,54 also called dysfunctional uterine bleeding. Other causes include pregnancy, oral contraceptives and endocrine disorders (PCOS, hypothyroidism, hyperprolactinemia).
DiagnosisPCOS should be considered in any adolescent with hirsutism or their equivalents, menstrual irregularities and/or obesity. The responsibility falls mainly on family physicians, pediatricians and gynecologists to make the diagnosis as soon as possible.55
According to the Endocrine Society guidelines43 the presence of isolated mild hirsutism (score between 8 and 15), without other changes such as menstrual irregularities, should not be investigated because the probability of making a diagnosis with therapeutic implications is very low.43,45
The following cases should be investigated.43,44
- •
Moderate or severe hirsutism or hirsutism equivalents (including refractory acne or androgenic alopecia);
- •
any degree of hirsutism when it is sudden in onset, rapidly progressive or when associated with any of the following:
- -
menstrual irregularities,
- -
central obesity,
- -
acanthosis nigricans,
- -
rapid progression,
- -
clitoromegaly;
- -
- •
adolescent with menstrual irregularities with more than 2 years of evolution or with severe dysfunctional uterine bleeding;
- •
adolescent with refractory obesity, irrespective of the presence of other changes.
The determination of androgens is important in PCOS assessment. Although one can consider the presence of either clinical or biochemical hyperandrogenism, the diagnosis is best documented after biochemical confirmation of hyperandrogenism. That is, although an adolescent with menstrual irregularity and moderate hirsutism already fulfils two of the three Rotterdam criteria, it is consensual to always determine levels of androgens.55 The initial work-up may be more or less extensive and different authors advocate different approaches.16,43,55–57
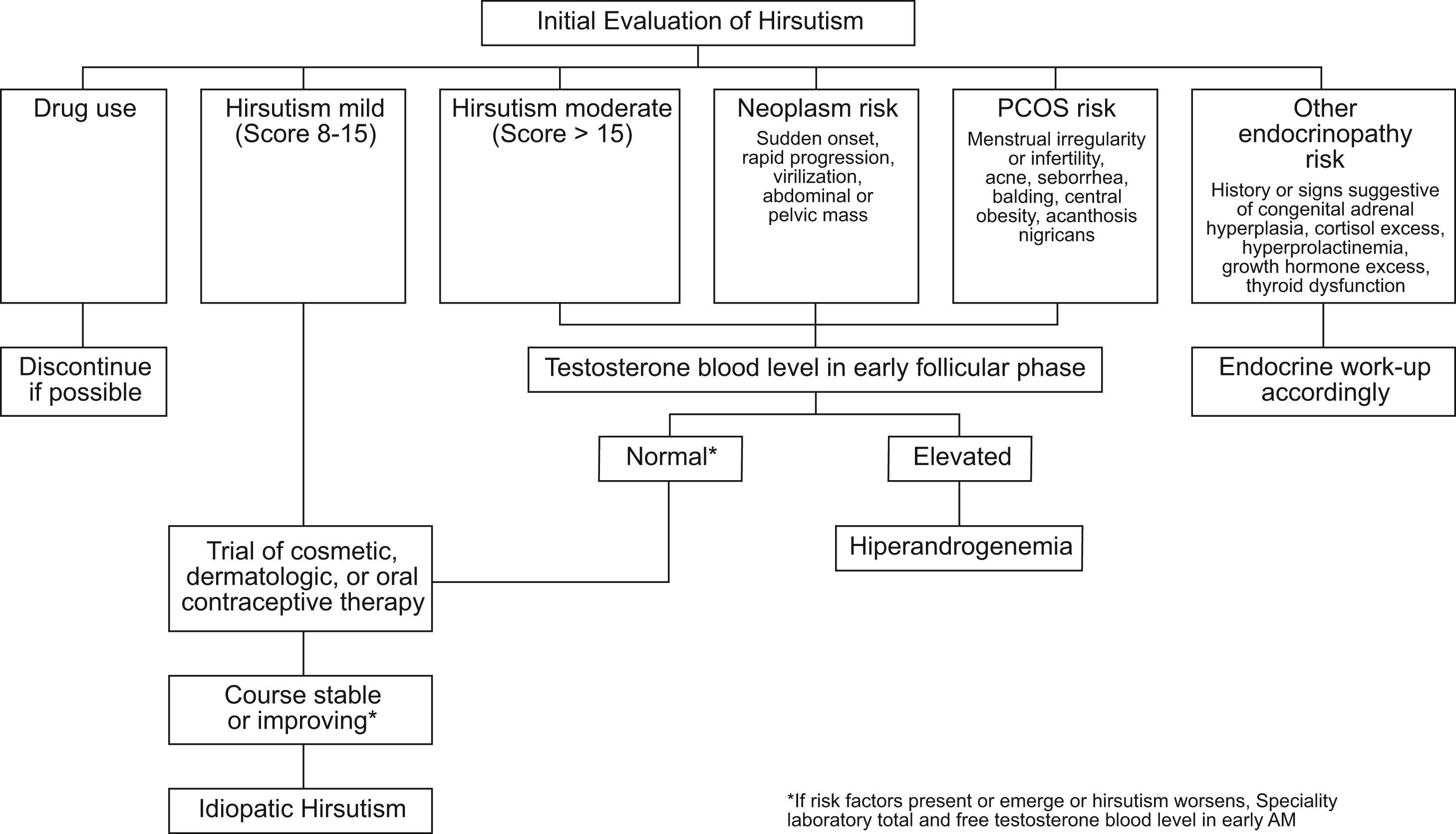
How to start the investigation of isolated hirsutism?Testosterone is the single most important androgen to measure when an investigation of isolated hirsutism is started.16 When testing for elevated androgen levels, the previously mentioned guidelines43 suggest measuring an early morning plasma total testosterone as the initial test. If the patient has symptoms of hyperandrogenism, hirsutism