There are two types of adipose tissue in the body whose function appears to be clearly differentiated. White adipose tissue stores energy reserves as fat, whereas the metabolic function of brown adipose tissue is lipid oxidation to produce heat. A good balance between them is important to maintain energy homeostasis. The concept of white adipose tissue has radically changed in the past decades, and is now considered as an endocrine organ that secretes many factors with autocrine, paracrine, and endocrine functions. In addition, we can no longer consider white adipose tissue as a single tissue, because it shows different metabolic profiles in its different locations, with also different implications. Although the characteristic cell of adipose tissue is the adipocyte, this is not the only cell type present in adipose tissue, neither the most abundant. Other cell types in adipose tissue described include stem cells, preadipocytes, macrophages, neutrophils, lymphocytes, and endothelial cells. The balance between these different cell types and their expression profile is closely related to maintenance of energy homeostasis. Increases in adipocyte size, number and type of lymphocytes, and infiltrated macrophages are closely related to the metabolic syndrome diseases. The study of regulation of proliferation and differentiation of preadipocytes and stem cells, and understanding of the interrelationship between the different cell types will provide new targets for action against these diseases.
En el organismo existen 2 tipos de tejido adiposo cuya función parece estar bien diferenciada. El tejido adiposo blanco almacena reservas energéticas en forma de lípidos, mientras que la función metabólica del tejido adiposo marrón es la oxidación de lípidos para producir calor. Un buen equilibrio entre ambos va a ser importante para mantener la homeostasis energética. En las últimas décadas ha cambiado radicalmente el concepto de tejido adiposo blanco, considerándose hoy en día un órgano endocrino que secreta numerosos factores con funciones autocrinas, paracrinas y endocrinas. Además, ya no podemos hablar del tejido adiposo blanco como un solo tejido, ya que las diferentes localizaciones muestran un perfil metabólico diferente con consecuencias también diferentes. Si bien la célula que caracteriza el tejido adiposo es el adipocito, este no es el único tipo celular presente en el tejido adiposo, ni siquiera el más abundante. Entre los otros tipos celulares del tejido adiposo se han descrito células madre, preadipocitos, macrófagos, neutrófilos, linfocitos y células endoteliales. El equilibrio entre estos diferentes tipos celulares, así como su perfil de expresión, están estrechamente relacionados con el mantenimiento de la homeostasis energética. El incremento del tamaño de los adipocitos, del número y del tipo de linfocitos y macrófagos infiltrados está estrechamente relacionado con las enfermedades del síndrome metabólico. El estudio de la regulación de la proliferación y diferenciación de preadipocitos, células madre, así como la comprensión de la interrelación entre los diferentes tipos celulares, nos van a aportar nuevas dianas para actuar frente a estas patologías.
Lipids are stored in the body in two types of adipose tissue: white adipose tissue (WAT) and brown adipose tissue (BAT). WAT is the main energy storage tissue in the body, and to it is also attributed the function of isolating and providing mechanical protection to some vital organs. Mature adipocytes in WAT show the expression profile required for the synthesis of triacylglycerols, glucose uptake, and lipogenesis, as well as lipolysis.1 It is this phenotype which ensures that, when energy supply to the body is excessive and/or energy expenditure is decreased, excess energy is efficiently deposited in WAT as triacylglycerols. On the other hand, when energy intake is inadequate and/or energy expenditure is increased, WAT mobilizes lipid deposits to release fatty acids and glycerol which are transported in blood to tissues, where they are oxidized to obtain energy.2 By contrast, BAT has the physiological role of metabolizing fatty acids to produce heat.3 This specific role of BAT is supported by the high proportion in its mitochondria of uncoupling protein-1 (UCP-1), which short-circuits the coupling of the respiratory chain to ATP synthetase, thus facilitating heat production.3 The functional differences and balance between WAT and BAT contribute to the maintenance of energy balance in the body.
