Vitamin D deficiency is becoming endemic in many parts of the world.
AimTo study vitamin D status in Egyptian females of different age groups.
Subjects and methodsA cross-sectional study was conducted on 404 females, who were categorized into group 1 (51 nursing females); group 2 (50 pregnant females); group 3 (208 females of childbearing age); group 4 (38 elderly females); and group 5 (57 geriatric females). Females completed a questionnaire regarding dietary calcium and vitamin D intake, sun exposure, and clothing habits, and performed laboratory tests including calcium, PO4, alkaline phosphatase, intact PTH, and 25-OH vitamin D levels.
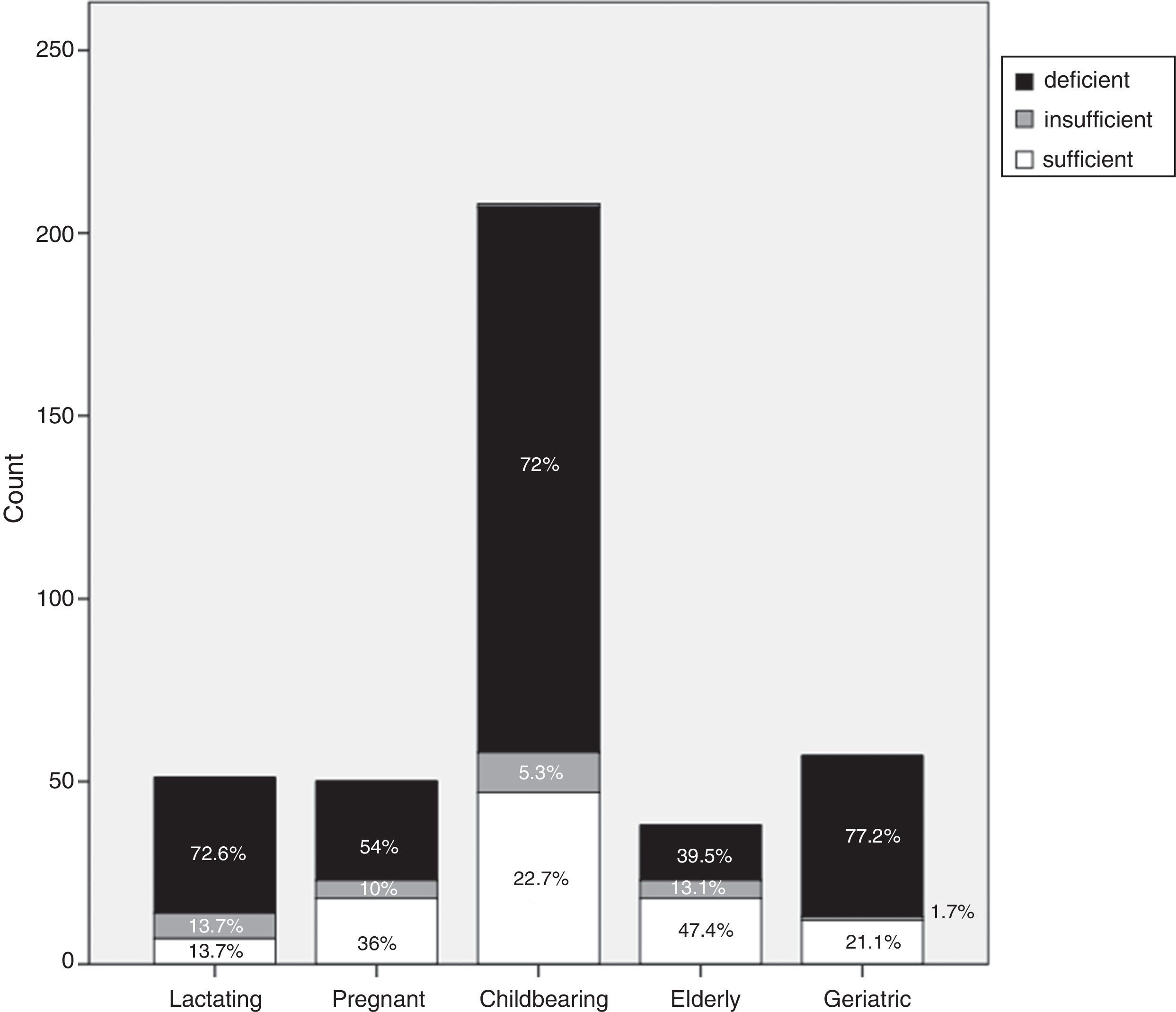
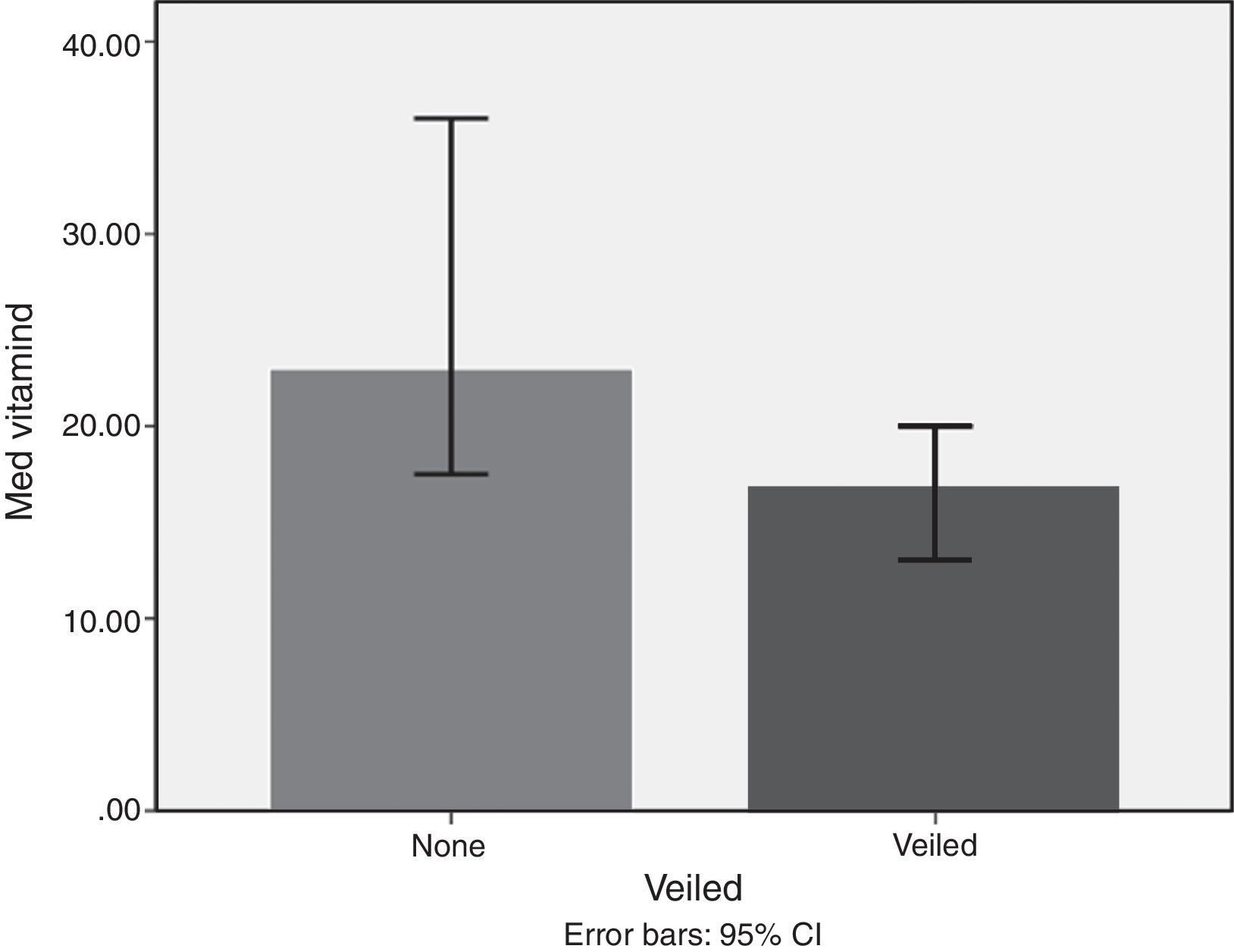
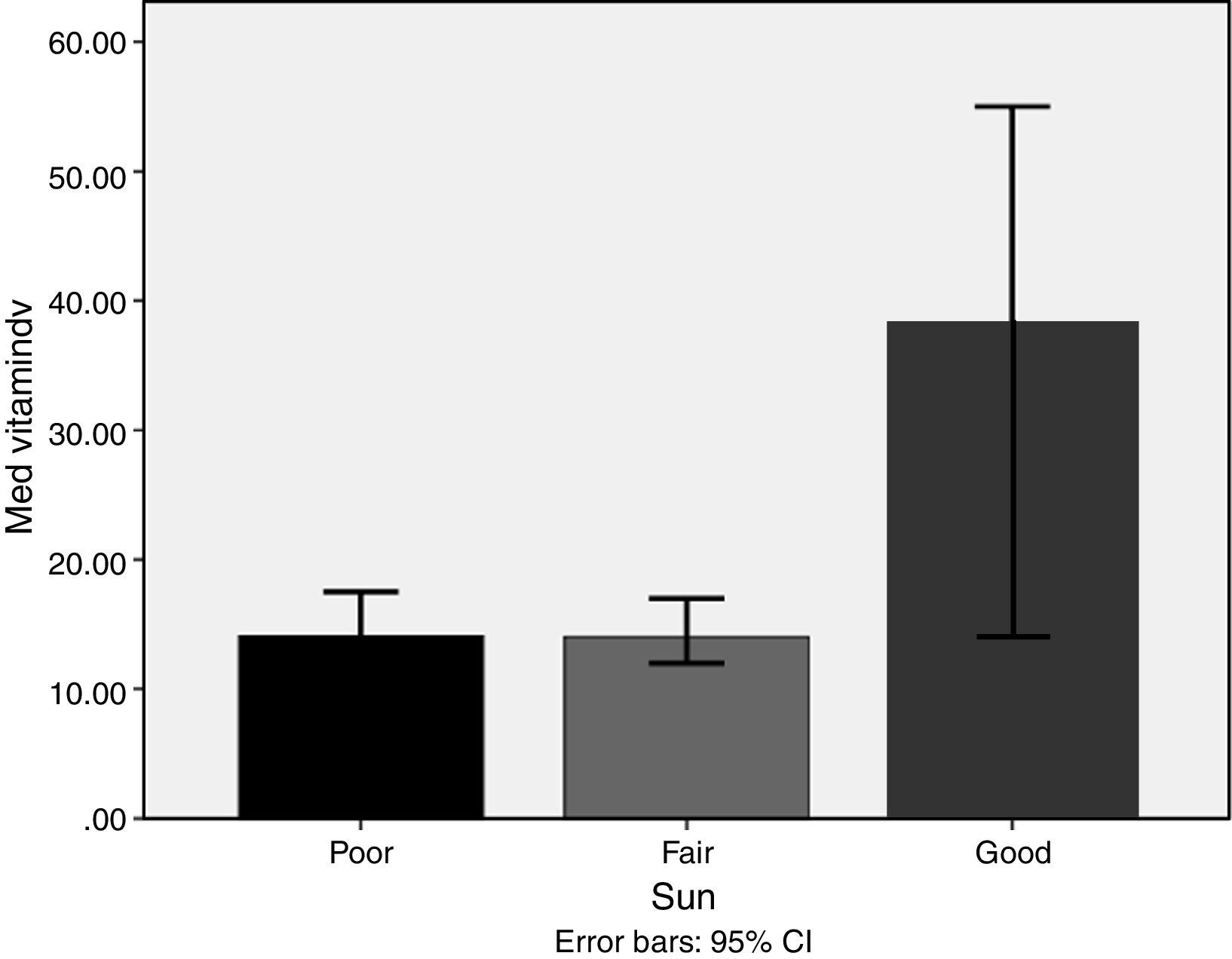
ResultsMedian and IQR of vitamin D levels across groups 1, 2, 3 and 5 were in the deficient range, being lowest in groups 3, 5, and 1, respectively. Vitamin D deficiency was found in 72.6% of the nursing group, 