Despite its benefits, conjugated linoleic acid (CLA) may cause side effects after long-term administration. Because of this and the controversial efficacy of CLA in humans, alternative biomolecules that may be used as functional ingredients have been studied in recent years. Thus, conjugated linolenic acid (CLNA) has been reported to be a potential anti-obesity molecule which may have additional positive effects related to obesity.
According to the results reported in obesity, CLNA needs to be given at higher doses than CLA to be effective. However, because of the few studies conducted so far, it is still difficult to reach clear conclusions about the potential use of these CLNAs in obesity and its related changes (insulin resistance, dyslipidemia, or inflammation).
Pese a sus efectos beneficiosos, se desconoce si el ácido linoleico conjugado (conjugated linoleic acid, CLA) podría producir efectos adversos al ser administrado de forma crónica. Considerando este hecho y dada la controvertida eficacia del CLA en humanos, en los últimos años el ácido linolénico conjugado (CLNA, conjugated linolenic acid) se ha descrito como alternativa al CLA, con un potencial funcional para la prevención de la obesidad, además de tener otros efectos positivos relacionados con la misma.
A la vista de los resultados descritos, en lo que respecta a la obesidad, no parece que el CLNA sea una molécula más prometedora que el CLA, dado que el efecto generalmente tiene lugar a dosis más elevadas que las dosis efectivas de CLA. No obstante, dado el escaso número de estudios realizados hasta la fecha, todavía resulta difícil llegar a conclusiones claras acerca del potencial uso de estos CLNA en obesidad y alteraciones relacionadas con ella (resistencia a la insulina, dislipidemias o inflamación).
The general term conjugated fatty acids refers to positional and geometric isomers of fatty acids with double conjugated bonds. Conjugated linoleic acid isomers, grouped under the acronym CLA (conjugated linoleic acid), are the most widely studied to date. Many physiological benefits have been attributed to this group of fatty acids (anticancer and antiatherogenic effects, immune function improvement, decreased fat accumulation, decreased inflammation, and increased muscle mass). These effects have been associated with the double conjugated bond and are commonly due to the individual actions of its two most abundant isomers, cis-9,trans-11, and trans-10,cis-12.
It should be noted that, despite its beneficial effects, CLA may have adverse effects. Although few studies have analyzed this potential toxicity, several authors have reported in experimental animals effects such as insulin resistance, increased C-reactive protein levels, or hepatic steatosis.1,2 However, the European Food Safety Authority (EFSA) demonstrated in 2010 that, in humans with normal weight or overweight with no history of diabetes, the administration of an equimolecular mixture of the cis-9,trans-11 and trans-10, -12 isomers of CLA at a dose of 3.5g for six months had no adverse effects on insulin sensitivitiy levels and blood glucose control or liver function.3 Further studies are, however, needed to ascertain its long term effects, as well as its safety in patients with type 2 diabetes. In fact, as concluded by the EFSA expert panel, in these specific patients, the equimolecular mixture of CLA isomers appeared to negatively affect dynamic (ISI, OGIS) and static (HOMA-IR) markers of insulin sensitivity and increased some subclinical inflammation markers (15-keto-dihydroprostaglandin F2 and C-reactive protein).
