Since the initial article by Epstein in 2006,1 several reports have highlighted the relationship between long-term treatment with proton pump inhibitors (PPIs) and hypomagnesemia. Although very few cases have been reported to date, this may be an adverse effect more common than expected because of the high number of patients who receive this treatment. We report the case of a patient with hypomagnesemia refractory to treatment with oral supplementation that resolved upon discontinuation of omeprazole treatment.
A 65-year-old male patient was admitted after experiencing an episode of generalized tonic-clonic seizures. His personal history included HBP treated with doxazosin and valsartan/hydrochlorothiazide, cryptogenic focal epilepsy diagnosed in 2005 treated with levetirazetam, and atrial flutter (on anticoagulation with warfarin). He had had gastroesophageal reflux, which was being treated with omeprazole 20mg, for 20 years.
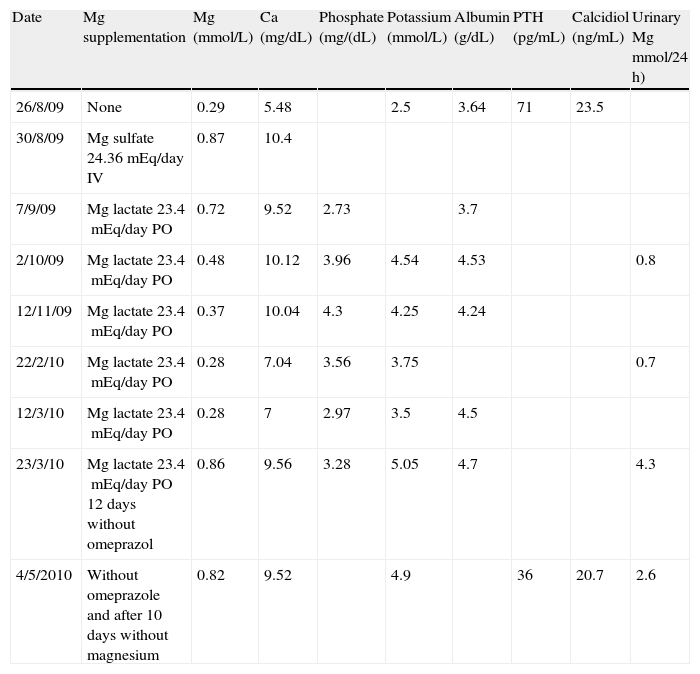
Significantly decreased plasma calcium magnesium, and potassium levels were found upon admission, and treatment with intravenous and subsequently oral magnesium supplements was therefore started. On the first three days, calcium and potassium supplements were also given. Magnesium levels normalized with intravenous supplementation, but gradually decreased later to plasma levels similar to baseline values despite high oral supplementation (Table 1). Urinary magnesium losses were very low, which suggested that hypomagnesemia was related to deficient absorption. The patient reported a balanced diet and no alcohol or laxative use. Celiac disease and pancreatic insufficiency were ruled out. An attempt was made to substitute magnesium pidolate for lactate, but it was discontinued due to intolerance. Hydrochlorothiazide treatment was also discontinued with no improvement in laboratory results. After a literature review, omeprazole was discontinued and replaced by ranitidine, after which calcium and magnesium levels normalized in 12 days. Oral supplements were subsequently discontinued, and the patient currently has normal plasma magnesium levels.
Changes in laboratory parameters over time.
| Date | Mg supplementation | Mg (mmol/L) | Ca (mg/dL) | Phosphate (mg/(dL) | Potassium (mmol/L) | Albumin (g/dL) | PTH (pg/mL) | Calcidiol (ng/mL) | Urinary Mg mmol/24 h) |
| 26/8/09 | None | 0.29 | 5.48 | 2.5 | 3.64 | 71 | 23.5 | ||
| 30/8/09 | Mg sulfate 24.36mEq/day IV | 0.87 | 10.4 | ||||||
| 7/9/09 | Mg lactate 23.4mEq/day PO | 0.72 | 9.52 | 2.73 | 3.7 | ||||
| 2/10/09 | Mg lactate 23.4mEq/day PO | 0.48 | 10.12 | 3.96 | 4.54 | 4.53 | 0.8 | ||
| 12/11/09 | Mg lactate 23.4mEq/day PO | 0.37 | 10.04 | 4.3 | 4.25 | 4.24 | |||
| 22/2/10 | Mg lactate 23.4mEq/day PO | 0.28 | 7.04 | 3.56 | 3.75 | 0.7 | |||
| 12/3/10 | Mg lactate 23.4mEq/day PO | 0.28 | 7 | 2.97 | 3.5 | 4.5 | |||
| 23/3/10 | Mg lactate 23.4mEq/day PO 12 days without omeprazol | 0.86 | 9.56 | 3.28 | 5.05 | 4.7 | 4.3 | ||
| 4/5/2010 | Without omeprazole and after 10 days without magnesium | 0.82 | 9.52 | 4.9 | 36 | 20.7 | 2.6 |
A review of the patient's history found no calcium or magnesium measurements before the reported admission, and the time of onset of the reported changes is therefore unknown.
