Type one diabetes mellitus (T1D) is often associated with autoimmune diseases, but pregnancies complicated by Addison's disease (AD) are rarely observed1 and glycemic control can be deteriorated leading to an increased rate of hyper- and hypoglycaemia. Even though there is little evidence of its use in pregnancy, combined treatment with continuous subcutaneous insulin infusion (CSII) and real-time continuous glucose monitoring (CGM) (SAP, sensor augmented pump) may help to improve glycemic control.
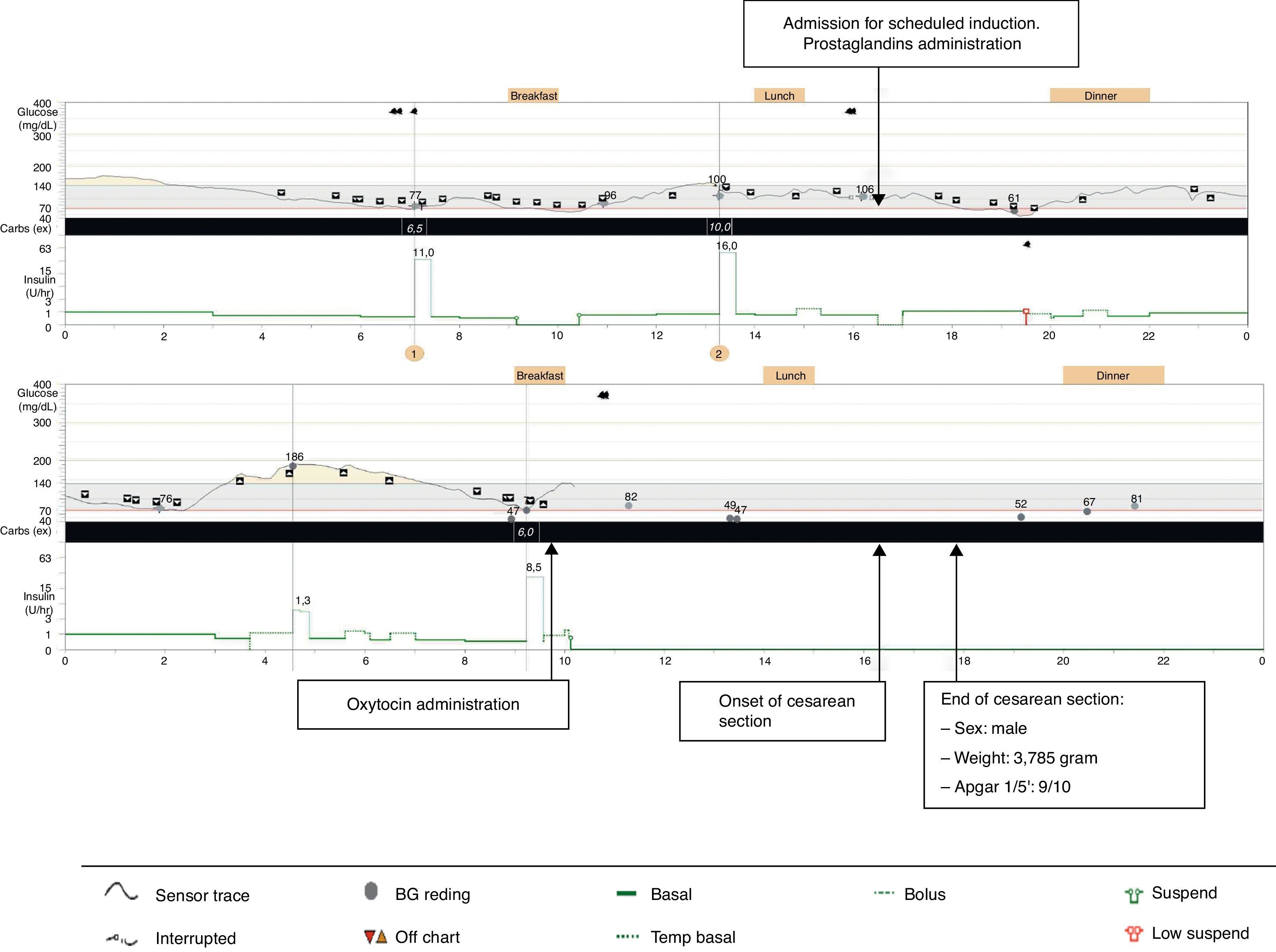
We report the case of a 34-year-old woman with AD and T1D, who was treated with SAP therapy (Medtronic® Paradigm Veo-754 system; including low-glucose-suspend (LGS) capability) because of labile glycemic profile and repeated episodes of non-severe (NS) and severe hypoglycaemia (SH). She was closely followed during the pregestational and gestational periods by tele-assistance using web-based diabetes management software (Carelink-Pro for Diabetes – Clinical, Medtronic). In her first visit at prepregnancy control, she had a glycated hemoglobin (A1c) of 7.7% with 2% of the readings below 70mg/dl. To achieve pregnancy objectives, blood glucose targets were strengthened and modifications of insulin treatment patterns were needed. She became pregnant with an A1c of 7.1% and 4% of the sensor readings below 70mg/dl. She was started on a multidisciplinary follow-up by an obstetrician and an endocrinologist with physical visits every fortnight and telemedical visits the remaining weeks. Throughout the first trimester of gestation, she complained of nausea, vomiting and orthostatic