WAT was once considered to be a poorly active tissue, but is now known to be highly dynamic, synthesizing and secreting multiple lipid and protein factors which are involved in the regulation of a wide range of physiological and metabolic processes. While WAT was already reported in the late 1980s to secrete steroid hormones,4 it was not until 1994—with the discovery of leptin,5 a satiety factor mainly synthesized by WAT—that WAT was recognized as an endocrine organ. Many factors released by WAT with autocrine, paracrine, and endocrine functions have subsequently been identified.
Among the multiple substances secreted by WAT, fatty acids are quantitatively the most important molecules and are released during periods of negative energy balance, such as during fasting.2 Other lipid molecules are also secreted by WAT, including prostanoids, synthesized by tissue itself, cholesterol and retinol, which are stored in order to be subsequently released,6 and steroid hormones (sex steroids and glucocorticoids), which may experience in WAT transformations from inactive to active forms and vice versa, with significant autocrine and paracrine roles.7
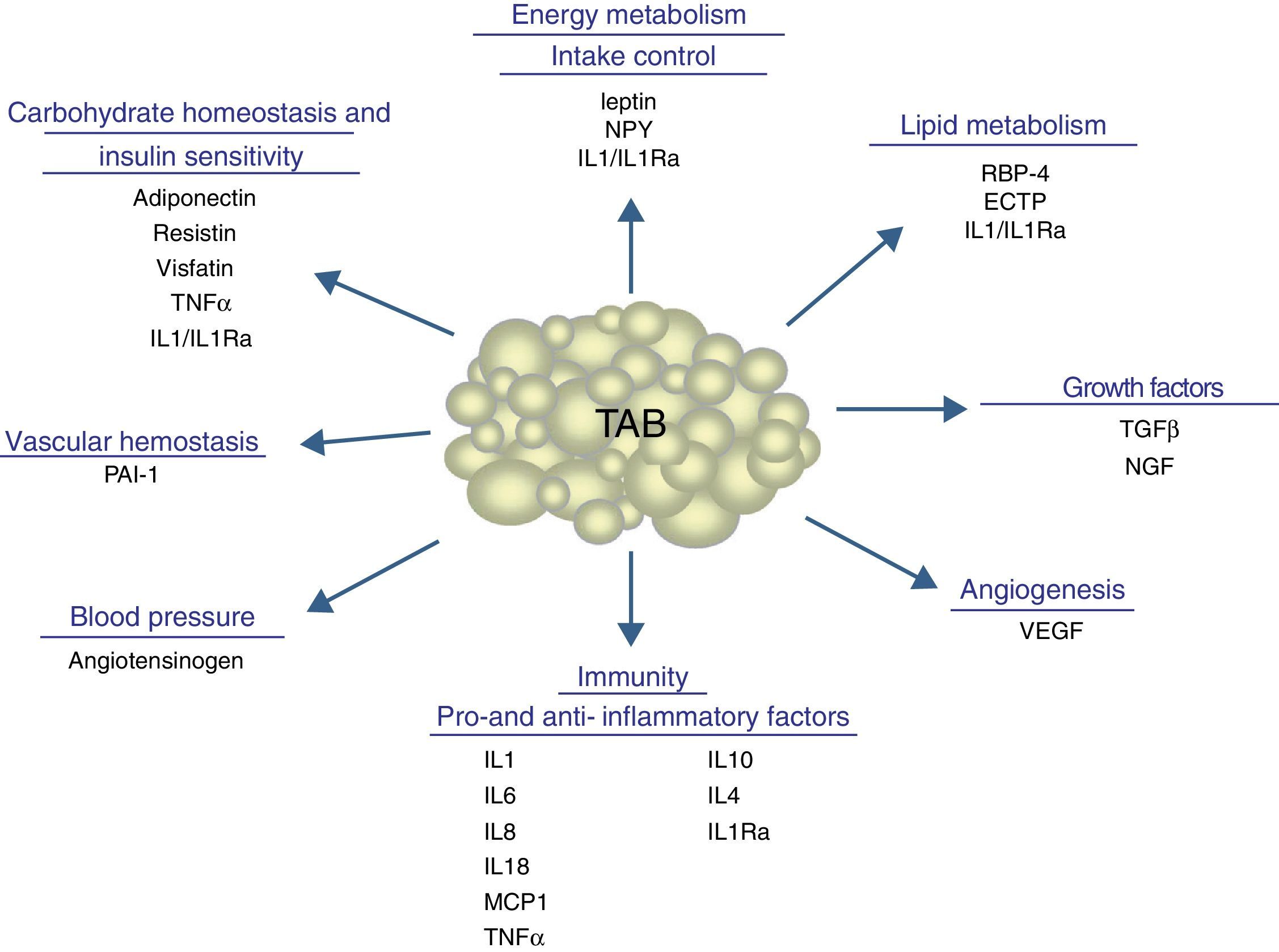
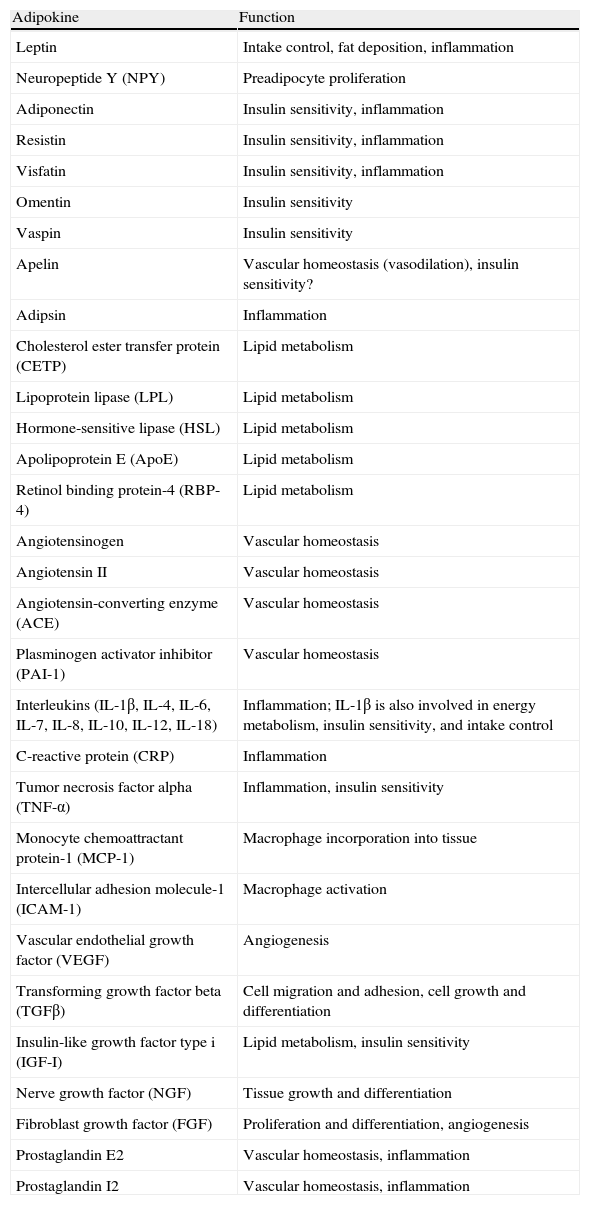
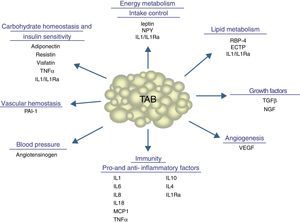
In addition to lipid substances, adipose tissue secretes a substantial number of protein factors (of which more than 50 have been reported) which are collectively called adipokines. Strictly speaking, the term “adipokine” should be used to designate the proteins synthesized and secreted by adipocytes.8 However, the term is generically used to refer to proteins synthesized and secreted by the whole WAT, although they are mainly synthesized by other cell types present in tissue, such as infiltrated macrophages. Adipokines have highly diverse chemical structures and physiological roles9–11 and, interestingly, many of them are related to the immune system, including classical cytokines such as TNF-α, IL-1β, IL-6, IL-8, IL-10, IL-4, IL-13, and MCP-1, and a link may have been established between inflammation and obesity, a condition where the secretion of proinflammatory adipokines is increased.12 Adipokines also include proteins involved in the regulation of energy intake and balance (leptin), in blood pressure regulation (angiotensinogen), in vascular hemostasis (PAI-1), in lipid metabolism (RBP-4, CETP), in carbohydrate homeostasis (adiponectin, resistin, visfatin), and in angiogenesis (VEGF), as well as growth factors (TGFβ) and acute phase and stress response proteins (haptoglobulin, α1-acid glycoprotein) (Fig. 1). As noted, many adipokines with wide and significant regulatory roles at different physiological levels have been reported. Table 1 lists some adipokines and their main roles. It should be noted that this endocrine function is not unique to WAT, because many of these factors are also synthesized by BAT.3
Physiological and metabolic processes regulated by WAT by adipokine secretion. CETP: cholesterol ester transfer protein; IL1: interleukin 1; IL1Ra: interleukin receptor antagonist-1; IL4: interleukin 4; IL6: interleukin 6: IL8: interleukin 8; IL10: interleucin-10; IL18: interleukin-18; MCP-1: monocyte chemoattractant protein-1; NGF: nerve growth factor; NPY: neuropeptide Y; RBP-4: retinol binding protein-4; TGFβ: transforming growth factor β; TNFα: tumor necrosis factor alpha: VEGF: vascular endothelial growth factor.
Adipokines secreted by white adipose tissue and physiological function.
| Adipokine | Function |
| Leptin | Intake control, fat deposition, inflammation |
| Neuropeptide Y (NPY) | Preadipocyte proliferation |
| Adiponectin | Insulin sensitivity, inflammation |
| Resistin | Insulin sensitivity, inflammation |
| Visfatin | Insulin sensitivity, inflammation |
| Omentin | Insulin sensitivity |
| Vaspin | Insulin sensitivity |
| Apelin | Vascular homeostasis (vasodilation), insulin sensitivity? |
| Adipsin | Inflammation |
| Cholesterol ester transfer protein (CETP) | Lipid metabolism |
| Lipoprotein lipase (LPL) | Lipid metabolism |
| Hormone-sensitive lipase (HSL) | Lipid metabolism |
| Apolipoprotein E (ApoE) | Lipid metabolism |
| Retinol binding protein-4 (RBP-4) | Lipid metabolism |
| Angiotensinogen | Vascular homeostasis |
| Angiotensin II | Vascular homeostasis |
| Angiotensin-converting enzyme (ACE) | Vascular homeostasis |