or equivalent, an initial approach may be the one described in Figure 2. Total testosterone should be measured in the morning between the 4th and 10th day of the menstrual cycle. If total testosterone is within the normal range in the presence of risk factors or if there is clinical worsening despite treatment, total and free testosterone should be rechecked in a reliable laboratory.
Suggested algorithm for the initial evaluation of hirsute women for hyperandrogenism. Risk assessment includes more than the degree of hirsutism. Medications that cause hirsutism include anabolic or androgenic steroids (considered in athletes and patients with endometriosis or sexual dysfunction) and valproic acid (considered in neurologic disorders). If hirsutism is moderate or severe or if mild hirsutism is accompanied by features that suggest an underlying disorder, elevated androgen levels should be ruled out. Disorders to be considered, as shown, include neoplasm and various endocrinopathies, of which polycystic ovary syndrome (PCOS) is the most common. Plasma testosterone is best assessed in the early morning, on days 4–10 of the menstrual cycle in regularly cycling women, the time for whose norms are standardized. Plasma total testosterone should be rechecked along with free testosterone in a reliable laboratory if the plasma total testosterone is normal in the presence of risk factors or progression of hirsutism on therapy. Simultaneous assay of 17-hydroxyprogesterone may be indicated in subjects at high risk for congenital adrenal hyperplasia. A small minority of women initially diagnosed with idiopathic hirsutism by this algorithm will later be found to have otherwise asymptomatic idiopathic hyperandrogenism or previously unsuspected infertility as their only non-cutaneous manifestation of PCOS. [Reproduced from Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society Clinical Practice Guideline.43].
Routine determination of dehydroepiandrosterone sulphate (DHEAS) in the initial evaluation of hyperandrogenism is of little use as it is increased in 16% of women that have normal free and total testosterone, and it is unlikely to change the approach to be taken.58 Except for very high values of DHEAS (>700ug/dL) suggesting an adrenal tumor, the predictive value of this androgen is low.
