The standard treatment of hypothyroidism (central and primary) consists of thyroxine (T4) administration alone. However, the normal thyroid gland produces a small proportion of triiodothyronine (T3) directly into the circulation.
AimWe aimed to study the free T3 to free T4 ratio in treated central hypothyroidism compared with euthyroidism and treated primary hypothyroidism.
MethodsEighty-three subjects were included in this cross-sectional study: 36 with central hypothyroidism, 20 with primary hypothyroidism and 27 healthy controls. A clinical history and a physical examination, including height and weight measurement, were performed and body mass index (BMI) was calculated. Fasting blood was drawn to measure T3, T4, free T3, free T4 and TSH.
ResultsThe free T3 to free T4 ratio was lower in treated central hypothyroidism than in euthyroidism but was similar to treated primary hypothyroidism. Free T4 was higher in treated central and primary hypothyroidism than in euthyroidism. Age, sex and BMI did not affect the free T3 to free T4 ratio.
ConclusionsTreated patients with central hypothyroidism had a lower free T3 to free T4 ratio, similar free T3 levels and higher free T4 concentrations than euthyroid controls, whereas all these parameters were similar in central and primary hypothyroid patients treated with T4. The question of whether these findings translate into adequate tissue concentrations of free thyroid hormones in all tissues remains to be answered. Further studies should aim to determine whether clinical outcomes could be improved by a treatment achieving more physiological plasma concentrations.
El tratamiento habitual del hipotiroidismo (central y primario) consiste en administrar sólo tiroxina (T4). Sin embargo, la glándula tiroides normal produce una proporción pequeña de triyodotironina (T3) que va directamente a la circulación.
ObjetivoEstudiar la razón entre las concentraciones de T3 /T4 circulantes en el hipotiroidismo central tratado respecto al eutiroidismo y al hipotiroidismo primario también tratado.
MétodosSe incluyeron 83 sujetos en este estudio transversal: 36 presentaban hipotiroidismo central, 20 hipotiroidismo primario y 27 eran controles sanos. Se realizó una historia clínica y una exploración física que incluía la altura y el peso, y se calculó el índice de masa corporal (IMC). Se extrajo sangre en ayunas para medir T3, T4, T3 libre, T4 libre y TSH.
ResultadosLa razón T3/T4 circulantes fue inferior en el hipotiroidismo central que en el eutiroidismo, pero similar a la del hipotiroidismo primario. La T4 libre fue mayor en el hipotiroidismo central y en el primario que en el eutiroidismo. La edad, el sexo y el IMC no afectaron la razón T3 /T4 circulante.
ConclusionesLos pacientes con hipotiroidismo central tratados presentan una razón T3/T4 circulante más baja, niveles de T3 circulante similares y concentraciones de T4 libre superiores a los controles eutiroideos; sin embargo, todos estos parámetros son similares en los pacientes con hipotiroidismo central y primario tratados con T4. No se sabe si esto se traduce en concentraciones tisulares adecuadas de hormonas tiroideas libres en todos los tejidos. Queda por investigar si un tratamiento que obtenga una concentración plasmática más fisiológica sería mejor desde el punto de vista de los resultados clínicos. Es de esperar que se diseñen estudios en esa dirección.
Triiodothyronine (T3) is the active thyroid hormone. Plasma T3 is generated by direct thyroid production (about 20%) and from peripheral conversion of thyroxine (T4) to T3, mainly in liver and kidneys (80%).1-4 The T3 found in the cell nucleus in distinct tissues is derived in a different proportion either from the plasma pool or from local T4 deiodination within the tissue.4
Patients with primary hypothyroidism correctly treated with levothyroxine (LT4) [based on normal thyroid-stimulating hormone (TSH) levels] are known to have a lower plasma T3 to T4 ratio than euthyroid individuals.5,6
Central hypothyroidism is usually part of a complex hormonal dysfunction. This disorder is rarely found as an isolated deficiency and is frequently combined with other pituitary deficiencies. Therefore, in addition to the clinical consequences of thyroid hormone deficiency and replacement, other hormone deficiencies and their treatments come into play. Cortisol and growth hormone (GH) play a role in T4 to T3 conversion;7-9 non-replacement or suboptimal replacement of these hormones affect T4 deiodination10 and may alter the plasma free T3 to free T4 ratio. In addition, TSH levels are not useful to adjust the LT4 dose in central hypothyroidism and consequently a potential T3 deficiency will be more difficult to detect.11
Whether the free T3 to free T4 ratio in patients with stable treated central hypothyroidism is similar to that of euthyroid individuals and to that of primary hypothyroid patients treated with T4 is unknown. Determining this issue would be the first step to evaluate the adequacy of LT4 alone in the treatment of central hypothyroidism and to define optimal thyroid replacement therapy for this disorder.