| Plasminogen activator inhibitor (PAI-1) | Vascular homeostasis |
| Interleukins (IL-1β, IL-4, IL-6, IL-7, IL-8, IL-10, IL-12, IL-18) | Inflammation; IL-1β is also involved in energy metabolism, insulin sensitivity, and intake control |
| C-reactive protein (CRP) | Inflammation |
| Tumor necrosis factor alpha (TNF-α) | Inflammation, insulin sensitivity |
| Monocyte chemoattractant protein-1 (MCP-1) | Macrophage incorporation into tissue |
| Intercellular adhesion molecule-1 (ICAM-1) | Macrophage activation |
| Vascular endothelial growth factor (VEGF) | Angiogenesis |
| Transforming growth factor beta (TGFβ) | Cell migration and adhesion, cell growth and differentiation |
| Insulin-like growth factor type i (IGF-I) | Lipid metabolism, insulin sensitivity |
| Nerve growth factor (NGF) | Tissue growth and differentiation |
| Fibroblast growth factor (FGF) | Proliferation and differentiation, angiogenesis |
| Prostaglandin E2 | Vascular homeostasis, inflammation |
| Prostaglandin I2 | Vascular homeostasis, inflammation |
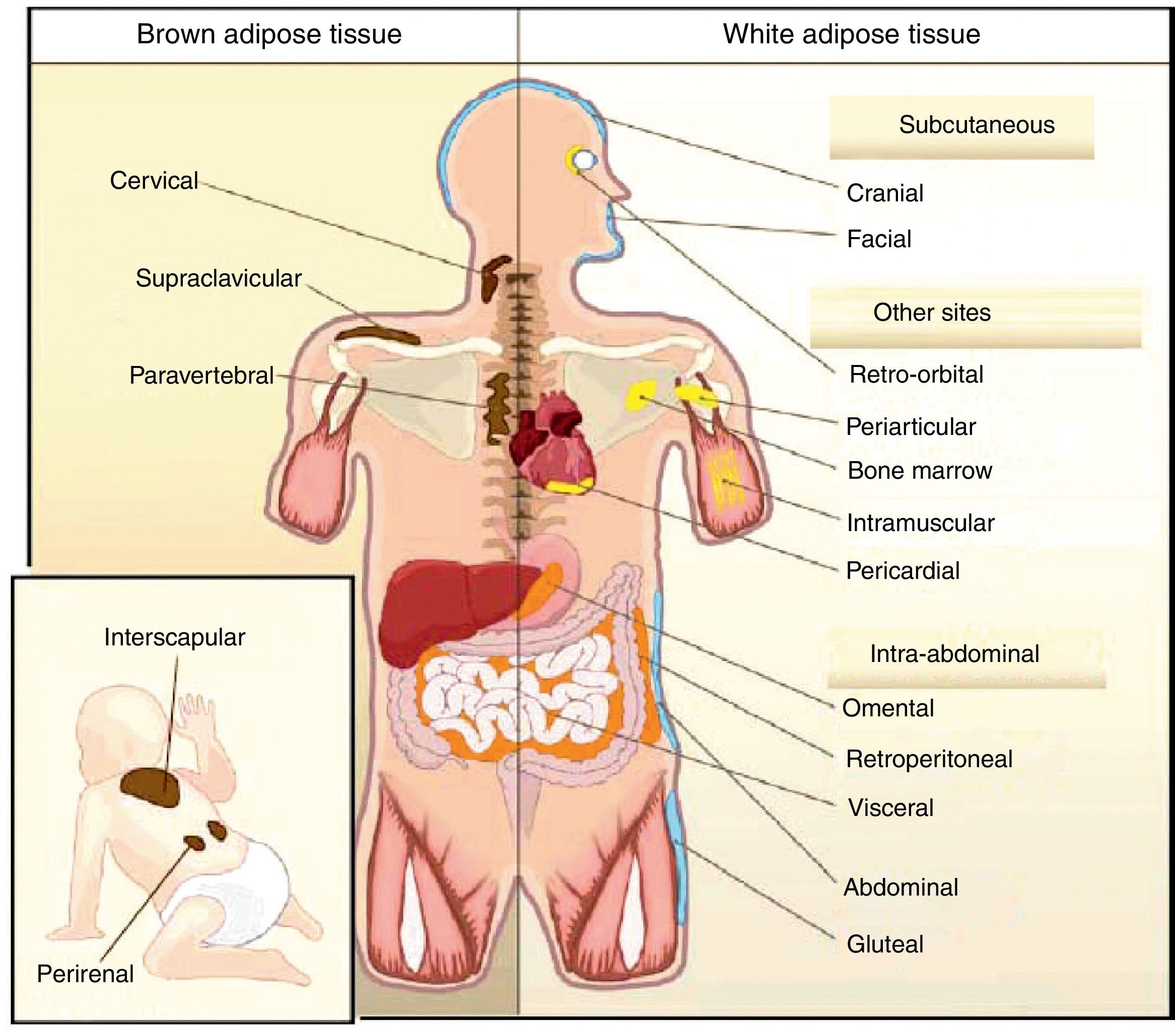
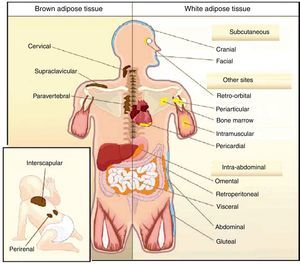
All animal species have found a way of storing excess energy for future needs. Thus, for example, the nematode Caenorhabditis elegans accumulates fat in bowel epithelium,13 while sharks accumulate it in the liver,14 both of them tissues of an endodermal origin. In most species, however, lipids are stored in WAT, which has a mesodermal origin. Despite having a common embryonic origin, WAT distribution varies depending on the species. In invertebrates, as well as amphibia and reptiles, intra-abdominal fat predominates. Subcutaneous fat predominates in seals and whales, while fat is distributed in both locations in all other mammals and in birds. This different distribution of WAT cannot simply be explained by evolutionary adaptation to warmer or colder climates, as it has been seen that for a same body mass, mammals adapted to arctic or tropical environments have a similar body fat distribution.15 In humans, WAT is distributed all over the body. Inside the abdomen, the greatest deposits are found around the omentum (omental), bowel (mesenteric), and perirenal areas (retroperitoneal), while at subcutaneous level the fat is mainly located in the buttocks, thighs, and abdomen. However, in addition to these main deposits, there are other areas in the body where WAT is found, including pericardial, perivascular or periarterial, periarticular, retro-orbital, intramuscular, bone marrow, and face deposits (Fig. 2). It is now known that the different WAT locations have different metabolic and endocrine characteristics.16,17 Thus, for example, WAT in the breasts and buttocks is highly sensitive to estrogens, while WAT in the upper back and neck is more sensitive to glucocorticoids. Visceral (intra-abdominal) WAT has an adipokine secretion profile related to inflammation and type 2 diabetes, while subcutaneous WAT shows less secretion of proinflammatory adipokines. In perivascular WAT, widely distributed throughout arterial circulation, a proinflammatory secretion profile similar to that of visceral WAT has been reported. It has been suggested that perivascular (periarterial) WAT may have originated as a physiological mechanism to regulate post-prandial blood flow distribution between and within organs. However, excess periarterial WAT may be harmful. Thus, under conditions of inactivity or excess energy, these deposits increase and, as a consequence, there is an increased production of proinflammatory adipokines that may promote atherosclerosis.18 WAT distribution varies with age, so that a trend to an increased intra-abdominal fat and a decreased subcutaneous fat is seen with advancing age. This occurs even in subjects with a stable weight and body mass index (BMI). Fat distribution is also influenced by genetic factors, as exemplified by the marked fat accumulation in the buttocks of women from the Hottentot/Khoisan South African ethnic group (Fig. 3). There is also sexual dimorphism in body fat distribution. Thus, males have a greater body accumulation in the upper body, the so-called android or apple-like distribution, while fat predominates in the lower body of females, leading to the so-called gynoid or pear-shaped distribution. The reverse situation may however occur depending on genetic background, and women with android fat distribution, and vice versa, may be found.