hypotension which were attributed to adrenal insufficiency, resulting in hydrocortisone (HC) dose increase of up to 50mg/day. Her glycemic profile then deteriorated and as a result of the greater use of correcting boluses and temporary basal rates, the rate of hypoglycaemia increased. Consequently, LGS duration and manual basal suspend function were incremented. After 13 weeks’ gestation, symptoms improved, edemas occurred and limit values of blood pressure were found. Therefore HC doses were progressively reduced to 30mg/day, which corresponds to an increase of 20% compared to the initial dose. Ionogram and blood pressure were monitored throughout gestation. The glycemic control during the follow-up is summarized in Table 1. Mean sensor use was 5 days per week during the follow-up and the number of blood glucose readings fluctuated between 6.1 at the beginning of pregnancy to 10.4 at the end. Non-SH occurred. A 3,785g boy was born by cesarean section (CS) because of stationary delivery at 38 weeks’ gestation. She used her SAP system until administration of oxytocin, when she was transferred to an endovenous insulin pump. Maternal and perinatal morbidity were avoided, except for mild neonatal jaundice. The CGM trace during stress of labor is shown in Fig. 1. When breastfeeding was initiated insulin doses were reduced by half, but insulin requirements continued to fall during the following weeks. One month after delivery, there was a recovery of usual insulin doses prior to gestation (Table 1).
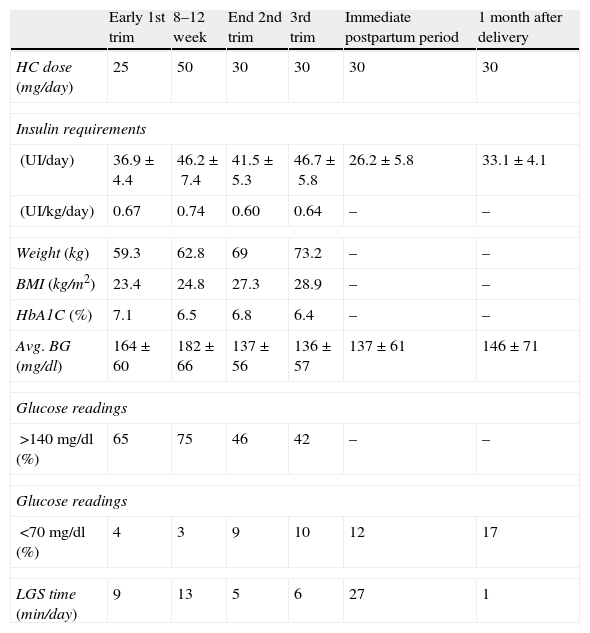
Follow-up during gestation and up-to one month after delivery.