We aimed to study serum free thyroid hormone concentrations in patients with treated central hypothyroidism in comparison with patients with treated primary hypothyroidism and euthyroid controls.
MethodsPatientsA total of 83 subjects participated in this cross–sectional study. Thirty-six patients with central hypothyroidism who were followed-up in our tertiary referral center were recruited. Patients were included if they were receiving stable treatment for thyroid and other pituitary deficiencies for at least 3 months prior to participating in the study and pituitary disease was inactive. Adequate central thyroid replacement was defined as free T4 levels within the normal range. Twenty patients with primary hypothyroidism were also included. These patients had to show normal TSH levels and have been receiving a stable LT4 dose for at least 3 months prior to entering the study. Twenty-seven control subjects were recruited from the patients’ relatives and hospital staff and their relatives. Exclusion criteria for patients and controls consisted of a serious concomitant medical disease, glucocorticoid treatment for reasons other than replacement therapy, and treatment with antiepileptic drugs.
StudyPatients with either central or primary hypothyroidism attending outpatient consultations who met the inclusion criteria were asked to participate in the study. A clinical history and a physical examination that included height and weight measurement were performed and body mass index (BMI) was calculated. Fasting blood was drawn to measure total T3 and T4, free T3 and free T4. TSH was measured only in patients with primary hypothyroidism and in controls. Premenopausal women in the patient and control groups were studied in the early follicular phase. Sera were collected and stored at –20°C for thyroid hormone measurement in the same batch. The study was approved by the Ethics Committee of Hospital Clinic in Barcelona and participants gave informed consent.
Biochemical assaysFree T4, Free T3, total T4, total T3 and TSH measurements.
Thyroid hormones were determined using the ADVIA CENTAUR (Bayer, NY, USA) with an automated direct chemiluminescence immunoassay. TSH was measured with a third-generation assay with a detection limit of 0.019 mIU/L. Interassay coefficients of variation were: 5.73 to 7.3% for free T4, 4.9 to 6.7% for free T3 and 5.4 to 6.1% for TSH.
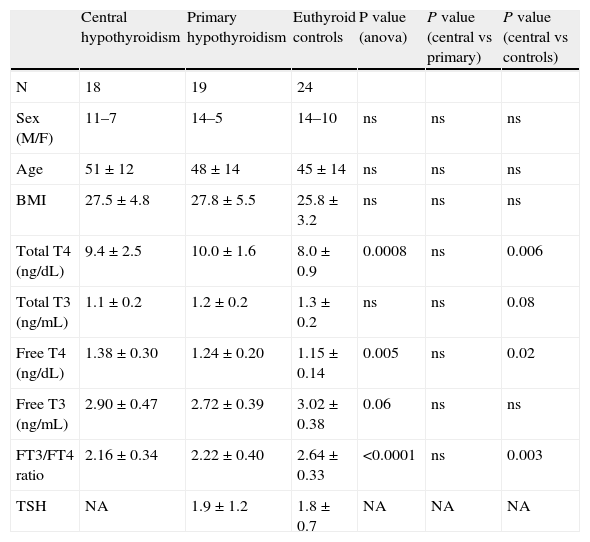
Statistical analysisANOVA was used to compare the three groups: patients with central hypothyroidism were compared with patients with primary hypothyroidism and healthy controls, while controlling for potential confounding covariates. Student's T-test was used to compare groups two-by-two. Spearman's correlation coefficients between outcome variables and age, sex and BMI were studied. As an additional check, since the three groups were not matched for all clinical variables, a matched subgroup was defined according to age, sex and BMI, which required random removal of the 17 oldest men and the most obese woman in the central hypothyroidism group, the three youngest women in the control group and the youngest woman in the primary hypothyroidism group. ANOVA and T-tests were performed to compare the results between groups in this subset. Two sided p≤0.05 was considered significant. All analyses were performed using JMP 3.2.2 (SAS Institute, Inc., Cary, NC) and STATA 9.2.