Obesity is defined as excess body fat, but based on the above, it is associated with a higher or lower risk of cardiovascular disease and type 2 diabetes depending on the localization of excess fat. When fat deposition is increased in the upper body (in other words, is of the apple-like type), specifically intra-abdominal fat, there is an increased risk of metabolic complications.19
Unlike WAT, BAT is only found in mammals. BAT occurs later in life and is related to thermal regulation capacity, that is, with homeothermy and the capacity of non-shivering thermogenesis. The occurrence of BAT is however not only related to homeothermy, because birds are endothermic (homeothermic) animals, but have no BAT, their capacity of non-shivering thermogenesis depending on UCP expression in muscle.20 In rodents, BAT is particularly abundant in the perinatal period, but persists throughout their adult life. BAT is distributed in different body locations, being most abundant in the interscapulara area.21 It has been reported in recent years that brown adipocytes are also found in typical WAT deposits.21
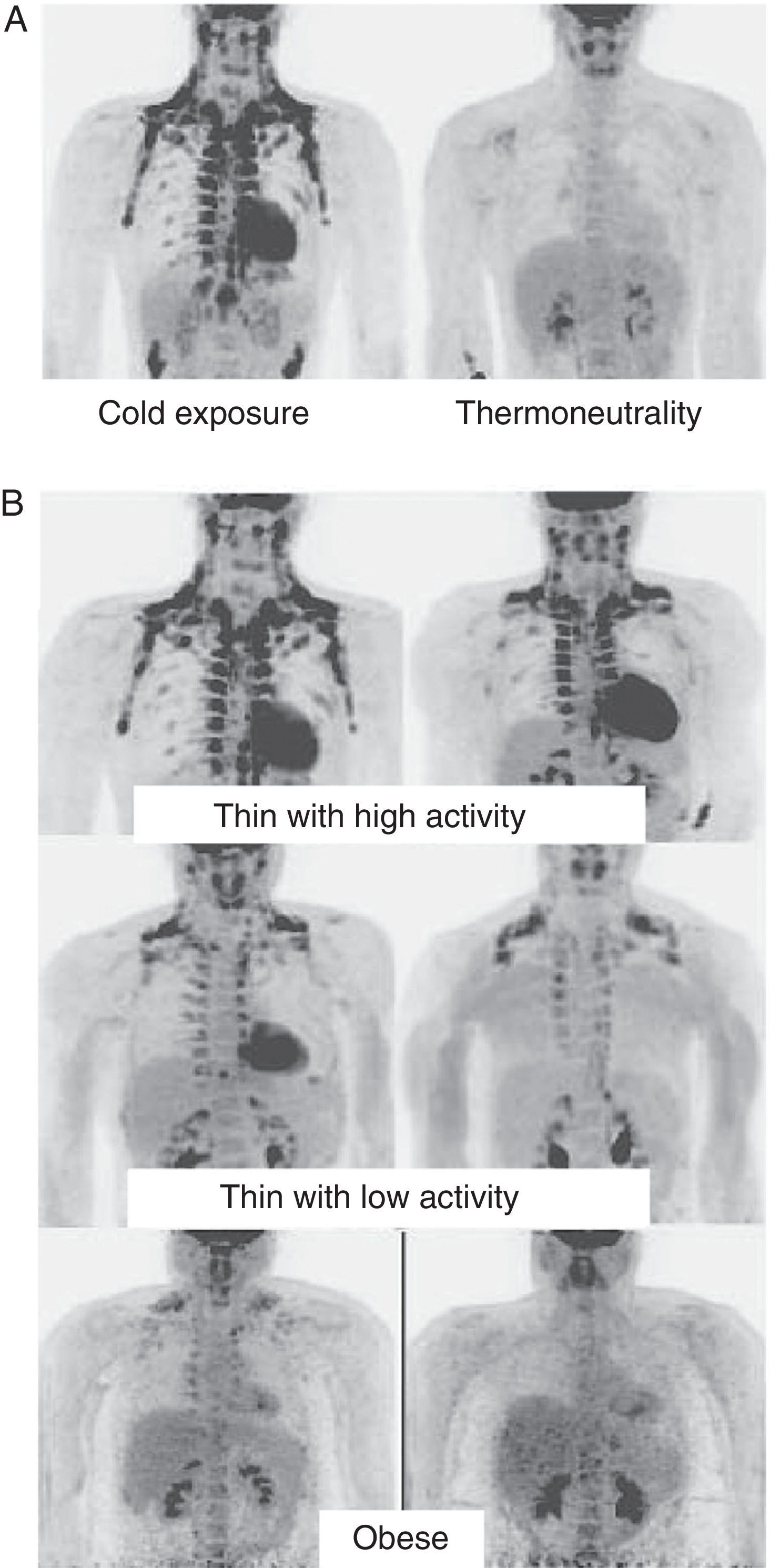
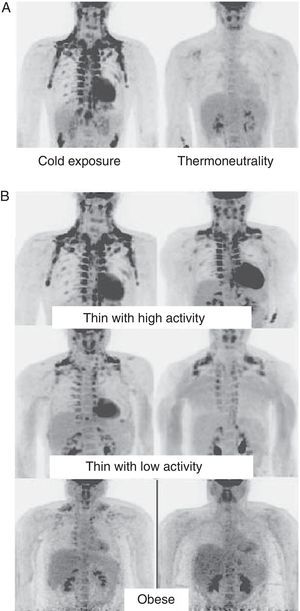
In humans, BAT is found in fetuses and newborns at axillary, cervical, perirenal, and periadrenal levels,3 but its presence rapidly decreases after birth and has traditionally been considered negligible in adults, except in patients with pheochromocytoma22 or subjects exposed to cold climates for a long time.23 However, recent studies using positron emission tomography (PET) have shown that the presence of BAT in adult humans may not be so rare as previously thought (Fig. 4). These studies found an increased uptake of 18F-fluorodeoxyglucose (18F-FDG) in PET in adipose tissue from the paracervical, supraclavicular, and paravertebral regions in healthy individuals exposed to cold,24,25 with the highest values found in the supraclavicular area. The presence of UCP-1 as a marker of BAT and the distinctive multivacuolar morphology of BAT were confirmed in biopsies.25,26 These BAT areas were seen to be highly innervated by the sympathetic nervous system, in contrast to adjacent WAT areas.27 Quantification of non-shivering thermogenesis by cold exposure using 18F-FDG-PET showed that regions with functional BAT were more common in women than men and that the amount of BAT decreased with age and was inversely correlated with BMI, particularly in the elderly.26 BAT is found in a higher proportion of young people as compared to elderly people, but BAT activity is decreased in overweight or obese young people.25 These studies appear to definitively show that BAT plays a role in energy metabolism in human adults, and the fact that it is found in lower amounts in overweight or obese people may suggest a new target for the treatment of obesity.
WAT distribution and activity in humans detected by positron emission tomography (PET) with 18F-FDG. (A) Increased TAM activity in a thin individual exposed to low temperature (16°C) or under thermoneutral conditions. (B) TAM activity in thin and obese individuals exposed to low temperature (16°C).
In addition to these metabolically active BAT regions immersed in different locations of adipose tissue, deposits of brown adipocytes positive for UCP-1 have also been reported interspersed between limb muscles in a mouse strain resistant to diet-induced obesity. This suggests that ectopic BAT locations may also play a significant role in the regulation of energy homeostasis.28
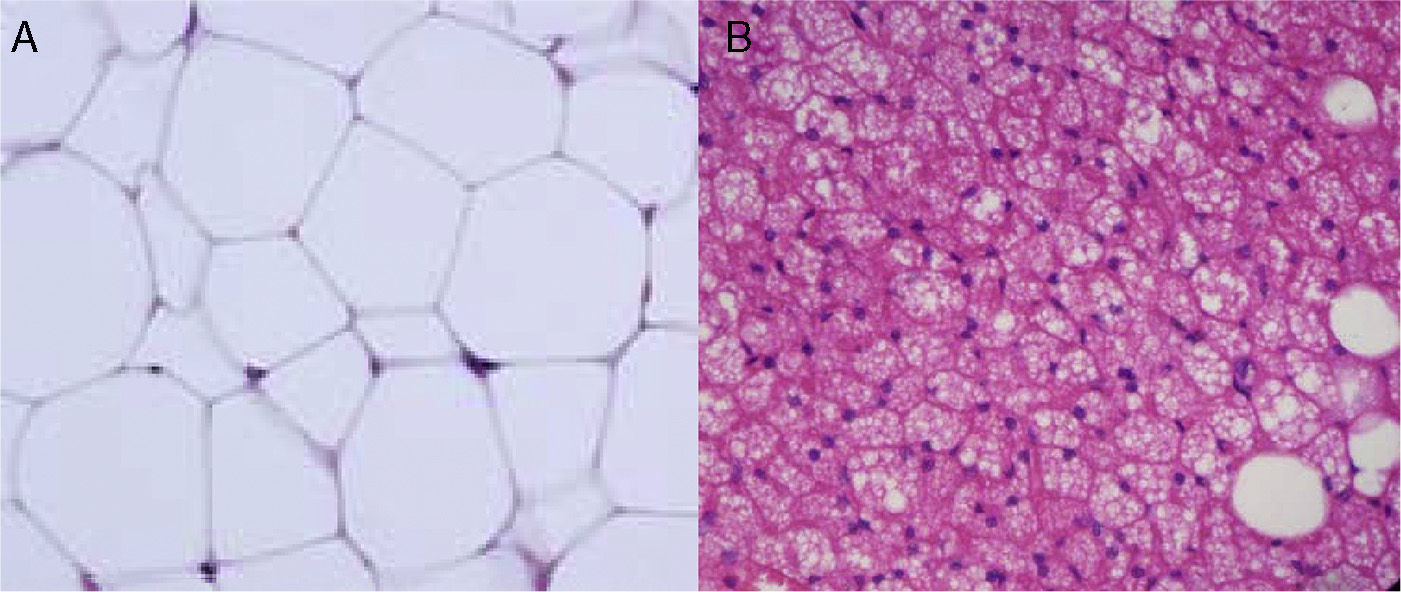
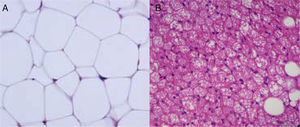
Cellular heterogeneity of adipose tissueAdipocytes are the characteristic cells of adipose tissue. They are big cells specialized in lipid accumulation, as previously stated. The difference between WAT and BAT adipocytes is that while white adipocytes have a single fat vacuole occupying the whole cytoplasm, brown adipocytes are characterized by the presence of multiple fat vacuoles, and also by an abundance of mitochondria in their cytoplasm (Fig. 5). Because of the size of mature adipocytes, histological observations formerly suggested that these were virtually the only cells in adipose tissue, or at least the most abundant ones by far. However, it is now known that although adipocytes virtually occupy the whole tissue size, they are not the only cell type present, or possibly even the most abundant type. It has been reported that approximately 60% (subcutaneous) to 80% (visceral) of WAT cells from obese humans are not mature adipocytes, but “vascular stroma” cells”.29 Cells forming vascular stroma include preadipocytes, macrophages, stem cells, and endothelial cells,29 and the presence of neutrophils30 and lymphocytes31 has also been reported. The proportions of the different cell types in vascular stroma may vary depending on the physiological situation (inflammation) and location of WAT. It has been estimated that, in WAT from obese humans, both preadipocytes and macrophages account for approximately 10%.