If the measurements of androgens are normal, subsequent determinations may be necessary because they sometimes become high a few years after menarche.44
If testosterone is increased, although the most likely hypothesis is PCOS, one should exclude other situations such as other endocrine diseases or pregnancy, for which several research strategies have been described.16,43,55,56,59 Typically this evaluation includes the following tests.
- •
Pregnancy test, if amenorrhoea.
- •
Pelvic ultrasound.
- •
Prolactin to exclude hyperprolactinemia. It can manifest with oligomenorrhea or amenorrhea and/or galactorrhea.
- •
DHEAS and 17-OH-progesterone (between 7 and 8 a.m.) in the follicular phase of the menstrual cycle to exclude adrenal hyperandrogenism.
- •
Assessment for Cushing's syndrome, thyroid dysfunction or growth hormone excess, if other clinical features are present:
- -
TSH if menstrual irregularities, symptoms or signs of thyroid dysfunction.16
- -
Insulin-like growth factor 1(IGF-1) if growth hormone excess is suspected.
- -
Urinary free cortisol (24-h urine) if hyperandrogenism, menstrual irregularities, violaceous striae, central obesity.
- -
However, the approach described will not identify some patients with rare disorders. It is important to follow the adolescents with PCOS, to make sure they respond to therapy as expected. If hyperandrogenism is documented but does not meet the criteria for PCOS, additional work-up will be useful. Some extremely rare cases will be clarified only with more specific tests such as dexamethasone suppression or Corticotropin (ACTH) stimulation tests, as suggested by the American Society for Reproductive Medicine.55,59
How to start the investigation of hirsutism and menstrual irregularities?When there are menstrual irregularities associated even with mild hirsutism, hyperandrogenemia is usually present.43 The initial approach to adolescents with abnormal uterine bleeding of puberty in addition to hirsutism should be more extensive from the beginning, including additional study for differential diagnosis of PCOS along with determination of testosterone. Oral contraceptives should not be prescribed before completing the investigation.
Other measurementsAlthough a lutropin (LH)/follitropin (FSH) ratio greater than 2 is often found, this is a nonspecific finding that occurs in only 30–50% of patients with PCOS.4 For confirmation of anovulation, measurement of progesterone between the 22th and 24th days of menstrual cycle can be useful.
Pelvic ultrasoundDespite the limitations previously described in these ages,15,60 in the presence of hyperandrogenemia, pelvic ultrasound is important not only for the definition of polycystic ovary syndrome but also for detecting androgens producing ovarian tumors4 and sexual development abnormalities. The ultrasound criteria for polycystic ovary are the presence of 12 or more follicles measuring 2–9mm in diameter or an ovarian volume greater than 10cm3 in at least one ovary. If a single follicle is greater than 10mm in largest diameter, the ultrasound should be repeated later to calculate again ovarian volume and area. Although the peripheral distribution of follicles in “string of pearls” and hyperechogenicity of the stroma are typical features, their descriptions are not required for this classification.61 Both hormonal work-up and ultrasound should be carried out without hormonal contraception.
Additional studyOther tests should be considered according to the clinical picture. In the presence of rapidly progressive hirsutism or signs of virilization, computed tomographic scans (CT) or magnetic resonance imaging (MRI) of adrenal glands are also essential for diagnosis of an adrenal tumor.