| Early 1st trim | 8–12 week | End 2nd trim | 3rd trim | Immediate postpartum period | 1 month after delivery | |
| HC dose (mg/day) | 25 | 50 | 30 | 30 | 30 | 30 |
| Insulin requirements | ||||||
| (UI/day) | 36.9±4.4 | 46.2±7.4 | 41.5±5.3 | 46.7±5.8 | 26.2±5.8 | 33.1±4.1 |
| (UI/kg/day) | 0.67 | 0.74 | 0.60 | 0.64 | – | – |
| Weight (kg) | 59.3 | 62.8 | 69 | 73.2 | – | – |
| BMI (kg/m2) | 23.4 | 24.8 | 27.3 | 28.9 | – | – |
| HbA1C (%) | 7.1 | 6.5 | 6.8 | 6.4 | – | – |
| Avg. BG (mg/dl) | 164±60 | 182±66 | 137±56 | 136±57 | 137±61 | 146±71 |
| Glucose readings | ||||||
| >140mg/dl (%) | 65 | 75 | 46 | 42 | – | – |
| Glucose readings | ||||||
| <70mg/dl (%) | 4 | 3 | 9 | 10 | 12 | 17 |
| LGS time (min/day) | 9 | 13 | 5 | 6 | 27 | 1 |
HC: hydrocortisone; BMI: body mass index; Avg. BG: blood glucose average; LGS: low-glucose-suspend; trim: trimester.
Regarding our case report, we add information concerning the usefulness of SAP therapy in the management of pregnancies complicated by T1D and AD. T1D in pregnancy increases the risk of adverse outcomes for mother and offspring. Obtaining and maintaining tight glycemic control are crucial for optimizing outcomes. However, the risk of NS and SH during pregnancy is a major obstacle. Patients with T1D and AD have difficulty in achieving good glycemic control, especially if the glucocorticoid doses are modified. They are prone to the development of hyper- and hypoglycaemia with its attendant morbidity and as such, this population requires even more meticulous attention to hypoglycaemia and its prevention. In addition, women with AD have an increased risk of CS and preterm deliveries, although if correctly treated, the majority have normal pregnancy outcomes. Therefore, the management of T1D complicated by AD during pregnancy could be considered particularly troublesome.
In 2005, Dolci et al. reported a case of a woman with T1D and AD who achieved tighter glycemic control with multiple doses of insulin, although HC doses were unchanged throughout gestation.1 Intensive insulin therapy still represents the goal standard in the treatment of T1D subjects during pregnancy, because there is a lack of evidence demonstrating that the use of CSII is associated with better maternal and perinatal outcomes.2 However, when glycemic targets are not achieved, CSII is indicated. Regarding our patient with T1D and AD, a history of labile glycemic profile and repeated episodes of NS and SH, the additional use of SAP helped us to improve glycemic control during pregnancy.
Real-time CGM provides sensor glucose levels that can be used for immediate and retrospective adjustments of treatment regimens. Retrospective analysis of CGM could be especially useful before and during pregnancy for detecting not only unnoticed hypoglycaemia, but also postprandial hyperglycaemia, and for reducing the risk of macrosomia.3 Although there are few studies reviewing the role of real-time CGM during gestation, it is considered to be helpful in achieving and maintaining good glycemic control without worsening the quality of life,4,5 especially when the use is not intermittent.6 With respect to our patient, we confirmed the difficulties in achieving an A1c <6.5% without hypoglycaemia, although finally we almost succeeded with a reasonable time spent in glucose values < 70mg/dl and without SH. Our patient received specific instructions on how to use the data from CGM in order to modify treatment patterns, resulting in more frequent insulin bolus administration, and more frequent usage of temporary basal rates, manual basal suspend function and LGS suspend function. Moreover, as she continued using the SAP therapy during and after delivery, we could follow-up insulin requirements also during breastfeeding. To our knowledge, no previous reports of CGM during lactation in T1D women have been published, although it is known that a reduction in total daily insulin is caused by increased glucose consumption during breastfeeding.7
In addition, telemedicine assistance using web-based diabetes management software helped us in the approach of this patient. We could improve insulin treatment without increasing the number of physical visits. A recent review of the literature suggested that telematic intensive care system improved the effectiveness of diabetes treatment during pregnancy, reducing patient visits and improving quality of life, without jeopardizing the outcome.8,9
In conclusion, the use of SAP therapy and telemedicine management in addition to an interdisciplinary approach may be useful in achieving an adequate glycemic control, especially avoiding hypoglycaemia, and successful outcomes in pregnancies complicated by labile T1D. However, limitations of its use are in relation to the price and make this treatment not available to all patients who might benefit.
Funding sourcesNo.
Conflict of interestNone.