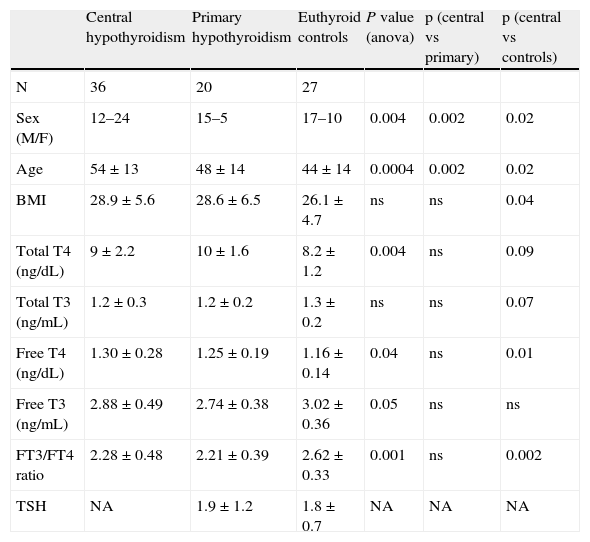
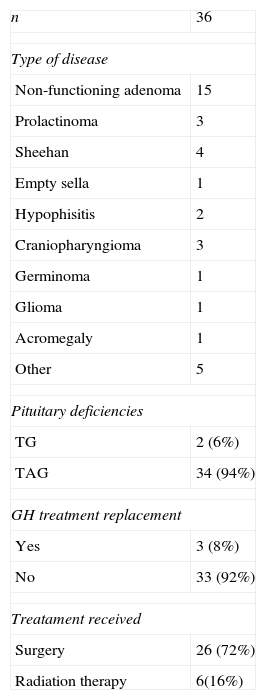
ResultsA total of 83 subjects participated in the study: 36 patients with central hypothyroidism, 20 with primary hypothyroidism and 27 controls (Table 1). Characteristics of pituitary disease are summarized in Table 2. In most patients (53%), central hypothyroidism was caused by a pituitary adenoma. All but two patients with central hypothyroidism (94%) had concomitant adrenal and gonadal deficiencies. Two patients had only gonadal deficiency associated with hypothyroidism. Growth hormone (GH) deficiency had been evaluated and diagnosed in 29 patients (86%) and diabetes insipidus was present in 12 patients (36%). All patients with hypopituitarism were treated with conventional replacement therapy, but only three patients were treated with GH. All participants were receiving the standard of care at our institution. For central hypothyroidism all patients were treated with levothyroxine alone, with a median dose of 100μg/day (mean±SD=115±42.7). Adrenal replacement consisted of hydrocortisone in all but two patients who were receiving prednisone. In most patients (60%), the hydrocortisone dose was 20mg/day, in distinct dose fractionation regimens; two patients were receiving 15mg/day, and another was receiving 30mg/day. Five premenopausal women (age<50 years) with hypopituitarism were receiving estrogen therapy and four women in the control group were taking oral contraceptives. None of the premenopausal women with primary hypothyroidism was receiving estrogen.