54% of the pregnant group, 72% of the childbearing age group, 39.5% of the elderly group, and 77.2% of the geriatric group. Vitamin D was significantly higher in non-veiled females [23ng/dl] as compared to veiled females [16.7ng/dl]. Vitamin D levels with poor, fair, and good sun exposure were 14.1, 14, and 37ng/dl, respectively.
ConclusionThese results show a high prevalence of vitamin D deficiency among healthy Egyptian females.
El déficit de vitamina D es ya una enfermedad endémica en muchas partes del mundo. El objetivo de este estudio era investigar el estado de la vitamina D en mujeres de distintos grupos de edad.
Sujetos y métodosSe realizó un estudio transversal en 404 mujeres, que se clasificaron en grupo 1 (51 mujeres lactantes); grupo 2 (50 mujeres embarazadas); grupo 3 (208 mujeres en edad fértil; grupo 4 (38 mujeres de edad avanzada), y grupo 5 (57 mujeres ancianas). Se les sometió a un cuestionario sobre la ingesta de calcio y vitamina D en la dieta, la exposición al sol y los hábitos de vestir. Se les practicaron además análisis de laboratorio que incluyeron calcio sérico, fósforo en suero PO4, fosfatasa alcalina, PTH intacta y 25-OH vitamina D.
ResultadosLa mediana y el IIC de los niveles de vitamina D en los grupos 1, 2, 3 y 5 estaban dentro del intervalo deficitario, siendo los más bajos los del grupo 3, 5 y 1, respectivamente. Se halló déficit de vitamina D en el 72,6% del grupo lactante, el 54% del grupo de embarazadas, el 72% del grupo en edad fértil, el 39,5% del grupo de edad avanzada y el 77,2% del grupo de ancianas. La concentración de vitamina D era significativamente mayor en las mujeres que no usaban velo [23ng/dl] que en las mujeres con velo [16,7ng/dl]. Se hallaron valores de 14,1ng/dl, 14ng/dl y 37ng/dl en las mujeres con exposición baja, media o buena al sol, respectivamente.
ConclusiónEstos resultados demuestran una alta prevalencia del déficit de vitamina D en las mujeres egipcias sanas.
