Type 2 diabetes mellitus prevalence and morbidity are increasing. Osteoporotic fractures are among the ‘non-classical’ complications of diabetes and been overlooked for a long time, maybe because of their complex diagnostic and therapeutic approach. The usual tools for preventing fragility fractures (such as the fracture risk assessment tool and bone densitometry) underestimate risk of fractures in type 2 diabetic patients. New techniques, such as trabecular bone score or bone turnover markers, could be useful, but greater scientific evidence is required to recommend their use in clinical practice. The special characteristics of their pathophysiology result in decreased bone remodeling with normal or even increased bone mineral density, but with low quality. These changes lead to the occurrence of osteoporotic fractures without evidence of densitometric changes, which could be called ‘the diabetic paradox’.
La diabetes mellitus tipo 2 es una patología con una enorme prevalencia y morbilidad, que van en aumento. La fractura osteoporótica se encuentra entre las denominadas complicaciones «no clásicas» de la diabetes y ha sido durante tiempo ignorada, tal vez por su complejo abordaje tanto diagnóstico como terapéutico. Las herramientas habituales para la prevención de la fractura por fragilidad, como el FRAX y la densitometría ósea, no han demostrado la suficiente eficacia en estos pacientes, ya que infraestiman el riesgo. Nuevas técnicas de evaluación ósea, como el trabecular bone score o los marcadores de remodelado óseo, podrían ser de utilidad, aunque requieren una mayor evidencia científica para recomendar su uso en la práctica clínica habitual. Las características especiales de su fisiopatología condicionan la aparición de fracturas sin existir alteraciones densitométricas, en lo que podemos calificar de «paradoja diabética».
Many epidemiological studies have addressed the management of the classical complications of diabetes. The great–and increasing–prevalence of this condition makes it a leading public health problem. However, osteoporosis in type 2 diabetes mellitus (T2DM) is a “silent epidemic”, it being difficult to assess as it causes no symptoms until fracture occurs.
The study and prevention of osteoporosis in these patients is a complex task, because the standard diagnostic procedures are not helpful.1 The pathophysiological mechanism underlying osteoporosis results in bone of normal or increased density, but with a low resistance due to changes in bone microarchitecture, the bone turnover rate, and the accumulation of lesions. Several studies have reported this “diabetic paradox”, showing an increased number of fragility fractures in these patients despite normal bone density.2–4 A meta-analysis of 12 studies conducted by Janghorbani et al.5 concluded that patients with T2DM had a relative risk of hip fracture of 1.7.
On the other hand, the significant morbidity, the economic costs, and the impact on quality of life in patients with fragility fractures, due to decreased physical capacity and greater dependence, also need to be taken into consideration.6,7 All these factors demand a better approach to, and solution of, this complication of diabetes.