After the diagnosis of polycystic ovary syndromeAs women with PCOS are particularly prone to develop metabolic syndrome (MS),62-64 we must not forget to measure blood presure, waist circumference, fasting glucose and lipid profile and to perform an oral glucose tolerance test (OGTT). One study showed that 37% of adolescents with PCOS had MS as opposed to the group with similar age without PCOS in which only 5% had MS, and this association was independent of obesity.63 Studies suggest that BMI is not enough to predict which patients with PCOS will have abnormal results in oral glucose tolerance test (OGTT). Insulin resistance accompanied by compensatory hyperinsulinemia is a common finding in both lean (incidence of 35%) and obese (incidence 70%) with PCOS.4,65 Even if the fasting glucose is normal, there may be glucose intolerance. Thus, in the presence of PCOS, OGTT should be performed even in the presence of a normal fasting glucose,4,66 regardless of the presence of obesity. These are also the recommendations of the American Society for Reproductive Medicine published in late 2008.67
TreatmentThe treatment of adolescents with PCOS has focused on short-term aspects, such as self-esteem, acne and hirsutism improvement, weight control and menstrual cycles regulation. The goal of treatment is not related to infertility in this age group. It is important, however, to stress that the treatment should also take into account long-term aspects such as reducing the risk of type 2 diabetes mellitus and cardiovascular disease.4
The treatment should be individualized according to co-morbid conditions (e.g. obesity) and the goals to be achieved. Given the strong evidence that hyperinsulinemia plays a key role in the pathogenesis of PCOS, targeted interventions aiming changes in lifestyle are essential. Weight reduction with dietary interventions is effective in reducing androgens levels and in regulating menstrual cycles.4 Reduction of testosterone concentrations and regulation of menstrual cycles are associated with losses of about 5–7% of body weight.68–70 Above all, changes in lifestyle are the cornerstone of treatment to reduce the risk of type 2 diabetes mellitus and cardiovascular disease.
Medical therapyOral contraceptivesFor most pediatric endocrinologists, oral contraceptives are considered first-line drugs in the treatment of adolescents with PCOS.1
The menstrual irregularities should be treated, as chronic anovulation increases the risk of endometrial hyperplasia, which is associated with endometrial carcinoma. Moreover, in some cases, dysfunctional bleeding or menorrhagia can lead to anemia. Oral contraceptives are the treatment of choice for control of menstrual irregularities in PCOS, particularly if signs of hyperandrogenism are present. The estrogenic component suppresses LH and thus production of androgens by ovaries and increases production of SHBG, resulting in lower concentrations of free testosterone. Estroprogestatives also inhibits 5-alpha-reductase in the skin, lowering levels of dihydrotestosterone.7,71 In most of the adolescents with mild elevation of androgens and a good clinical response to estroprogestatives it is not necessary to repeat androgen measurements. In those with poor clinical response or high androgen levels these can be repeated to assess the degree of response.16 There is a normalization of androgen levels in 18–21 days of treatment in most cases.72
Estroprogestatives can be used with 30–35μg or lower doses of 20μg of ethinyl estradiol for improvement of hyperandrogenism.43 Both preparations appear to be similar in terms of effectiveness at least regarding the improvement of acne.
Anti-androgensAnti-androgens are used to control the growth of terminal hair and, because of its teratogenic potential, they are generally associated with oral contraceptives. When hirsutism remains despite oral contraceptive therapy for at least 6 months, an anti-androgen should be added.43 It may take 9–18 months of treatment to check effects due to the long cycle of growth of terminal hair.73 Cyproterone acetate is a potent anti-androgen and is available in combination with ethinyl estradiol.
Spironolactone is associated with a subjective improvement of hirsutism and also with a decrease in Ferriman–Gallwey score scale.74 It is generally administered at a dose of 50mg bid, although some authors initially use 100mg bid for a greater effect.16
Flutamide is an anti-androgen with similar efficacy as that of cyproterone acetate and spironolactone. However, the risk of liver toxicity has limited its use, and it is even discouraged by the Endocrine Society in its recommendations for treatment of hirsutism.43 Finasteride is a 5 alpha reductase type 2 inhibitor, which seems to be less effective than spironolactone,74 with 30–60% reduction in hirsutism scores. There is only limited information about the use of flutamide and finasteride in adolescence4.