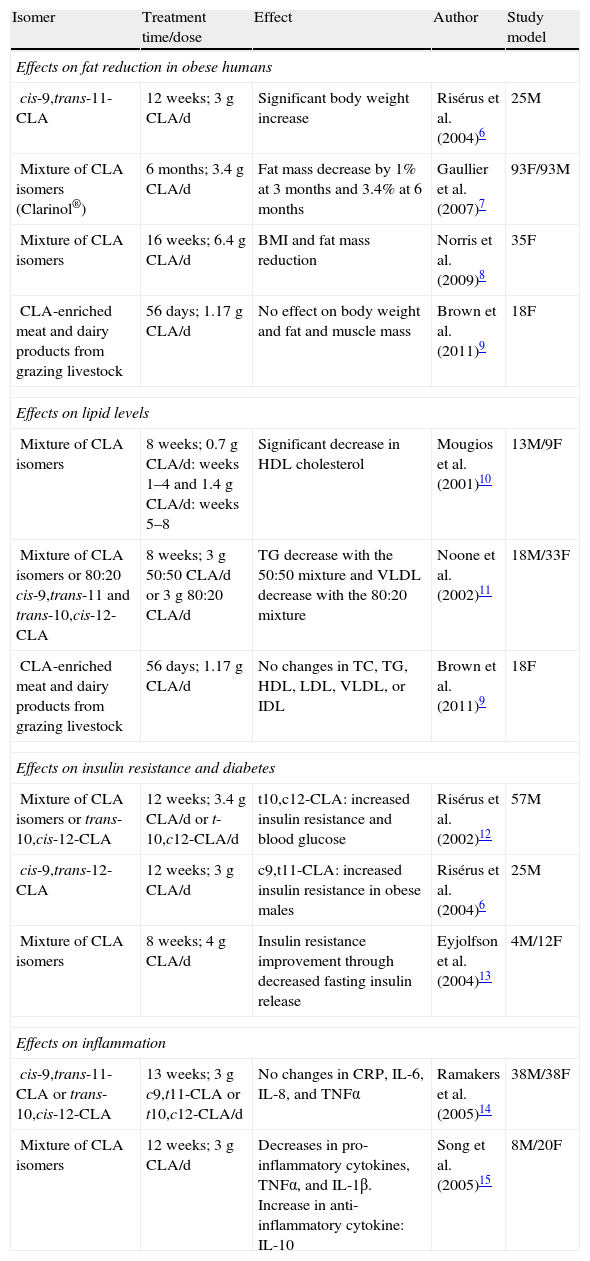
Multiple studies have shown that CLA, and more specifically the cis-9,trans-11 and trans-10,cis-12 isomers, have benefits in different animal species (mice, rats, hamsters, pigs).4,5 By contrast, the effectiveness of CLA in humans is more controversial (Table 1).6–15 As an example, it may be noted that while several authors found effects such as decreased fat accumulation, others did not.6–9 Moreover, when this effect was reported, it was less prominent than in rodents.
Effects of CLA in humans.
| Isomer | Treatment time/dose | Effect | Author | Study model |
| Effects on fat reduction in obese humans | ||||
| cis-9,trans-11-CLA | 12 weeks; 3g CLA/d | Significant body weight increase | Risérus et al. (2004)6 | 25M |
| Mixture of CLA isomers (Clarinol®) | 6months; 3.4g CLA/d | Fat mass decrease by 1% at 3months and 3.4% at 6 months | Gaullier et al. (2007)7 | 93F/93M |
| Mixture of CLA isomers | 16weeks; 6.4g CLA/d | BMI and fat mass reduction | Norris et al. (2009)8 | 35F |
| CLA-enriched meat and dairy products from grazing livestock | 56days; 1.17g CLA/d | No effect on body weight and fat and muscle mass | Brown et al. (2011)9 | 18F |
| Effects on lipid levels | ||||
| Mixture of CLA isomers | 8weeks; 0.7g CLA/d: weeks 1–4 and 1.4g CLA/d: weeks 5–8 | Significant decrease in HDL cholesterol | Mougios et al. (2001)10 | 13M/9F |
| Mixture of CLA isomers or 80:20 cis-9,trans-11 and trans-10,cis-12-CLA | 8weeks; 3g 50:50 CLA/d or 3g 80:20 CLA/d | TG decrease with the 50:50 mixture and VLDL decrease with the 80:20 mixture | Noone et al. (2002)11 | 18M/33F |
| CLA-enriched meat and dairy products from grazing livestock | 56days; 1.17g CLA/d | No changes in TC, TG, HDL, LDL, VLDL, or IDL | Brown et al. (2011)9 | 18F |
| Effects on insulin resistance and diabetes | ||||
| Mixture of CLA isomers or trans-10,cis-12-CLA | 12weeks; 3.4g CLA/d or t-10,c12-CLA/d | t10,c12-CLA: increased insulin resistance and blood glucose | Risérus et al. (2002)12 | 57M |
| cis-9,trans-12-CLA | 12weeks; 3g CLA/d | c9,t11-CLA: increased insulin resistance in obese males | Risérus et al. (2004)6 | 25M |
| Mixture of CLA isomers | 8 weeks; 4g CLA/d | Insulin resistance improvement through decreased fasting insulin release | Eyjolfson et al. (2004)13 | 4M/12F |
| Effects on inflammation | ||||
| cis-9,trans-11-CLA or trans-10,cis-12-CLA | 13 weeks; 3g c9,t11-CLA or t10,c12-CLA/d | No changes in CRP, IL-6, IL-8, and TNFα | Ramakers et al. (2005)14 | 38M/38F |
| Mixture of CLA isomers | 12weeks; 3g CLA/d | Decreases in pro-inflammatory cytokines, TNFα, and IL-1β. Increase in anti-inflammatory cytokine: IL-10 | Song et al. (2005)15 | 8M/20F |
TC: total cholesterol; H: males; IL: interleukin; BMI: body mass index; F: females; mixture of CLA isomers; 50:50 cis-9, trans-11-, and trans-10, cis-12-CLA; TG: triglycerides, TNF: tumor necrosis factor.