Preadipocytes and adipocytesPreadipocytes are small cells with a morphology similar to fibroblasts which, after adequate stimulation, are converted into mature adipocytes. Adipose tissue mass in adults is determined by the increase in adipocyte size (hypertrophy) up to a certain point, and by the increase in adipocyte number (hyperplasia). Adipocyte volume reflects the balance between lipogenesis and lipolysis, while adipocyte number reflects the balance between preadipocyte proliferation, differentiation, and apoptosis, and apoptosis of adipocytes. The ability of preadipocytes to proliferate and differentiate, as well as their susceptibility to apoptosis, differ depending on tissue location, which may account for the differences found in fat deposition in obese humans.32 The Spalding et al. study33 found that approximately 10% of adipocytes in the whole body are regenerated every year. This study found that the number of adipocytes is fixed during childhood and adolescence and remains relatively constant in adults. Obese individuals already have a higher number of adipocytes in childhood, but adipocyte turnover is similar in thin and obese subjects.
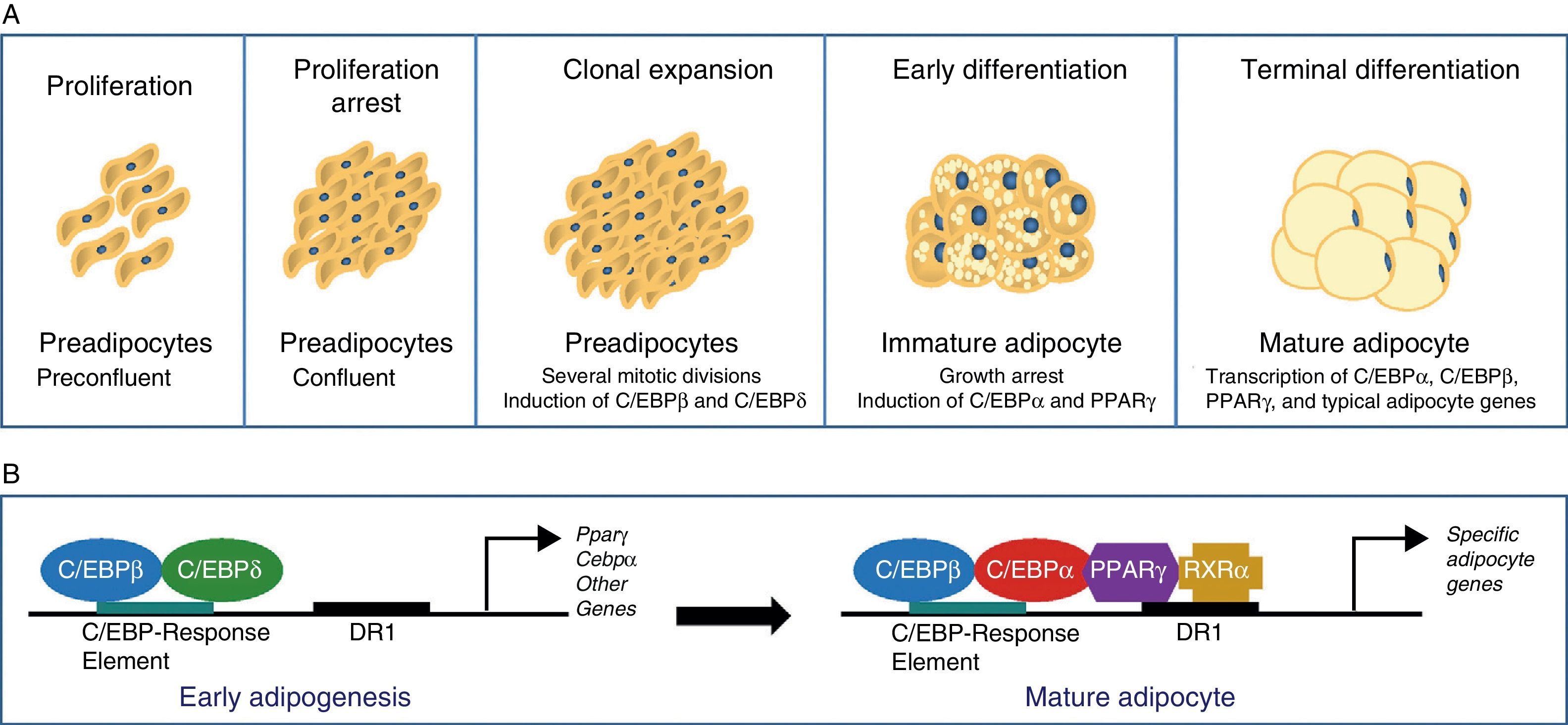
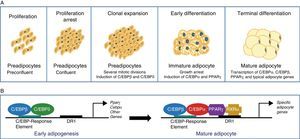
The differentiation process from preadipocytes to adipocytes has been widely investigated in various cell models of white preadipocytes (3T3-L1, 3T3-F442A) and immortal lines of brown preadipocytes.34 Thus, four stages are reported in the transition process from preadipocyte to mature adipocyte. Proliferation arrest induced by contact inhibition indicating that confluence has been reached first occurs, after which the preadipocyte is committed to differentiating into an adipocyte. Clonal expansion induced by hormone signals and represented by a few mitotic divisions intended to synchronize the cell cycle follows. An early differenciation stage is reported where cell division is arrested, characteristic adipocyte genes start to express themselves, and lipid accumulation starts. The typical adipocyte morphology is finally reached with the terminal differentiation phase, and the transcription of genes typical of mature adipocytes is induced (Fig. 6). The coordination of these stages is under the control of a complex transcriptional cascade of regulatory factors where nuclear receptor PPARγ and various members of the C/EBP family of transcription factors play an essential role.35 PPARγ has been shown to be required for adipocyte differentiation and also for the maintenance of differentiation. When PPARγ is silenced in already differentiated 3T3-L1 adipocytes, dedifferentiation with lipid loss and decreased expression of mature adipocyte markers are induced.36 Of the 2 isoforms of PPARγ, PPARγ2 is preferentially expressed in adipose tissue.36 The C/EBP family of transcription factors includes 5 members: C/EBPα, C/EBPβ, C/EBPδ, C/EBPγ, and CHOP. The sequential expression of these factors has been seen during adipocyte differentiation, so that early expression is required of C/EBPβ and C/EBPδ, which promote the expression of C/EBPα and PPARγ, essential for total adipocyte differentiation.35 C/EBPβ expression therefore appears to be essential in the early differentiation stages. Thus, animals deficient in C/EBPβ show a decreased adipose tissue mass.37 In contrast, C/EBPα is required for adipogenesis together with PPARγ, although the latter appears to be dominant in the process38 (Fig. 6).