Over the past two decades, vitamin D deficiency has emerged as a major worldwide health concern encompassing all age groups. This issue also affects those residing in countries of low latitude, who were previously thought to be protected.1 Even with the implementation of vitamin D fortification policies in industrialized countries, it has been estimated that 20–100% of US, Canadian and European elderly men and women are vitamin D deficient. Additionally, children, young adults and the middle aged are also at risk of vitamin D deficiency.1,2
In many countries, data pertaining to vulnerable populations, such as pregnant women, adolescents and the geriatric, are still lacking.1 For example, vitamin D deficiency is a common problem among Egyptian adolescent girls,3 for whom contributing factors include inadequate sun exposure possibly related to cultural/social factors, and insufficient dietary calcium.3 Several risk factors are implicated in vitamin D deficiency, including lack of sun exposure, extreme age, seasonal variations, being female, dark skin pigmentation, clothing style and obesity.4 A paucity of dietary sources of vitamin D, such as fatty fish, liver, eggs, milk and dairy products, renders skin synthesis of vitamin D as the main source and determinant of a person's vitamin D status. Vitamin D photosynthesis in turn depends upon latitude, time of day, season and UVB intensity.4
Skeletal manifestations of hypovitaminosis D present clinically as bone and muscle pain, poor muscle strength and low bone mineral density (BMD). Extraskeletal effects have been implicated in cancer, cardiovascular risk, type 2 diabetes, autoimmune diseases, infectious diseases and respiratory diseases such as asthma.2,4,5 The most recent Endocrine Society guidelines recommend the use of the 25-OH vitamin D test for the screening and diagnosis of vitamin D deficiency.2 Other parameters, such as low serum calcium and phosphate levels or elevated alkaline phosphatase, can also indicate vitamin D deficiency.6 Based on the most recent Endocrine Society guidelines, vitamin D deficiency has been defined as a 25-OH vitamin D3 level below 50nmol/L (20ng/dl), while an insufficiency is considered to exist at levels of 51–74nmol/L (21–29ng/dl). Females, especially those pregnant or lactating, are at a higher risk of vitamin D deficiency or insufficiency, as highlighted by several studies in the Middle East.2,7 Accumulating data on the vitamin D status of Egyptians over the last two decades indicate a resurgence of vitamin D deficiency among otherwise healthy members of the population.8–11 As such, it became prudent to study vitamin D statuses across all age groups of healthy Egyptian females in order to detect possible effectors, which will help policy makers develop prevention and treatment strategies.
Aim- 1.
To obtain data on the vitamin D status of Egyptian females at different age groups and in relation to pregnancy and lactation.
- 2.
To define the determinants of vitamin D status in this population sample.
A cross-sectional study was conducted during spring and summer in the period from 2013 to 2014, targeting otherwise healthy Egyptian females of different age groups in Cairo (30.0500N°, 31.2333E° latitude) and Port Said (31.2600N°, 32.2900E° latitude); this study was approved by the local ethical committee. Healthy females accompanying inpatients and females visiting Ain Shams University and Port Fouad General Hospital outpatient clinics for otherwise minor intercurrent problems were invited to participate in the study. The aim of the study was explained to them and their consent requested. Pregnant and lactating females were recruited from the Obstetric Outpatient Clinic and Maternity Unit at Ain Shams University Hospital.
After obtaining a signed consent, a questionnaire regarding dietary calcium and vitamin D intake, vitamin D and calcium supplements, sun exposure and clothing habits was given to the volunteers to answer. In addition, a detailed clinical history was taken and an examination conducted on each volunteer. Volunteers with a history of renal disease, malabsorption syndrome, malignancy, musculoskeletal diseases, fractures, steroid therapy, hepatic diseases or long-term intake of anticonvulsants, glucocorticoids, rifampin and highly active antiretroviral therapy (HAART) were excluded from the study.
Lifestyle factorsVolunteers were asked to describe their dietary calcium and vitamin D intakes, which were then designated as poor, fair or good (poor for no intake; fair for <600IU/day; good for >600IU/day) according to the latest IOM recommendations.2 Additionally, vitamin D and calcium supplement intakes were designated as either ‘yes’ for taking a supplement or ‘no’ for not taking a supplement. Exposure to sun was designated as poor for no exposure, fair for once per week exposure of arms and legs (which is equivalent to 20–25% of the body surface) to 0.5 minimal erythemal dose (MED)or good for more than twice per week exposure of arms and legs to 0.5 MED between the hours of 10am and 3pm.12 Clothing habits were designated as ‘yes’ for being veiled and ‘no’ for being non-veiled (according to coverage provided).
Laboratory studiesLaboratory studies included total serum calcium, serum PO4, alkaline phosphatase (ALP), intact PTH and 25-OH vitamin D. 25-OH vitamin D was measured using RIA (radioimmunoassay), while intact PTH was assayed using IEMA (immunoenzymetric assay) (DIAsource ImmunoAssays S.A, Belgium). The intra-assay coefficient of variation (CV) for PTH was 1.1–2% while the inter-assay coefficient of variation was 2.1–7.9%, with a normal range of 10–65pg/ml. Patients were classified, according to the Endocrine Society guidelines for 2011, into vitamin D deficient if serum 25 was ≤20ng/dl, vitamin D insufficient if it was 21–29ng/dl and vitamin D sufficient if it was ≥30ng/dl.2
Sampling and analysisAll subjects who completed the assessment had fasting blood samples drawn in the morning. Blood samples were collected by venipuncture in a 5 or 10ml evacuated glass tube. Blood samples were allowed to clot at room temperature (15–25°C) then centrifuged for 15min and stored at −20°C.