DiscussionMagnesium is the fourth most abundant cation in the body, and ranks second in the intracellular environment. Ninety-nine percent of body magnesium is found in the intracellular space, mainly in bone tissue (60%), followed by skeletal muscle (20%). Extracellular magnesium only accounts for 1% of total magnesium and circulates in plasma mainly in free form (50%), while 30% is bound to plasma protein and 10% occurs as mineral salts.
Under normal conditions, plasma magnesium levels range from 0.66 to 1.05mmol/L and are influenced by the balance between intestinal absorption and renal excretion.
Magnesium absorption mainly occurs in proximal jejunum and ileum. An average diet provides 360mg daily, of which only 50% are absorbed. In addition, 40mg of magnesium are secreted daily in the small bowel to the intestinal lumen, of which 20mg are reabsorbed in the colon and rectum.2 Magnesium absorption in the gastrointestinal tract occurs through two different mechanisms. The first and main mechanism is an active, saturable process occurring through the magnesium channel, transient receptor potential melastatin 6 (TRPM6).3 This receptor is also found in the distal renal tubule, favoring the reabsorption of magnesium not assimilated at the loop of Henle. Ninety percent of intestinal absorption occurs through this route. The second mechanism is passive and non-saturable and occurs through the paracellular route. Eighty percent of plasma magnesium is filtered by the glomerulus. Of this amount, 95% is reabsorbed by the nephron (60–70% at the loop of Henle, 15–25% at the proximal tubule, and 5–10% at the distal tubule).
Table 2 lists the different causes of hypomagnesemia. Differential diagnosis is usually simple because most of these conditions are easy to recognize.
Causes of hypomagnesemia.
| • Causes of hypomagnesemia |
| - Decrease intake |
| • Malnutrition |
| • Alcoholism |
| • Long-term parenteral nutrition |
| - Redistribution |
| • Hungry bone syndrome |
| • Refeeding syndrome |
| • Treatment of diabetic ketoacidosis |
| - Gastrointestinal losses |
| • Diarrhea |
| • Intestinal resection |
| • Hypomagnesemia with secondary hypocalcemia (HSH): TRPM6 gene mutation |
| - Renal losses |
| • Several tubular changes (Gitelman, Bartter III) |
| • Drugs (diuretics) |
Since 2006, several publications have reported isolated cases or small series of patients with severe hypomagnesemia refractory to oral treatment related to the long-term use of PPIs.4–9 A total of 14 cases have been reported, which is a very low incidence considering the widespread use of these drugs by the population. However, we may be detecting only a small proportion of cases, because routine measurement of plasma magnesium levels is not usually done, and patients may have few symptoms.
The mechanism causing hipomagnesemia has not been elucidated yet. However, it appears clear that it is an absorption defect, because urinary magnesium excretion is decreased in virtually all patients. A majority of patients have in common prior long-term use for years of PPIs, which suggests that chronic depletion of body deposits should occur before the clinical picture appears. Other drugs able to cause hypomagnesemia act through an increased urinary magnesium excretion.
Cundy and Dissanayake4 suggested that there is a failure in the active absorption mechanism, while the non-saturable passive system remains unchanged, as is shown by partial correction with very high doses of oral magnesium supplements. These authors also performed a test of venous infusion of magnesium on two patients and showed that severe magnesium occurred and, on the other hand, urinary excretion continued to be extremely low until plasma levels exceeded 1.0mmol/L, which supports the concept of the existence of an absorption defect with renal compensatory mechanisms.
Since hypomagnesemia associated with PPIs is to date an uncommonly reported effect, it is thought that there may be a genetic susceptibility to develop this complication, and it has been postulated that TRPM6 gene mutations may be implicated in pathogenesis, although there have been no reports on this subject.
It is probably a class effect, because it has not only been reported with omeprazole, but also with esomeprazole, pantoprazole, and lansoprazole,7 while the use of ranitidine is not associated with this phenomenon.
Prior studies had found no short-term effects of PPIs on magnesium absorption.10 This may be related to the need for long-term treatments for these changes to occur, or to the low probability of the occurrence of this condition. In our patient, the problem did not become evident until he had been treated with omeprazole for 15 years.
This defect is clearly reversible, and magnesium levels return to normal a few days after the discontinuation of PPIs, as occurred in the reported patient.
To sum up, PPIs may cause hypomagnesemia refractory to oral supplementation. This possibility should be considered in differential diagnosis of hypomagnesemia, particularly when there are no other apparent causes.
Please cite this article as: Martínez Faedo C, et al. Hipomagnesemia severa refractaria a la suplementación oral asociada al tratamiento con omeprazol. Endocrinol Nutr. 2012;59(7):463–5.