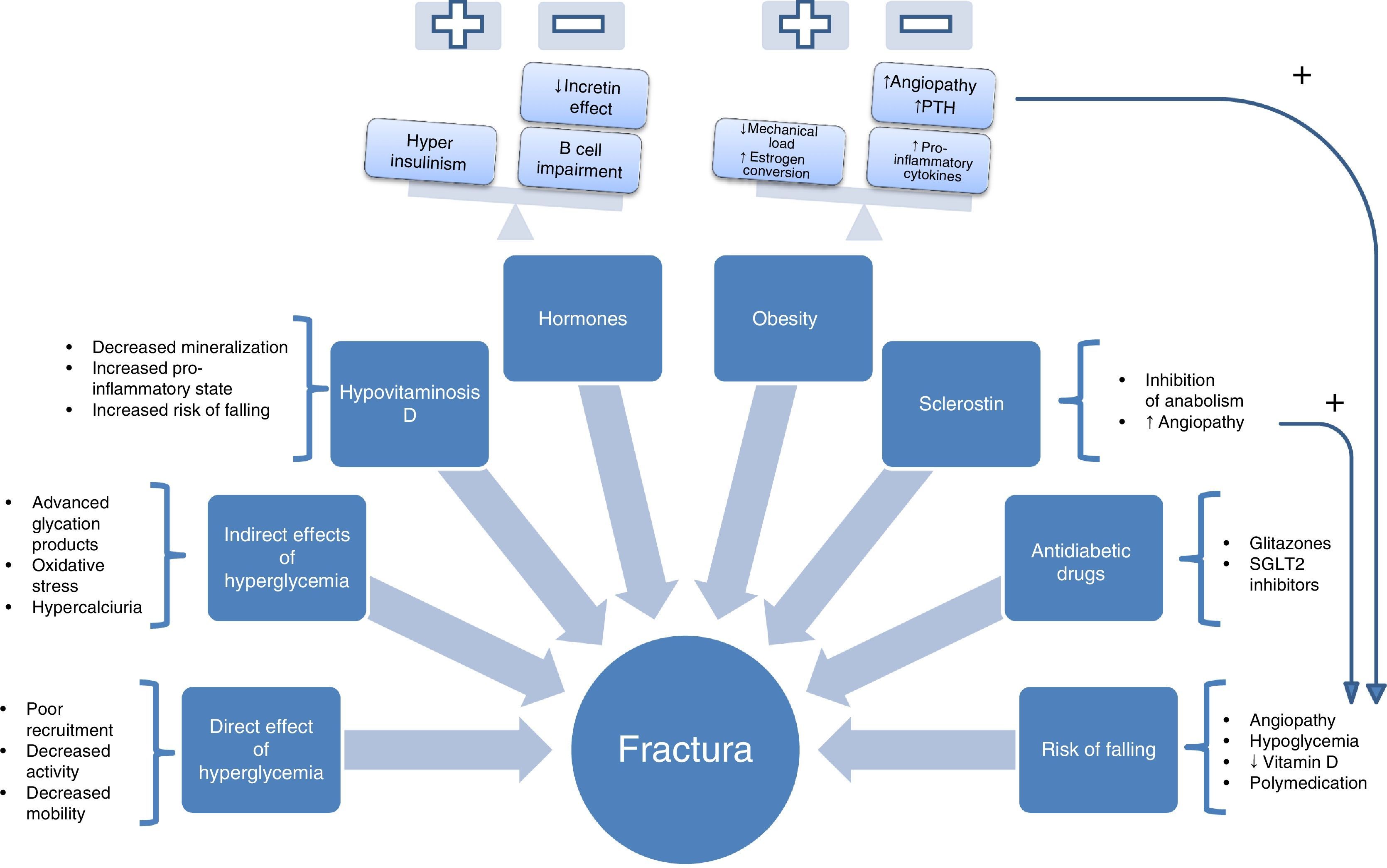
Pathophysiology. “The ominous octet of fractures in patients with T2DM”The pathophysiological characteristics of fractures in T2DM are special and extremely heterogeneous. The diverse factors involved appear to cause decreased bone turnover. Several studies have reported decreases in bone remodeling markers in patients with T2DM.8 Unfortunately, histomorphometric analysis has rarely been performed, most probably because it is an invasive procedure. In a study of 26 patients with T2DM (13 males and 13 females with normal kidney function), decreases were found in osteoid volume and osteoblasts.9 These results agree with those of another study conducted on 8 subjects with diabetes (6 T2DM and 2 T1DM) on whom iliac crest biopsies were performed for histomorphometric analysis.10 In a healthy person (without diabetes), bone is a dynamic tissue that undergoes continuous remodeling in order to maintain the biomechanical competence of the skeleton, prevent the occurrence of lesions, and contribute to mineral homeostasis. Each year, approximately 25% of trabecular bone and 3% of cortical bone are renewed in a process regulated by different mechanical, hormonal, and local factors. However, if this remodeling process is impaired, the result is a bone of normal or high density but with an increased risk of fracture.
To understand this complex set of factors involved in pathogenesis, a scheme of the genesis of fragility fractures can be drawn (Fig. 1) containing the following “ominous octet”:
- 1.
Direct effects of hyperglycemia on bone cells. Hyperglycemia has been associated with decreased osteoclast maturity, function, and motility. This was seen, for example, in a study conducted with cell models that measured the impact of exposure to a medium with high glucose concentration on osteoclastogenesis induced by RANK-L. For this, the activation of osteoclastogenesis was assessed by measuring tartrate-resistant acid phosphatase, calcitonin receptor expression (characteristic of mature osteoclasts), cathepsin K, and caspase 3 activity. All these markers of osteoclastic activity were decreased in the presence of hyperglycemia. Migration capacity and the motility of osteoclasts were also decreased in those exposed to greater glucose concentrations. It was, therefore, concluded that exposure to hyperglycemia causes different changes in osteoclasts, including decreased formation, activity reduction, and decreased motility, resulting in difficulties in reaching microfractures and repairing them.11
- 2.
Indirect effects of hyperglycemia. The production of advanced glycation products, which is greatly increased in patients with diabetes, is another indirect mechanism through which hyperglycemia acts. Glycation modifies the structure, physico-chemical properties, and biological functions of native proteins. The case of pentosidine is among the most widely studied in patients with diabetes. Studies such as the one reported by the Yamamoto et al. group12 showed pentosidine levels to be associated with vertebral fractures irrespective of diabetes complications and duration, insulin or glitazone therapy, or other known risk factors, such as alcohol, smoking, etc.