Statistical analysisAfter the collection of data, revision and tabulation analysis was performed using SPPS, version 21 (SPSS, Inc.). Continuous data were expressed as mean and ±SD, while non-parametric numerical data were expressed as median and IQR, with the value of categorical data presented as a number (percentage). The Kruskal–Wallis and Mann–Whitney tests were used to compare non-parametric and quantitative variables between groups. Stepwise regression analysis was conducted to detect predictors of vitamin D status among the study population.
ResultsVitamin D status and demographic data404 healthy females were included in this study. They were categorized into five groups, according to age group, pregnancy status and lactation status. Group 1 (lactating) included 51 females; group 2 (pregnant) included 50 females; group 3 (childbearing age) included 208 females; group 4 (the elderly) included 38 females; and group 5 (geriatric) included 57 females.
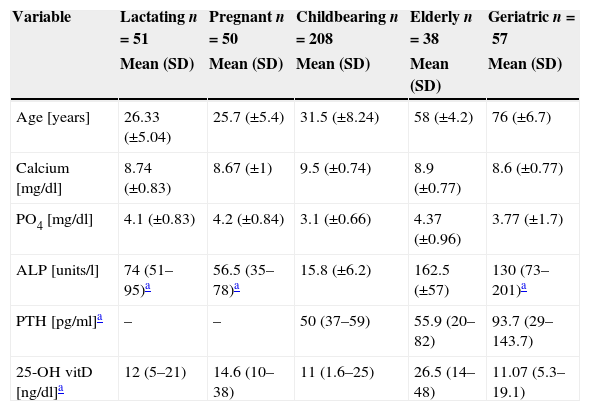
Median and IQR of 25-OH vitamin D levels across groups 1, 2, 3 and 5 were in the deficient range, being lowest in group 3 (childbearing age), with a median of 11ng/dl and an IQR of 1.6–25ng/dl, followed by group 5 (Geriatric group) a median of 11.07ng/dl and an IQR of 5.3–19.1ng/dl and group 1 (lactating group) a median of 12ng/dl and an IQR of 5–21ng/dl. Group 4 (the elderly) was in the insufficient range, with a median of 26.5 and an IQR of 14–48 (Table 1).
Vitamin D levels and other studied parameters among the study subgroups.
| Variable | Lactating n=51 | Pregnant n=50 | Childbearing n=208 | Elderly n=38 | Geriatric n=57 |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age [years] | 26.33 (±5.04) | 25.7 (±5.4) | 31.5 (±8.24) | 58 (±4.2) | 76 (±6.7) |
| Calcium [mg/dl] | 8.74 (±0.83) | 8.67 (±1) | 9.5 (±0.74) | 8.9 (±0.77) | 8.6 (±0.77) |
| PO4 [mg/dl] | 4.1 (±0.83) | 4.2 (±0.84) | 3.1 (±0.66) | 4.37 (±0.96) | 3.77 (±1.7) |
| ALP [units/l] | 74 (51–95)a | 56.5 (35–78)a | 15.8 (±6.2) | 162.5 (±57) | 130 (73–201)a |
| PTH [pg/ml]a | – | – | 50 (37–59) | 55.9 (20–82) | 93.7 (29–143.7) |
| 25-OH vitD [ng/dl]a | 12 (5–21) | 14.6 (10–38) | 11 (1.6–25) | 26.5 (14–48) | 11.07 (5.3–19.1) |
ALP: alkaline phosphatase.
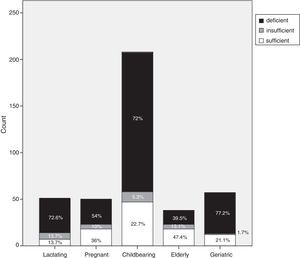
Each group was categorized, according to vitamin D cut-off values, into vitamin D deficient, vitamin D insufficient or vitamin D sufficient. Vitamin D deficiency accounted for 72.6% of the lactating group (37/51), 54% (27/50) of the pregnant group, 72% (149/208) of the childbearing age group, 39.5% (15/39) of the elderly group and 77.2% (44/57) of the geriatric group (Fig. 1).
Laboratory indicesSerum calcium was in the low to normal range in groups 1, 2 and 5, with a mean of 8.74±0.83, 8.67±1 and 8.6±0.77mg/dl, respectively. Groups 3 and 4 had a mean of 9.5±0.74mg/dl and 8.9±0.77mg/dl, respectively. Serum PO4 values were in the normal range across all groups, while ALP was highest among the elderly and geriatric groups (162.5±57 and 130 (73–201), respectively). Serum PTH was measured among the childbearing, elderly and geriatric groups and was highest in the geriatric group at 93.7pg/ml, IQR (29–143.7).