Characteristics of patients in the study
| Central hypothyroidism | Primary hypothyroidism | Euthyroid controls | P value (anova) | p (central vs primary) | p (central vs controls) | |
| N | 36 | 20 | 27 | |||
| Sex (M/F) | 12–24 | 15–5 | 17–10 | 0.004 | 0.002 | 0.02 |
| Age | 54±13 | 48±14 | 44±14 | 0.0004 | 0.002 | 0.02 |
| BMI | 28.9±5.6 | 28.6±6.5 | 26.1±4.7 | ns | ns | 0.04 |
| Total T4 (ng/dL) | 9±2.2 | 10±1.6 | 8.2±1.2 | 0.004 | ns | 0.09 |
| Total T3 (ng/mL) | 1.2±0.3 | 1.2±0.2 | 1.3±0.2 | ns | ns | 0.07 |
| Free T4 (ng/dL) | 1.30±0.28 | 1.25±0.19 | 1.16±0.14 | 0.04 | ns | 0.01 |
| Free T3 (ng/mL) | 2.88±0.49 | 2.74±0.38 | 3.02±0.36 | 0.05 | ns | ns |
| FT3/FT4 ratio | 2.28±0.48 | 2.21±0.39 | 2.62±0.33 | 0.001 | ns | 0.002 |
| TSH | NA | 1.9±1.2 | 1.8±0.7 | NA | NA | NA |
NA: non assessed, ns=non-significant.
Characteristic of pituitary disease
| n | 36 |
| Type of disease | |
| Non-functioning adenoma | 15 |
| Prolactinoma | 3 |
| Sheehan | 4 |
| Empty sella | 1 |
| Hypophisitis | 2 |
| Craniopharyngioma | 3 |
| Germinoma | 1 |
| Glioma | 1 |
| Acromegaly | 1 |
| Other | 5 |
| Pituitary deficiencies | |
| TG | 2 (6%) |
| TAG | 34 (94%) |
| GH treatment replacement | |
| Yes | 3 (8%) |
| No | 33 (92%) |
| Treatament received | |
| Surgery | 26 (72%) |
| Radiation therapy | 6(16%) |
GH: growth hormone; TAG: thyroid + adrenal+gonadal; TG: thyroid+gonadal.
The clinical and biochemical characteristics of patients with central hypothyroidism, primary hypothyroidism and controls are shown in Table 2. Patients with central hypothyroidism were older than patients with primary hypothyroidism and healthy controls and had higher BMIs than controls. There were more males in the central hypothyroidism group.
Free T3, Free T4 and free T3/ freeT4 ratioPatients with central hypothyroidism had a lower free T3 to free T4 ratio than healthy controls (2.28±0.48 vs 2.62±0.33, p=0.002), both in the analysis of the whole group and in the analysis of the matched subset (2.16±0.34 vs 2.64±0.33. p=0.003) (Tables 2 and 3). No differences were found in the free T3 to free T4 ratio between patients with central and primary hypothyroidism either in the analysis of the whole group (2.28±0.48 vs 2.21±0.39, p=0.6) or in the matched subset (2.16±0.34 vs 2.22±0.40, p=0.6) (Table 3).
Characteristics of the matched subset
| Central hypothyroidism | Primary hypothyroidism | Euthyroid controls | P value (anova) | P value (central vs primary) | P value (central vs controls) | |
| N | 18 | 19 | 24 | |||
| Sex (M/F) | 11–7 | 14–5 | 14–10 | ns | ns | ns |
| Age | 51±12 | 48±14 | 45±14 | ns | ns | ns |
| BMI | 27.5±4.8 | 27.8±5.5 | 25.8±3.2 | ns | ns | ns |
| Total T4 (ng/dL) | 9.4±2.5 | 10.0±1.6 | 8.0±0.9 | 0.0008 | ns | 0.006 |
| Total T3 (ng/mL) | 1.1±0.2 | 1.2±0.2 | 1.3±0.2 | ns | ns | 0.08 |
| Free T4 (ng/dL) | 1.38±0.30 | 1.24±0.20 | 1.15±0.14 | 0.005 | ns | 0.02 |
| Free T3 (ng/mL) | 2.90±0.47 | 2.72±0.39 | 3.02±0.38 | 0.06 | ns | ns |
| FT3/FT4 ratio | 2.16±0.34 | 2.22±0.40 | 2.64±0.33 | <0.0001 | ns | 0.003 |
| TSH | NA | 1.9±1.2 | 1.8±0.7 | NA | NA | NA |
NA: non-assessed, ns=non-significant.