Understanding of the preadipocyte to adipocyte differentiation process is therefore an essential precondition for responding to problems such as obesity. Preadipocytes from WAT in different body sites have been seen to have specific characteristics when cultured in vitro. In humans, preadipocytes from subcutaneous abdominal WAT have been reported as having greater proliferation ability and differentiation and less susceptibility to apoptosis than preadipocytes from omental WAT,16,32 thus making it possible to differentiate between populations of preadipocytes with rapid and slow proliferation. Both types of preadipocytes were subsequently reported to occur simultaneously in the same location, but one or the other type predominated depending on the location. Thus, subcutaneous abdominal WAT has been found to contain approximately 50% of each subtype, while slowly proliferating preadipocytes account for 90% of those present in omental WAT.32 The presence of 2 preadipocyte subpopulations with different characteristics but with the ability to differentiate could be a potential mechanism explaining the plasticity in adipose tissue development. The abundance of each subpopulation could be regulated in response to specific stimuli, e.g. proinflammatory cytokines.
In addition to the heterogeneity of the preadipocyte population, differences between mature adipocytes in a same WAT site have also been reported. Thus, 2 adipocyte populations have been reported in fat-specific insulin receptor knockout (FIRKO) mice or hormone-sensitive lipase (HSL) knockouts: one of small diameter (<50μm) and the other with a longer diameter (>150μm).39–41 Two adipocyte populations of different size have also been found in the abdominal subcutaneous tissue of healthy subjects.42 The two populations have different expression profiles. Bigger adipocytes show an increased expression of proteins related to inflammation (IL-8, CXCL2 [chemokine (C-X-C motif) ligand 2], E-selectin, SAA2 [serum amyloid A2], C1QR1 [complement component 1 q subcomponent receptor 1]) and, thus, metabolic syndrome. Other studies have reported an inverse relationship between adipocyte size and the expression of lipogenic genes, as well as a strong relationship between small adipocytes and greater insulin sensitivity.43
In both humans and rodents, BAT deposits have been observed to be gradually replaced by WAT with age. Under certain conditions, such as adrenergic stimulation or cold exposure, an increase is also seen in the number of brown adipocytes immersed in WAT.3,24,25 There is therefore a marked plasticity between WAT and BAT suggesting the possibility that among the heterogeneous vascular stromal cells of WAT there are also preadipocytes targeted to differentiate into brown adipocytes. Controlling the number of each of these populations and their specific differentiation could provide new treatments for obesity and its complications. In this regard, it may be noted that there is some difficulty involved in their study in that there are no markers that may identify preadipocytes with a different potential to differentiate and which are morphologically indistinguishable from any cell type with fibroblast morphology.
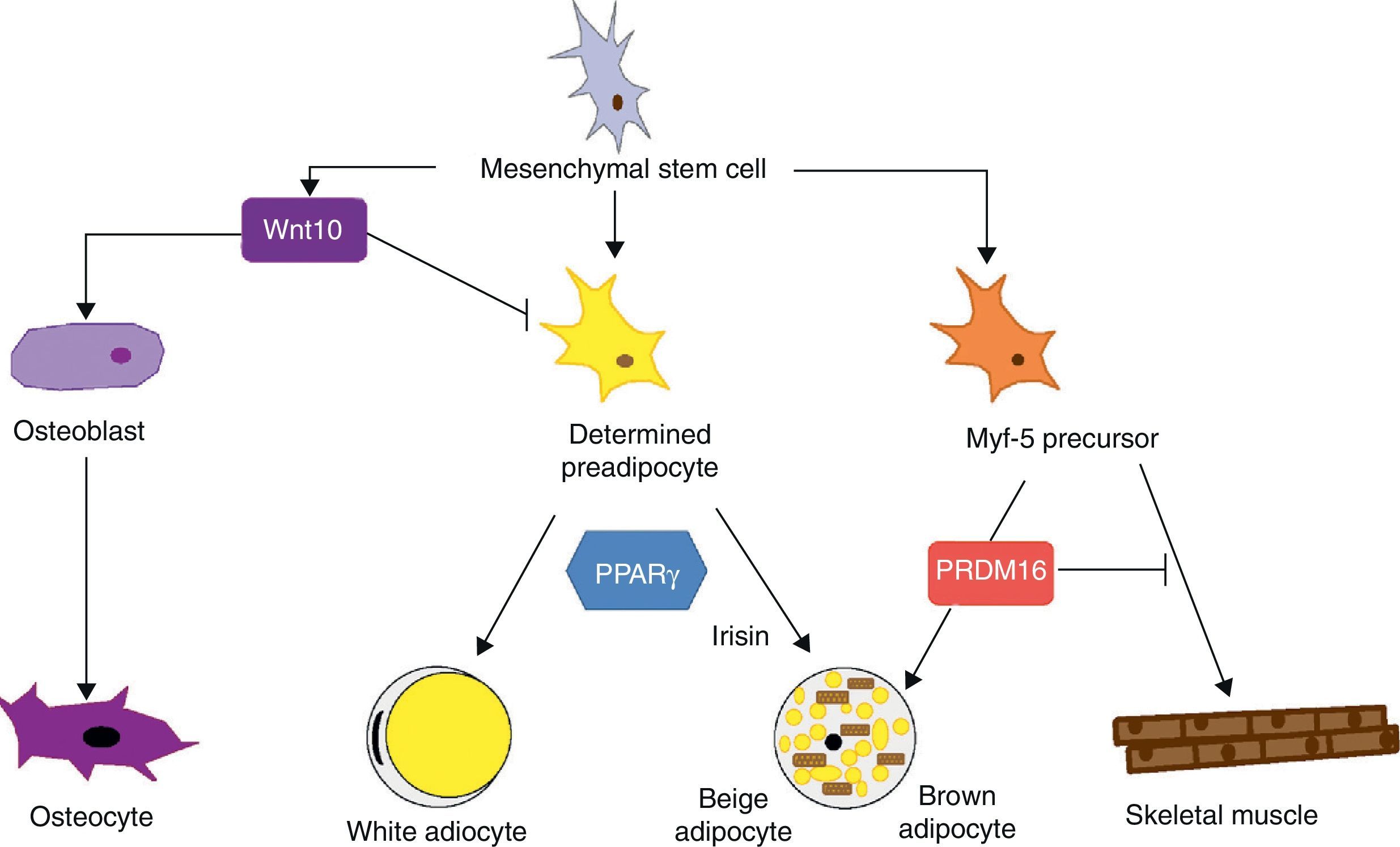
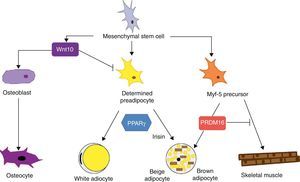
Mesenchymal stem cells (MSCs) isolated from bone marrow have been widely used to study the differentiation of tissues of a mesodermal origin, such as adipose tissue. MSCs are able to differentiate into adipocytes, osteoblasts, chondrocytes, myoblasts, and connective tissue (Fig. 7) depending on the environment where they are found.44 The intermediate stages between a mesenchymal stem cell and the mature adipocyte are not well known, and the theory initially considered as most feasible was that MSCs result in a common precursor (adipoblast) that is subsequently able to differentiate into a white or brown adipocyte depending on the specific stimuli it receives. This theory is however not fully clear, because the lack of specific markers makes it difficult to establish whether a single precursor exists or whether there are different precursors for adipoblasts and/or white and brown preadipocytes, or whether this precursor is different even for preadipocytes from different WAT sites. The notion that preadipocytes from different WAT deposits have a different origin is supported by some observations. First, the different WAT deposits show chronological variations in their embryonic development. In rodents, for example, periovarian and subcutaneous deposits develop first, followed by the omental deposit. Second, it has been widely reported that subcutaneous and visceral WAT have substantially different expression patterns.45 And third, this differential expression profile appears to be an intrinsic characteristic of tissue. As previously stated, it has been noted in preadipocyte cultures that subcutaneous preadipocytes are more able to proliferate and differentiate than visceral preadipocytes.32 It has also been reported that some factors may affect the development of the different fat deposits in different ways depending on their response capacity and, thus, on the intrinsic characteristics of tissue. For example, transgenic mice overexpressing 11βhydroxysteroid dehydrogenase in WAT have the same enzyme level in several tissues but only develop visceral obesity.7
Differentiation of mesenchymal stem cell into different cell types. Recent studies support the theory that there is no common precursor for white and brown preadipocytes. Brown preadipocytes have a “myogenic signature”. However, brown adipocytes immersed in WAT masses appear to come from a different precursor than those located in BAT masses. These adipocytes immersed in WAT with UCP-1 expression have been called “beige adiocytes” and are especially sensitive to the hormone irisin.