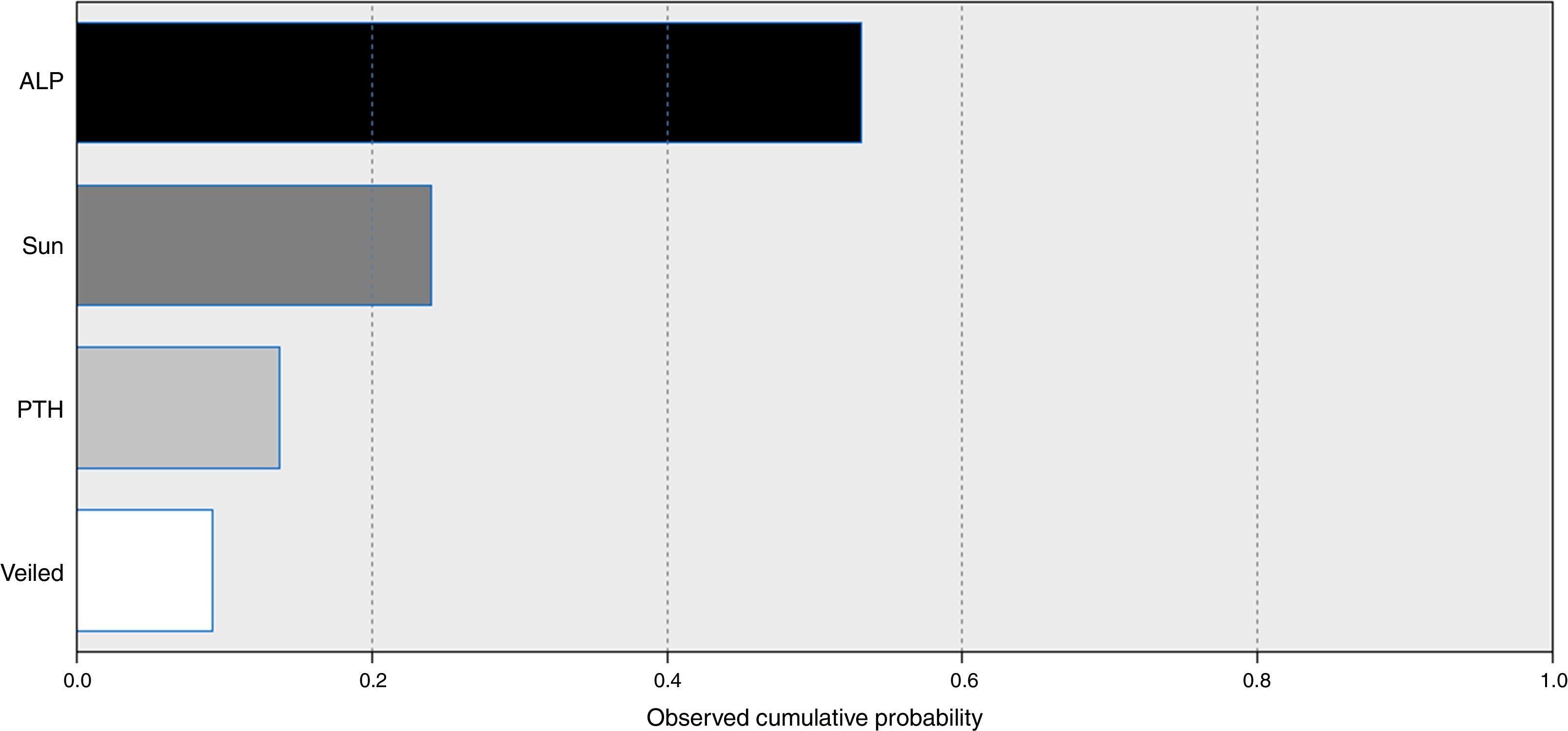
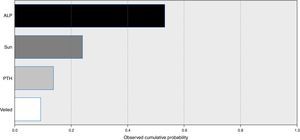
A model for stepwise regression analysis was conducted to detect predictors for vitamin D that included laboratory and lifestyle variables and indicated that predictors were ALP, sun exposure, PTH and veiling, respectively (Fig. 2).
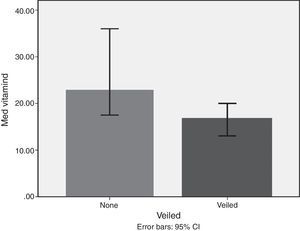
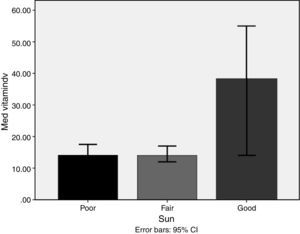
Lifestyle factorsRegrouping of the sample according to lifestyle factors indicated that veiled females had vitamin D values within the deficient range (16.7ng/dl), while non-veiled females had vitamin D values within the insufficient range (23ng/dl), with a highly significant difference between the two groups (Fig. 3). Those with a history of poor sun exposure had a median vitamin D level of 14.1ng/dl, those with a history of fair sun exposure had a median vitamin D level of 14 and those with a history of good sun exposure had a median vitamin D level of 37ng/dl (Fig. 4). Grouping according to calcium and vitamin D supplementation indicated that those with a history of supplementation had a median vitamin D level of 12.25ng/dl, while those with no history of supplementation had a median vitamin D level of 1.25ng/dl.