Free T4 levels were higher in patients with central hypothyroidism than in euthyroid persons (1.30±0.28 vs 1.16±0.14, p=0.01) but were similar to those of patients with primary hypothyroidism (1.30±0.28 vs 1.25±0.19, p=0.4).
Free T3 levels were similar among the three groups: 2.88±0.49 vs 2.74±0.38 vs. 3.02±0.36 (p=0.2) for patients with central hypothyroidism, those with primary hypothyroidism and euthyroid controls, respectively. In the two-by-two comparison, free T3 levels were similar between the central and primary hypothyroidism groups and between the euthyroid and central hypothyroidism groups (Table 2). However, patients with primary hypothyroidism had lower free T3 than euthyroid controls (2.74±0.38 vs. 3.02±0.36, p=0.05).
The results of free T4 and free T3 did not change according to whether the whole patient group (Table 2) or the matched subset (Table 3) was considered.
Correlations between free T3, free T4, free T3/freeT4 and age, sex and BMI were studied. We found only a weak negative correlation between free T3 and age (r2=0.13; p=0.0008). When the effect of age was controlled for in the whole group, the results for free T3, free T4 and the free T3/freeT4 were unchanged.
DiscussionThe present study shows that the proportion of free T3 relative to free T4 in patients with treated central hypothyroidism is lower than in euthyroid controls and similar to treated patients with primary hypothyroidism. It is important to evaluate whether thyroid replacement therapy in hypopituitarism does or does not mimic normal thyroid physiology and to design optimal thyroid replacement regimens. Our results suggest that current replacement therapy with LT4 alone does not mimic normal physiology, but whether this finding translates into adequate or inadequate tissular free T3 concentrations is unknown.
Several studies have reported that patients with hypopituitarism have higher mortality rates and lower quality of life than healthy individuals.12-17 Whether the cause is a particular type of pituitary deficiency and suboptimal or non-physiological hormone replacement for one or other pituitary axis remains to be elucidated.14,17 However, in the last few years, central thyroid replacement has received little interest, particularly in comparison with other pituitary hormones such as GH. The present study suggests that the standard treatment of central hypothyroidism does not mimic normal physiology and could potentially influence the quality of life and clinical characteristics of treated patients with hypopituitarism.
In central hypothyroidism in the context of hypopituitarism, other pituitary deficiencies and replacement treatments may affect T4 to T3 conversion. Cortisol and growth hormone modulate thyroid hormone metabolism.7-9 High doses of glucocorticoids inhibit T4 to T3 deiodination7-9 whereas GH stimulates the conversion of T4 to T3.10,18 A human model of isolated GH deficiency due to GH-releasing hormone receptor mutation has been reported to have low levels of T3 and higher free T4 levels than euthyroid controls,19 suggesting that GH deficiency plays a role in lower T3 concentrations. In addition, GH replacement in patients with GH deficiency resulted in increased free T3 and normalization of T3 levels in patients with central hypothyroidism.18 Non-replaced GH deficiency and supraphysiological adrenal replacement would result in lower T3 levels, whereas GH overreplacement or glucocorticoid underreplacement would theoretically cause higher free T3.
Treatment of hypoadrenalism is known not to mimic physiological levels and the use of distinct doses and regimens of hydrocortisone or prednisone may alter T4 to T3 conversion. In the present study, hypoadrenal patients (97% of the hypopituitary group) were receiving hydrocortisone at doses ranging from 15-20 (60% of patients) to 30mg/day, but dose fractionation differed among patients and two patients were receiving prednisone. Notably, nine patients were receiving 30mg/day of hydrocortisone, which might have been supraphysiological. There were also a high number of non-treated patients with GH deficiency (90%). Therefore, the type of adrenal replacement and non-replacement of GH may possibly have influenced the results. However, this was the real situation of treatment in our patients with hypopituitarism at the time of the study. The overall results of treatment for hypopituitarism in our patients was a free T3 to free T4 ratio lower than that in euthyroidism but similar to that in primary hypothyroidism.