Metformin in adolescents with polycystic ovary syndromeOral contraceptives do not address and may even worsen metabolic complications of PCOS, although these data are controversial.1,10,65 Although the use of estroprogestatives, with or without anti-androgens, is effective in symptoms control, it seems prudent and appropriate to consider a long-term strategy for reducing type 2 diabetes and cardiovascular disease risks. Metformin is the most commonly used drug in the treatment of type 2 diabetes.10 It inhibits hepatic glucose production and improves sensitivity of peripheral tissues to insulin. It also decreases appetite and promotes weight loss.
Studies such as the Diabetes Prevention Program (DPP) demonstrated that the use of metformin in patients with abnormalities of glucose tolerance prevents development of type 2 diabetes.75
Although there are no studies demonstrating effectiveness of metformin in preventing type 2 diabetes (T2DM) in patients with PCOS in particular, several studies have shown an improvement in metabolic parameters.10,76,77 Studies show that metformin improves insulin levels, reduces androgens and regulates menstrual cycles in patients with PCOS, not only in adulthood.10,78 but also in adolescence.79
Metformin may be beneficial in some children even before menarche. Studies in children with premature pubarche and a history of low birth weight demonstrated the efficacy of metformin in delaying menarche.80 Some studies have shown the effectiveness of metformin in preventing PCOS progression for non-obese individuals with a history of low birth weight and premature pubarche.25,81 Improved metabolic profile disappeared after discontinuation of therapy in these adolescents.25 In a study of obese adolescents with PCOS, metformin has been effective in improving menstrual cycles, decreasing androgens, improving hirsutism and acne, and in weight reduction, and these effects persisted 6 months after discontinuation.82 In adult non-obese with PCOS, there was an improvement in insulin levels and regulation of menstrual cycles with the use of metformin78. However, studies specifically targeted for hyperandrogenism manifestations (hirsutism, acne and hair loss) are limited, and the benefit in controlling these symptoms has not been documented in meta-analysis and recent reviews.43,67,83 Nevertheless, metformin has been used in adult women with PCOS.10 It is usually used in a dose of 1500–2000mg daily, starting with 500mg at dinner4 with gradual dose titration.
According to the recommendations of the American Society for Reproductive Medicine67:
- •
adolescents with PCOS should perform an OGTT every 2 years for screening glucose tolerance abnormality;
- •
metformin may be considered in patients with PCOS and abnormal glucose tolerance;
- •
we do not have enough long-term data in order to recommend metformin as first-line therapy in PCOS when the sole purpose is to prevent long-term complications such as T2DM or cardiovascular disease; in the meanwhile, lifestyle changes should take that place;
- •
the first-line therapy for ovulation induction in patients with PCOS who wish to become pregnant is clomiphene citrate, and combination with metformin can be useful in refractory cases.
Other insulin sensitizers drugs (glitazones) have been tested in adult women with PCOS, but have not been studied in adolescence. In any case, their side effects make them unlikely to replace metformin.1
Cosmetic treatmentsPatients often want to be counselled about permanent hair removal. Techniques commonly referred to as “permanent hair removal” are the hair removal laser/pulsed light and electrolysis.43 Topical eflornithine,84,85 which slows hair growth, is approved by the Food and Drug Administration (FDA) for reduction of unwanted facial hair in women.
Topical treatments for acne can be used in combination with oral contraceptives. Topical retinoids are indicated in all types of acne. Topical antibiotics such as benzoyl peroxide have been helpful in mild to moderate inflammatory acne. Oral isotretinoin is associated with several side effects and has a high teratogenic potential that remains at 6 weeks after completion of treatment; it is reserved for severe cases.