Finally, grouping according to dietary vitamin D and calcium intake indicated that those with a poor intake had a median vitamin D level of 13ng/dl, while those with a fair intake had a median vitamin D level of 10ng/dl. The group with poor intake had a 21% poor sun exposure, a 69% fair sun exposure and 9.6% good sun exposure compared to 37% poor sun exposure, 56% fair sun exposure and 7% good sun exposure in the fair intake group.
DiscussionVitamin D deficiency and insufficiency have become pandemic and are now seen in every country in the world. It has been estimated that more than one billion people worldwide are either vitamin D deficient or insufficient.13
Despite the fact that the reported prevalence of vitamin D deficiency among high-risk groups has been documented in several studies, the magnitude of prevalence among healthy populations is still less well-defined.14 Moreover, due to lack of information concerning the magnitude of the problem in a sunny country such as Egypt, vitamin D deficiency remains largely undiscussed. A recent systematic review of hypovitaminosis D in the Middle East & North Africa (MENA) region noted that data and knowledge concerning vitamin D status are still lacking in North Africa. In this systematic review, the few studies retrieved from Egypt were conducted among patients with other diseases, such as systemic lupus erythrematosis (SLE), fibromyalgia and HCV (hepatitis C virus). In the same review, being of the female gender was among the predictors of hypovitaminosis D.15 Thus, the goal of this study was to check the vitamin D status of healthy adult Egyptian females and determine possible contributing factors affecting the vitamin D status of the study groups.
Our study confirms that a large proportion of healthy adult Egyptian females have low vitamin D levels. The prevalence of vitamin D deficiency in our study was as follows: 72.6% of the lactating group; 54% of the pregnant group; 72% of the childbearing group; 39.5% of the elderly group; and 77.2% of the geriatric group. The prevalence of vitamin D insufficiency in our study was as follows: 13.7% of the lactating group; 10% of the pregnant group; 5.3% of the childbearing group; 13.1% of the elderly group; and 1.7% of the geriatric group.
Similar results regarding the pregnant group were reported in a recently published study conducted on 135 pregnant Egyptians; prevalence of deficiency and insufficiency among this sample was 40% and 28.9%, respectively.8 Another recent study conducted on males and females with a mean age of 20±1.96 years indicated a vitamin D deficiency prevalence of 79.2% among females (n=96), which is similar to our results in the childbearing period group (72%).9 Another study conducted among 75 adolescent females with a mean age of 14–17 years indicated a vitamin D deficiency and insufficiency prevalence of 21.3% and 24%, respectively.3
Another study conducted on a group of 105 healthy females subgrouped into premenopausal and postmenopausal indicated that 25.7% of the premenopausal group was vitamin D deficient, while 68.6% of the postmenopausal group was vitamin D deficient. It is worth noting that this study was conducted between Cairo and a rural district called Fayoum (130km southwest of Cairo). However in our study the elderly group showed only a prevalence of 39.5% vitamin D deficiency which could be attributed to exclusion of females who took vitamin D supplements in the study conducted by Wael et al.10 while in our study vitamin D supplement intake among the elderly group was 66%. Also the mean age of this group was 58±4.2 years where many females are still working and have good outdoor activity which permits sun exposure compared to the female geriatric group with a mean age of 76±6.7 who are less likely to be outdoor, more homebound and less likely to practice exercise due to cultural and social factors in addition to limited access to institutions that care for this age group. A recent study showed that there are sex differences for predictors of 25-OH vitamin D levels; time spent outdoors and intake of vitamin D supplement were among the predominant predictors of 25-OH vitamin D level in elderly females.16
A recently published study in a rural area of Egypt (Dakahlia) had contrary results to our study pertaining to the elderly population. The recently published study reported no deficiency in their sample, which included males and females aged over 60 years, compared to a prevalence of 13.1% deficiency in our study. Additionally, prevalence of insufficiency in the recently published study was only 26% compared to 39.5% in our study. The recently published study also indicated a higher prevalence of insufficiency in males than in females.11
Most of the studies conducted in Egypt concerning vitamin D status recruited subjects suffering of chronic illnesses like SLE, fibromyalgia, and HCV infection.16 Also these studies included both males and females, while only five studies were conducted on healthy females.3,8–11 Our study included only healthy females, with a larger sample size and including females of all age groups in addition to including vulnerable female groups (pregnant and lactating).17–21
Though abundant with sunshine, accumulating data from other Middle East countries indicate a prevalence of vitamin D deficiency and insufficiency. A report on the global vitamin D status published by the Scientific Advisory Committee of the IOF (International Osteoporosis Foundation) presented earlier data from some Middle East countries indicating that 70–80% of adolescent girls in Saudi Arabia and Iran had vitamin D levels of < 25nmol/L, while in Lebanon the figure was 32% in the same age group. Studies conducted among adults indicate a prevalence of 60–65% for vitamin D values <25nmol/L in Lebanon, Iran and Jordan and 48% for cut-off below 37.5nmol/L in Tunisia.22 Additionally, investigations in Saudi Arabia, Kuwait, the United Arab Emirates and Iran indicate that 10–60% of mothers and 40–80% of their neonates have undetectable or low 25-OH vitamin D levels (0–25nmol/L) at the time of delivery.15
Published studies from North Africa are rather scarce; two studies were reported from Morocco (one study included 178 elderly females in the spring, the other included 415 females aged 24–77)23,24 and two from Tunisia (one study included 261 females and 128 males, the other included 134 females)25,26; two of the four studies were conducted in spring and winter and the other two in summer. The relation to various variables as sun exposure, veiling and dietary intake was reported in only two studies.