Our results also show that the free T3 to free T4 ratio was similar in two very different models of hypothyroidism (primary and central), which shared the same type of treatment (levothyroxine alone). This finding suggests that a key factor in free T3 and free T4 serum concentrations is probably thyroid replacement therapy with LT4 alone.
Patients with primary hypothyroidism treated with LT4 are already known to have a lower plasma T3 to T45,6 and free T3 to freeT4 ratio20 than healthy euthyroid individuals. In agreement with the present study, other studies have found lower free T3 levels but higher free T4 concentrations in patients with primary hypothyroidism than in euthyroid controls.20 Therefore, a higher freeT4 is considered necessary in primary hypothyroidism to achieve a physiological free T3 concentration and a normal TSH value. However, whether this translates into physiological thyroid replacement to all tissues in humans is unknown. In thyroidectomized rats, Escobar-Morreale et al. showed that only the combination of T4+T3 treatment resulted in physiological tissue T3 concentrations in all tissues.21 Van Doorn et al. showed that each tissue had a distinct proportion of free T3 obtained from peripheral and local free T4 to free T3 conversion, possibly suggesting that some tissues are more sensitive to changes in the free T3/free T4 circulating ratio.3,4
In theory, a combination treatment of T4+ T3 may better mimic physiology, and free thyroid hormone concentrations would be more appropriate to provide optimal thyroid replacement to all tissues. Currently, only one study has evaluated combined treatment with T3+ T4 compared with T4 alone in central hypothyroidism.22 However, the combination resulted in supraphysiological free T3 levels and no conclusions on the superiority of one treatment over the other could be reached. In contrast, several clinical trials have tested combined T3+ T4 regimens compared with T4 alone in primary hypothyroidism23-29 and most have not proved the superiority of combination therapy.24-29
Another important consideration in central hypothyroidism is the difficulty of treatment monitoring.11 Only peripheral free T4 and free T3 concentrations can be used in treatment evaluation. TSH suppression may indicate adequate treatment but is not as sensitive or as reliable as in primary hypothyroidism.30,31 Furthermore, free T3 is not a reliable parameter as it can be normal in 75% of cases of thyroid underreplacement;11 freeT4 is more reliable than free T3 but can also be normal in a proportion of cases of suboptimal therapy (up to 25%) according to Ferreti et al.11 and Alexopoulou et al..30 In the present study, free thyroid hormone concentrations were similar between patients with primary and central hypothyroidism.
One limitation of this study is the lack of TSH data in patients with central hypothyroidism. Another possible limitation is the distinct sex ratios among groups, although sex was not correlated with any thyroid hormone variable. This study shows that under the standard of care, patients with central hypothyroidism in the context of hypopituitarism have a lower free T3 to free T4 ratio than euthyroid controls. The consequences of non-physiological thyroid hormone replacement may be clinically relevant. Small changes in thyroid status may affect resting energy expenditure,32 which in the long term may substantially affect body weight. Lipid levels, homocysteine, cardiovascular risk and bone metabolism may also be affected by thyroid hormones. Future studies should aim to investigate the possible clinical consequences of more physiological thyroid replacement in hypopituitarism.
In summary, patients with treated central hypothyroidism have a lower free T3 to free T4 ratio, similar free T3 levels and higher free T4 concentrations than euthyroid controls, whereas free thyroid hormone concentrations and the free T3 to free T4 ratio are similar to those in patients with primary hypothyroidism. Whether these results translate into adequate tissue concentrations of free thyroid hormones to all tissues is unknown. Research into whether a treatment that obtains a more physiological plasma concentration could improve clinical outcomes is warranted.
Conflicts of interestsThe authors have no conflict of interest to declare.
Grant supportSupported by a grant from Societat Catalana d’Endocrinologia i Nutrició.
This work was supported by a grant from the “Societat Catalana d’Endocrinologia i Nutrició”.