Hyperglycemia is also indirectly involved in increased oxidative stress and hypercalciuria, which could cause impairments in bone structure and mineralization.13
- 3.
Hypovitaminosis D. In addition to hyperglycemia, there are other important factors involved in the bone fragility of patients with T2DM, including vitamin D deficiency. Several studies have shown an inverse association between vitamin D and T2DM, as was reported in a recent review.14 This deficiency causes decreased mineralization, increases the pro-inflammatory state, and increases the risk of falling, because vitamin D has been shown to play a role in skeletal muscle.15 Moreover, a meta-analysis of randomized trials concluded that vitamin D supplementation (at doses of 700-1000IU/day) could decrease the risk of falling by 19%.16
- 4.
Hormones. Hyperinsulinism, present in the early stages of T2DM, has been postulated as one of the crucial factors for understanding the diabetic paradox.17 Insulin could be involved in bone mineral density increase in these patients, in contrast to density loss occurring in type 1 diabetes mellitus, which starts with insulinopenia in the early stages of life, so preventing the achievement of adequate peak bone mass. At more advanced disease stages, in which beta cell impairment occurs, this anabolic effect would be lost.
On the other hand, there is increasing scientific evidence regarding the role played by the incretin system in the bone tissue of these patients. Studies in animal models appear to show an anabolic effect. Thus, the study conducted in ovariectomized rats by Ma et al.18 showed that the administration of exendin-4 promoted bone formation (by increasing the number of osteoblasts by bone surface and formation markers procollagen type 1 N-terminal peptide and osteocalcin) and inhibited bone resorption (by decreasing osteoblast count and deoxypyridinoline and carboxiterminal telopeptide of type 1 collagen levels and increasing the osteoprotegerin/RANK ligand ratio). Another study conducted in an animal model of leptin receptor-deficient mouse also showed a positive effect on bone of a new triple analog of gastric inhibitory polypeptide (GIP) receptors, glucagon-like peptide-1 (GLP-1), and glucagon.19
- 5.
Obesity. This is undoubtedly one of the most controversial factors. It was traditionally held to have a positive effect on bone tissue due to the increase in mechanical load, stimulating osteocytes, or the conversion of androgens to estrogens. However, in a study conducted in an animal model of ovariectomized rat, weight and estrogen increases induced by a diet with a high fat percentage did not improve bone tissue properties.20 On the other hand, most studies of the relationship between weight and bone density were cross-sectional. The Health, Aging, and Body Composition Study was a longitudinal study of a sample of 2570 subjects where densitometry showed that obese patients lost 0.003g/cm2 per year more than adults with normal weight (p<0.001).21 This beneficial effect has since been questioned for several reasons, including the pro-inflammatory state induced by the release of multiple cytokines (IL-6) TNF-alpha, etc.). These promote both bone resorption and angiopathy,22,23 with a resultant increase in the risk of falling. All of these could contribute to increasing the risk of fracture in these patients.24
- 6.
Increased sclerostin levels. The role of the canonical Wnt pathway in bone tissue, where through a signal cascade it activates the transcription of genes with anabolic effects and controls osteoblasts at the differentiation, proliferation, and final function levels, is well known.25 Sclerostin, which has been shown to be significantly increased in patients with T2DM, inhibits this bone anabolic pathway. Recently, several studies have also shown a relationship between increased sclerostin levels in females with T2DM and vertebral fractures.26,27
No less important is the role played by this pathway in other biological processes related to cardiovascular disease. A study by Morales-Santana et al. demonstrated the relation between endogenous levels of the Wnt pathway inhibitor (sclerostin) and atherosclerotic lesion in patients with T2DM.28 Whether this relationship between sclerostin and arterial disease contributes to a greater risk of falling has yet to be elucidated.
- 7.
Risk of falling. Increased falls in patients with T2DM mainly result from angiopathy (retinopathy, polyneuropathy, etc.) and undoubtedly promote the occurrence of fractures. Angiopathy has a very close relationship with osteoporotic fracture in a sort of “pathogenic dyad” which has been shown in various studies where they have been seen to share not only common risk factors, but also identical pathogenic pathways, such as the already mentioned Wnt or the OPG/RANK-L pathway.28–31
In addition to artery disease, there are other factors that promote falls in these patients, such as the previously discussed vitamin D deficiency, hypoglycemia, or the polymedication of these patients.
- 8.