Several studies from Latin America have indicated similar findings. One study conducted in São Paulo, Brazil indicated a prevalence of 80% vitamin D insufficiency at the end of winter in healthy populations with an age range of 18–80 years.27 Results of a recent meta-analysis from 28 studies conducted in Latin America and the Caribbean indicated that vitamin D insufficiency was prevalent across all age groups.28
Environmental and cultural factors that may have contributed to such a high prevalence were included in subsequent analysis, which indicated that sun exposure is among the top predictors, while veiling is the lowest predictor. Additionally, subgrouping according to sun exposure indicated that groups with fair and poor sun exposure were vitamin D deficient compared to the vitamin D sufficiency recorded in the good sun exposure group.
Additionally, environmental factors such as air pollution could influence the amount of UVB reaching the earth's atmosphere, which in turn influences the amount of UVB ground-level. It has been demonstrated that the level of air pollution is inversely proportionate to the amount of UVB reaching the earth.29 A number of studies have investigated the effect of air pollution on vitamin D levels among healthy populations. One study conducted in India found that atmospheric pollution rendered children in this area more prone to vitamin D deficiency.30 Another study conducted in Belgium supported a positive correlation between hypovitaminosis D and air pollution.31 Lastly, a study conducted in Tehran among healthy females indicated that the odds of having a 25-OH vitamin D level<20ng/dl was 5.22 (CI 95% 2.2–12.2).29
The most recent WHO report for the ambient air pollution database showed the PM10 and PM2.5μg/m3 (which is a measure of fine particulate matter of 10/2.5 microns or less that has been associated with health risks) in Cairo and the Delta region to be PM10 135 and 140, respectively, and PM2.5 73 and 76, respectively. They are considered to be among the areas with the highest ambient air pollution as designated by the most recent WHO map for ambient air pollution in 1600 countries.32
Subgrouping of study populations into veiled and non-veiled showed a highly significant difference between both subgroups, with veiled being deficient in vitamin D and non-veiled being insufficient in vitamin D. However, veiling emerged as the least important factor in the regression analysis model. Studies from other Middle Eastern countries indicated that veiling and conservative dressing negatively impacted 25-OH vitamin D levels.33,34
Yet, in a study conducted in São Paulo, Brazil, authors noted that although their population is not conservative in their clothing style, they suffered from an 80% prevalence of vitamin D insufficiency and attributed this to a tendency in their population toward sun-protective behavior which included the use of sunblock and indoor work.27
The results of our study indicated that those who took supplementary calcium and vitamin D had a median vitamin D level of 12.25ng/dl, while those with no history of supplementation had a median vitamin D level of 1.25ng/dl. Consequently, we found that even those taking calcium and vitamin D supplements had a vitamin D insufficiency, which could be attributed to an inadequate supplementary dose. Another finding concerning dietary intake showed that fair intake subgroup had lower vitamin D level than those with poor dietary intake which is explained by the influence of sun exposure where the poor dietary intake subgroup showed higher rates for sun exposure than the fair intake group, this is also supported by the results of the regression analysis that showed sun exposure as a predictor for vitamin D status. Vitamin D is present in many food products but rather in low concentrations so that non-fortified dietary intake cannot suffice the needs and influence vitamin D status.35
LimitationsOur study did not include questionnaires pertaining to socioeconomic status and parity, which have been previously reported in other studies as predictors of vitamin D status. In addition, our study was primarily hospital-based and the need to establish local guidelines necessitates large population-based studies.
ConclusionAlarming levels of vitamin D deficiency among Egyptian females across all age groups have been demonstrated in our study. Therefore, we recommend the implementation of large, population-based studies that could lead to reformative action by policy makers regarding vitamin D food fortification and the establishment of evidence-based local guidance to address this epidemic.
Declaration of interestThere is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
FundingThis research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author contribution statementAll authors have participated sufficiently in the conception and design of this work or the analysis and interpretation of the data, as well as the writing and reviewing of the final version of the manuscript.