Differences between WAT location and expression profile have also been reported in relation to genes implicated in embryonic development, specifically for the Glyp4, Tbx15, and HoxA5 families. Mouse adipocytes and preadipocytes isolated from subcutaneous and visceral WAT show a different expression pattern of Glyp4, Tbx15 and HoxA5 that is maintained when these preadypocites are subcultured and differentiated in vitro.45 The expression profile of these genes in humans has been seen to be closely correlated to the BMI and the fat distribution pattern.45 Specifically, HoxA5 and Tbx15 expression predominate in visceral WAT, while Glyp4 is more strongly expressed in subcutaneous WAT. In visceral WAT, Glyp4 and HoxA5 expression positively correlate to BMI and waist circumference (WC), while Tbx15 expression shows a negative correlation.
In subcutaneous WAT, HoxA5 expression positively correlates to BMI and WC, while Glyp4 expression negatively correlates to both parameters, and Tbx15 expression positively correlates only to BMI. In addition, Glyp4 expression levels in subcutaneous WAT are extremely low for BMI values above 30. It has therefore been suggested that the expression of these genes represents an excellent marker of visceral fat acumulation.45
As regards the lineage specificity of WAT and BAT, differences in their development pattern supporting a specific tissue determination are also seen. However, the ability of WAT to trans-differentiate to BAT21 supports the hypothesis of a common precursor for both adipose tissues. Recent studies argue that white and brown preadipocytes originate from a different cell lineage and are predetermined to a single differentiation.46 These studies found that white and brown preadipocytes develop a different expression pattern, which in the case of brown preadipocytes has a “myogenic signature” (Myf-5-lineage). Other studies, in addition to supporting these findings, show that precursor cells of skeletal muscle may also differentiate into brown adipocytes.47 The decision to differentiate this progenitor into brown adipocyte or muscle cell is regulated by the transcription factor PRDM16: its expression induces differentiation into a brown adipocyte, and its silencing induces differentiation into a muscle cell.47 This interlineage between myocyte and brown preadipocyte could indicate a different origin of WAT and BAT which would in turn provide a plausible answer to the question of why brown adipocytes specialize in lipid catabolism rather than lipid storage, a more similar profile to the oxidative metabolism of skeletal muscle. The concept that BAT and skeletal muscle have a common precursor would also explain why in some species, such as birds, skeletal muscle has a BAT-like function.20
Recent studies have shown that brown adipocytes which appear immersed in WAT in both rodents and humans have characteristics different from those of brown adipocytes in typical BAT sites. It would thus appear that there are two different types of BAT. These are, on the one hand, the classical BAT, whose adipocytes have a “myogenic signature” (Myf-5-lineage), and, on the other hand, cells positive for UCP-1 emerging from WAT and having no myogenic signature. This second type of adipocytes shows an expression profile highly similar to that of white adipocytes, namely low baseline UCP-1 expression. However, as occurs with brown adypocites, an overexpression of UCP-1 after adrenergic stimulation is observed. These brown-like cells immersed in WAT with no myogenic signature have been called “beige adipocytes” and are particularly sensitive to the hormone irisin.48
Macrophages, neutrophils, and lymphocytesMacrophages are found among cells of the vascular stroma fraction of adipose tissue. The abundance of macrophages in WAT is related to their size, and a direct correlation exists between WAT size and the number of macrophages which have infiltrated into this tissue, so that obese individuals have a higher number of macrophages in their WAT than subjects with normal weight.49 These macrophages are the main factor responsible for the secretion of pro-inflammatory cytokines such as TNF-α, IL-6, IL-8, and IL-1b, and are related to the inflammation state present in obesity.12 The secretion of adipokines such as leptin, resistin, and adiponectin mainly results from adipocyte activity.50
It has also been reported that preadipocytes from WAT, as well as those from immortal lines such as 3T3-L1 cells, are able to differentiate into macrophages when inoculated into the peritoneal cavity of mice (Swiss nu/nu).51 Both cell types (preadipocytes and macrophages) have common characteristics, such as the ability to secrete cytokines and a phagocytic capacity; the latter is lost when preadipocytes differentiate into mature adipocytes.52 This feature of preadipocytes suggests that macrophages occurring in WAT may be derived from preadipocytes. There is however evidence to support the hypothesis that WAT macrophages come from circulating monocytes.53 Thus, the synthesis of chemokines such as monocyte chemoattractant protein-1 (MCP-1) by adipocytes and in proportion to adiposity induces an increased WAT infiltration by blood monocytes.54 Different secretion products of adipocytes, including leptin, increase the production of adhesion proteins (ICAM-1) by endothelial cells, inducing the transmigration and adhesion of blood monocytes.53 Secretion of colony-stimulating factor-1 (CSF-1) by adipocytes provides a favorable microenvironment for monocytes to differentiate and become established as mature macrophages in WAT.49
Chronic inflammation in obesity, together with increased macrophage infiltration in WAT, suggests a close relationship between the immune system and obesity. It should be borne in mind that neutrophils are the first white blood cells selected in inflammatory response, and as this response progresses and damage is repaired, a gradual change occurs in the type of cells present in inflamed tissue leading to an increased proportion of macrophages which resolve damage and inflammation. However, when the inflammatory stimulus persists, a chronic inflammatory state unbalancing the relationship between pro-inflammatory and anti-inflammatory cells is reached. It has recently been reported that, in the process of obesity induction in mice using a high-lipid diet, there is a first phase (three days) where neutrophil infiltration occurs in WAT, while at 16weeks, when obesity is already established, macrophage infiltration is seen.55
Macrophages may show a pro-inflammatory or anti-inflammatory profile depending on their stimulation by different cytokines. Classical macrophage activation is promoted by the secretion of molecules produced by T helper lymphocytes, particularly IFNγ, in response to damage or infection. Macrophages thus activated are called type 1 macrophages (M1), are pro-inflammatory, and have high microbicidal activity. Macrophages may be activated in an alternative form (M2) in response to interleukins IL-4 and/or IL-13 (M2a profile), secreted by adipocytes, among other cell types. The M2a profile shows an anti-inflammatory gene expression program and actively contributes to the resolution of inflammation.56 The nuclear receptor PPARδ/β has recently been reported as playing a key role by controlling alternative activation in macrophages from WAT and Kupffer cells in the liver.57,58
As noted above, obesity is characterized by an increased macrophage infiltration in WAT. The initial stimulus triggering this infiltration is unknown, but factors such as hypoxia due to WAT increase59 or damage resulting from adipocyte hypertrophy have been suggested.42 The phenotype of WAT macrophages in thin animals has been reported as corresponding to the M2a profile, while the profile of adipokines released by WAT in obese animals suggests the infiltration of M1 macrophages. Animals subject to a lipid-high diet and with PPARδ−/− macrophages, which cannot activate to M2, develop more marked obesity and insulin resistance. These animals show increased lipogenesis leading to steatosis and an inflammatory macrophage (M1) profile (Kupffer cells). Decreased insulin sensitivity and infiltration of M1 macrophages is seen in WAT.57,58 Studies where a decrease in M1 macrophages was induced in obese animals showed decreases in WAT and systemic inflammatory markers, while insulin sensitivity normalized.60 Thus, the presence of M1 macrophages and the inability to activate them by the alternative route play a significant role in the maintenance of inflammation and the occurrence of insulin resistance and obesity.