Oral antidiabetic drugs. A review this year by Meier et al.32 emphasized the importance of the use of certain antidiabetic drugs for bone safety. In this regard, special mention should be made of glitazones33,34 and type 2 sodium-glucose cotransporter (SGLT-2) inhibitors because of the potential increase in the risk of fractures.35 SGLT-2 inhibitors have been shown to decrease trabecular bone volume in a non-diabetic mouse model,36 while they did not induce bone improvements in the diabetic mouse model despite the improvement seen in blood glucose control. The Canagliflozin Cardiovascular Assessment Study (CANVAS), with 4327 participants, also showed an increase in fracture risk, 4% versus 2.6%, in patients with T2DM treated with canagliflozin compared to placebo.37
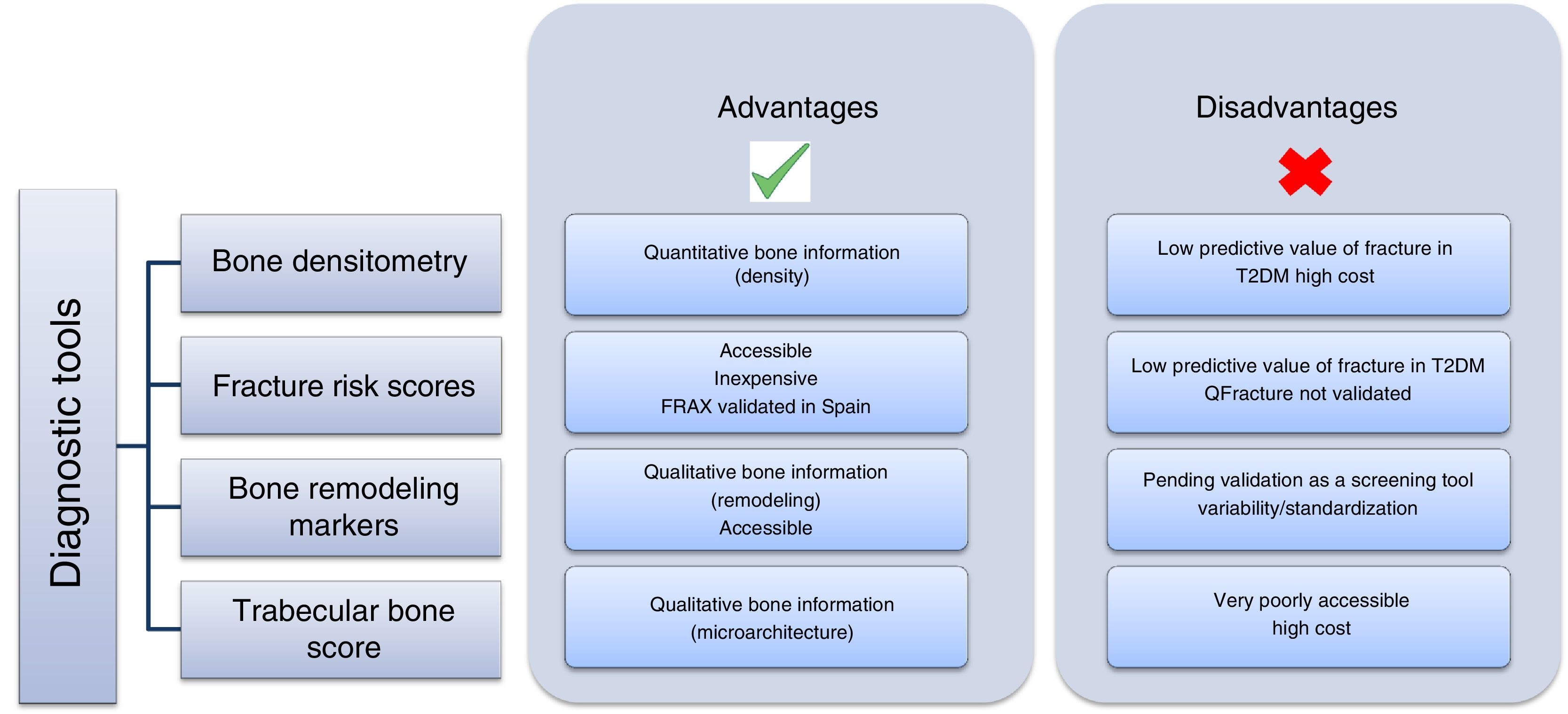
Once both the prevalence and complexity of the problem have been discussed, the next step is how to approach it and prevent it in patients with T2DM (Fig. 2). This is no simple task, because the traditional method for diagnosing osteoporosis has been the use of DEXA according to the criteria established by the World Health Organization (WHO) in 1994,38 based on the measurement of bone mineral density in the lumbar spine, femoral neck, hip, or distal third of the radius. According to these criteria, density values greater than −1 standard deviation (SD) as compared to the mean in young adults (T-score) are normal; values ranging from −1 to −2.5 SD are defined as low bone mass; and values less than −2.5 SD are diagnostic of osteoporosis. This procedure has not been shown to be helpful in patients with T2DM because the increase in the risk of fracture is independent of density, and these patients have in fact greater lumbar and femoral bone mineral density (BMD).39
Fracture risk scores are increasingly used, accessible, and inexpensive tools. The most widely used of these tools is FRAX, developed by the WHO collaborating center at the University of Sheffield as a tool for identifying the population at risk and performing primary prevention (intervention: if risk >3% in the hip or >20% in other site). The main disadvantage of this tool is that it does not assess T2DM itself as an item. In studies conducted to assess the value of FRAX in patients with diabetes, it was concluded that it is not a valid tool, because it underestimates the risk of fracture in this population.40,41 A possible alternative could be the use of the QFractureScore, developed in a large British cohort42 and including T2DM as a risk factor, but this has only been validated for the United Kingdom.