ConclusionPCOS is a common disease among adolescents and has been increasingly associated with metabolic and cardiovascular complications in the long term, going beyond cosmetic and fertility concerns. The prevalence of obesity is increasing, thereby aggravating the symptoms of these adolescents. The control of obesity and overweight is essential and should begin as early as possible PCOS may remain unnoticed because symptoms such as menstrual irregularities and acne are relatively common, with an overlap between symptoms of the syndrome and physiological changes of puberty. Changes in lifestyle are the first-line therapy. Depending on symptoms and presence of glucose intolerance, therapeutic weapons include oral contraceptives, anti-androgens and metformin, but more studies are needed to clarify the best strategy in the prevention of metabolic complications in the long term.
Conflicts of interestsThe authors have no conflict of interest to declare.




![Ferriman–Gallwey hirsutism scoring system. Each of the nine body areas most sensitive to androgen is assigned a score from 0 (no hair) to 4 (frankly virile), and these separate scores are summed to provide a hormonal hirsutism score. [Reproduced from Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society Clinical Practice Guideline.43] Ferriman–Gallwey hirsutism scoring system. Each of the nine body areas most sensitive to androgen is assigned a score from 0 (no hair) to 4 (frankly virile), and these separate scores are summed to provide a hormonal hirsutism score. [Reproduced from Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society Clinical Practice Guideline.43]](https://static.elsevier.es/multimedia/15750922/0000005700000007/v1_201305082226/S1575092210001087/v1_201305082226/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Suggested algorithm for the initial evaluation of hirsute women for hyperandrogenism. Risk assessment includes more than the degree of hirsutism. Medications that cause hirsutism include anabolic or androgenic steroids (considered in athletes and patients with endometriosis or sexual dysfunction) and valproic acid (considered in neurologic disorders). If hirsutism is moderate or severe or if mild hirsutism is accompanied by features that suggest an underlying disorder, elevated androgen levels should be ruled out. Disorders to be considered, as shown, include neoplasm and various endocrinopathies, of which polycystic ovary syndrome (PCOS) is the most common. Plasma testosterone is best assessed in the early morning, on days 4–10 of the menstrual cycle in regularly cycling women, the time for whose norms are standardized. Plasma total testosterone should be rechecked along with free testosterone in a reliable laboratory if the plasma total testosterone is normal in the presence of risk factors or progression of hirsutism on therapy. Simultaneous assay of 17-hydroxyprogesterone may be indicated in subjects at high risk for congenital adrenal hyperplasia. A small minority of women initially diagnosed with idiopathic hirsutism by this algorithm will later be found to have otherwise asymptomatic idiopathic hyperandrogenism or previously unsuspected infertility as their only non-cutaneous manifestation of PCOS. [Reproduced from Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society Clinical Practice Guideline.43]. Suggested algorithm for the initial evaluation of hirsute women for hyperandrogenism. Risk assessment includes more than the degree of hirsutism. Medications that cause hirsutism include anabolic or androgenic steroids (considered in athletes and patients with endometriosis or sexual dysfunction) and valproic acid (considered in neurologic disorders). If hirsutism is moderate or severe or if mild hirsutism is accompanied by features that suggest an underlying disorder, elevated androgen levels should be ruled out. Disorders to be considered, as shown, include neoplasm and various endocrinopathies, of which polycystic ovary syndrome (PCOS) is the most common. Plasma testosterone is best assessed in the early morning, on days 4–10 of the menstrual cycle in regularly cycling women, the time for whose norms are standardized. Plasma total testosterone should be rechecked along with free testosterone in a reliable laboratory if the plasma total testosterone is normal in the presence of risk factors or progression of hirsutism on therapy. Simultaneous assay of 17-hydroxyprogesterone may be indicated in subjects at high risk for congenital adrenal hyperplasia. A small minority of women initially diagnosed with idiopathic hirsutism by this algorithm will later be found to have otherwise asymptomatic idiopathic hyperandrogenism or previously unsuspected infertility as their only non-cutaneous manifestation of PCOS. [Reproduced from Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society Clinical Practice Guideline.43].](https://static.elsevier.es/multimedia/15750922/0000005700000007/v1_201305082226/S1575092210001087/v1_201305082226/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