Chronic inflammation is a complex process that implies, in addition to macrophage accumulation, an impaired T lymphocyte function. These T lymphocytes are one of the main components in atherosclerotic plaques and are important in plaque development through their communication with macrophages, either cell to cell or through inflammatory mediators.61 The presence of T lymphocytes in WAT and their potential implication in inflammation occurring in obesity has recently been studied. Wu et al.62 and Rausch et al.63 reported an increased infiltration of T lymphocytes (CD3), in addition to macróphages, in the perigonadal WAT of mice fed a long-term lipid-high diet as compared to thin animals. This infiltration of T lymphocytes into WAT was subsequently found to be prior to macrophage infiltration. In mice with obesity induced by a lipid-high diet, an infiltration of T lymphocytes occurring after five weeks of treatment and persisting up to 10 weeks is seen. The presence of macrophages is not seen at five weeks of treatment, but is seen after 10 weeks.31 These results suggest that T lymphocytes may play a significant role in the start and perpetuation of WAT inflammation.
An increased expression of T lymphocyte markers (CD3) is found in visceral WAT from obese humans as compared to thin individuals.62 An abundance of T lymphocytes (CD3) in WAT directly correlates to waist cirumference.31 Characterization of the T lymphocyte population by immunohistochemistry in visceral WAT shows the presence of CD4 lymphocytes, and no CD8 lymphocytes are found. CD4 lymphocytes express pro-inflammatory cytokines such as IFNγ and may be responsible for macrophage activation in this tissue.
Subsequent studies have also reported differences in the type of population of lymphocytes infiltrated in WAT of thin and obese individuals.64 Ten percent of cells of the vascular stromal fraction of perigonadal WAT of adult thin mice correspond to T lymphocytes, with a 3 to 1 ratio between CD4+ and CD8+. Surprisingly, more than half the CD4+ T lympochytes are positive for Foxp3, a higher proportion than that found in lymphoid tissues such as the spleen and lymph nodes. This phenotype corresponds to a type of regulatory T lymphocytes (Treg) which appears to be one of the most important body defenses against inadequate immune response, operating in autoimmunity, allergy, inflammation, infection, and tumorigenesis settings.65 It has also been reported that the number of Treg lymphocytes infiltrated is similar at birth in perigonadal, subcutaneous, and spleen WAT, but in perigonadal WAT, unlike in the other two sites, the number of T lymphocytes gradually increases with age in thin mice.64 In mice genetically obese (ob/ob) or with obesity induced by a lipid-high diet, a lower proportion of Treg lymphocytes is found in perigonadal WAT, while the proportion in subcutaneous and spleen WAT is normal, so that a direct relationship has been established between a lower infiltration of Treg lymphocytes of visceral location and the presence of obesity, insulin resistance, inflammation, and metabolic syndrome in general. Treg cells express a profile of non-inflammatory adipokines increasing the expression of IL-10, while conventional T lymphocytes (Tconv) not expressing Foxp3 show a pro-inflammatory profile with increased IFNγ expression. In omental WAT from obese humans, a lower expression of Foxp3 versus CD3 is found as compared to subcutaneous WAT, suggesting that visceral WAT also has a lower proportion of Treg lymphocytes in obese humans. There have been too few studies attempting to find the solutions to such problems as, for example, why Treg lymphocytes accumulate in visceral WAT and why there is a lower proportion of Treg lymphocytes in visceral WAT in obesity. In any case, it appears to be clear that a significant relationship exists between the type of lymphocyte and the type of macrophage present in WAT. That is, there is a crosstalk between lymphocytes and macrophages that determines the anti-inflammatory or pro-inflammatory profile of these cell types.
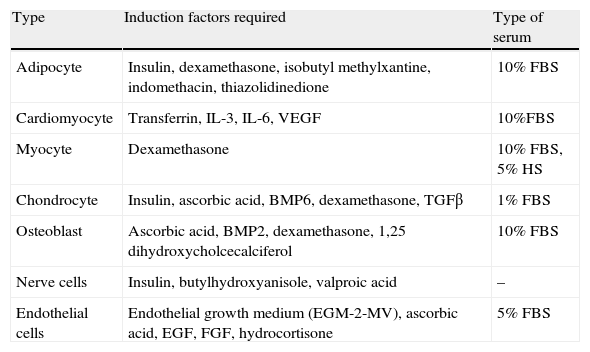
Stem cells and adipose tissue plasticityCells from WAT vascular stroma also include adipose-derived stem cells (ADSCs) with very similar characteristics to MSCs from bone marrow.66 There are multiple studies in the literature reporting the potential of these ADSCs to differentiate in vitro into different cell types depending on the stimulus received (Table 2). Thus, in addition to adipocytes, bone, cartilage, skeletal and cardiac muscle, nerve, and endothelial cells have been obtained from ADSCs.67 The presence of stem cells in adult WAT shows the persistence of its capacity to generate new adipocytes, a feature that represents another target for studies related to energy homeostasis and obesity. On the other hand, this great plasticity of ADSCs and their angiogenic capacity currently represent a focus of great interest for regenerative medicine, because they are a source of easy to obtain stem cells as compared to MSCs.68
In vitro differentiation potential of white adipose tissue stem cells (ADSCs).
| Type | Induction factors required | Type of serum |
| Adipocyte | Insulin, dexamethasone, isobutyl methylxantine, indomethacin, thiazolidinedione | 10% FBS |
| Cardiomyocyte | Transferrin, IL-3, IL-6, VEGF | 10%FBS |
| Myocyte | Dexamethasone | 10% FBS, 5% HS |
| Chondrocyte | Insulin, ascorbic acid, BMP6, dexamethasone, TGFβ | 1% FBS |
| Osteoblast | Ascorbic acid, BMP2, dexamethasone, 1,25 dihydroxycholcecalciferol | 10% FBS |
| Nerve cells | Insulin, butylhydroxyanisole, valproic acid | – |
| Endothelial cells | Endothelial growth medium (EGM-2-MV), ascorbic acid, EGF, FGF, hydrocortisone | 5% FBS |
Our understanding of WAT has dramatically changed over the past two decades. WAT is no longer regarded as a tissue whose basic role is to store lipids, but rather as an endocrine organ secreting multiple regulatory factors. In addition, its cellular complexity further demonstrates its dynamic and active role in the regulation of energy homeostasis. All this knowledge opens up new study goals aimed at understanding and so acting upon metabolic syndrome pathologies. Greater understanding of preadipocyte differentiation will provide new perspectives on why there are different preadipocyte populations in the same anatomical location of WAT and on what factors contribute to the predominance of one or the other. It is of interest to understand why visceral WAT accumulates Treg lymphocytes and why the number of these decreases in obesity. WAT is currently, and will possibly be for the next decades, a significant target organ for research from all perspectives, because increased knowledge about WAT will provide new ways of understanding such conditions as obesity and metabolic syndrome in general.
Conflict of interestThe author declares no conflict of interest.
Please cite this article as: Esteve Ràfols M. Tejido adiposo: heterogeneidad celular y diversidad funcional. Endocrinol Nutr. 2014;61:100–112.