The role of bone remodeling markers (BRMs) should also be mentioned. Several studies have reported decreased BRM levels in patients with T2DM due to decreased bone anabolism. They received support from a meta-analysis of 22 studies where significantly lower levels of the bone formation marker, osteocalcin, were found in patients with T2DM.8 Its authors have recently published a systematic review of the potential use of BRMs for bone assessment in patients with TDM2.43 BRMs have been shown to be significantly associated with vertebral fractures in T2DM.26 In 2014, the working group of the International Osteoporosis Foundation (IOF) and the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) published a meta-analysis which attributed to BRMs a potential predictive role for osteoporotic fracture in the general population.44 However, although these results are promising, additional studies are needed to establish the value of BRMs as a screening and monitoring tool for osteoporosis in T2DM. Moreover, their use has been questioned because of the need for standardization, on which the IOF-IFCC group is working. A study conducted in a Spanish population,45 designed to minimize the potential factors of preanalytical variability, established robust ranges in the Caucasian population that agreed with those reported for larger cohorts. This similarity of results from several studies using an assay on an automated platform for measurement at different laboratories, shows that variability may be minimized using adequate methods, thus reinforcing the clinical value of BRMs in the assessment of bone disease.
Finally, the Trabecular Bone Score (TBS) has been proposed as a potentially useful tool, especially in secondary osteoporosis such as the one caused by T2DM.46 A significant decrease in trabecular bone has been found in patients with diabetes, as shown by the Dhaliwal et al. study,47 conducted in 100 women, where those with T2DM had greater BMD and a lower TBS (p=0.001 and 0.01 respectively), or the retrospective study conducted in a Canadian cohort of 29,407 women (2356 with T2DM), in which the TBS was predictive of fracture.48 This decrease in trabecular bone appears to result from poor blood glucose control47 and the presence of insulin resistance, and a negative correlation was found between the TBS and glycosylated hemoglobin A1c, basal blood glucose, and insulin resistance.49
The recommendations for the evaluation and treatment of osteoporosis associated with endocrine and nutritional diseases of the working group on osteoporosis and mineral metabolism of the SEEN50 face up to this challenge and recommend the evaluation of this complication in patients with T2DM, especially those with chronic complications, insulin therapy, or glitazone treatment. The use of FRAX or the TBS, if available, is also suggested with a very low level of evidence. Therapeutically, the same recommendations as for the population without diabetes are made, although it is suggested that deficient osteoblastic function in this condition makes the use of anabolic drugs attractive in high-risk or secondary prevention patients.
ConclusionsThe population with T2DM has an increased risk of fracture, despite having normal or increased BMD, as a consequence of a complex pathophysiology. Because of this, standard prevention tools underestimate the risk. Careful routine evaluation of the factors involved, such as vitamin D deficiency, the prevention of the risk of falling, drugs, etc. is also needed to minimize its incidence. The TBS appears to be promising for diagnosis, but more prospective information is needed before its routine use for the prevention of diabetic fractures can be recommended.
Conflict of interestThe authors state that they have no conflict of interest.
Please cite this article as: Botella Martínez S, Varo Cenarruzabeitia N, Escalada San Martin J, Calleja Canelas A. La paradoja diabética: densidad mineral ósea y fractura en la diabetes tipo 2. Endocrinol Nutr. 2016;63:495–501.