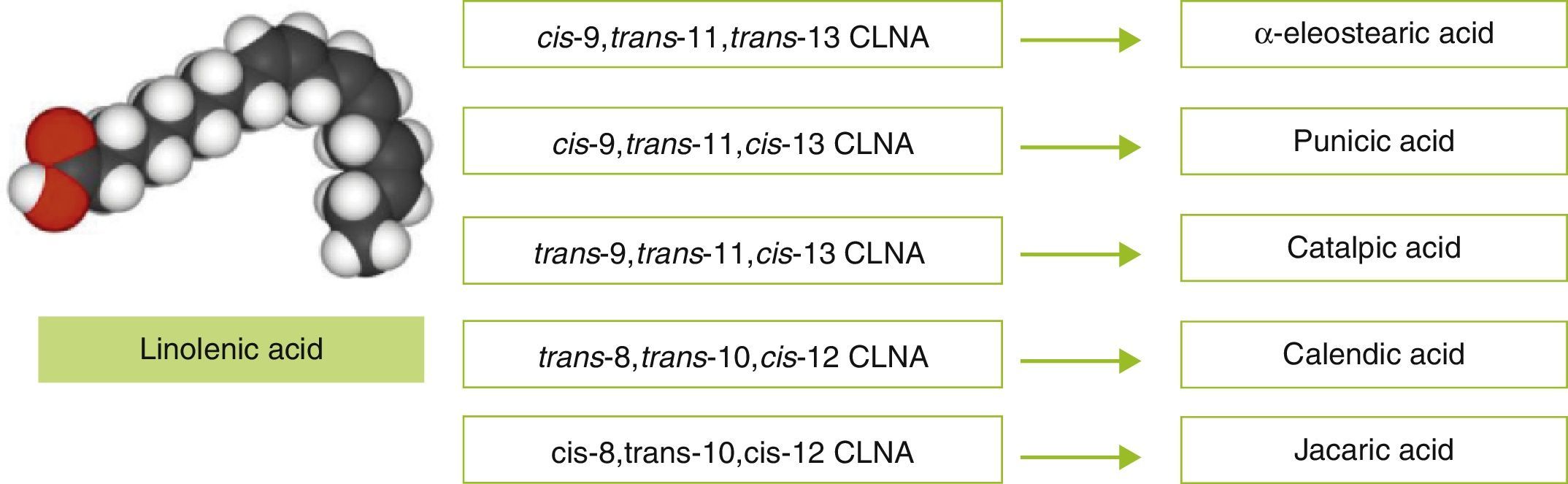
Because of the controversial efficacy of CLA in humans and the lack of reliable information on its effects after long-term administration (longer than six months), it appears appropriate to search for alternative biomolecules that may be used as functional ingredients for the prevention of obesity. One such biomolecule, conjugated linolenic acid (CLNA), has been reported to be a molecule able to decrease body fat, as well as having other positive effects on health. Actually, CLNA is a collective term for a group of positional and geometric isomers of linolenic acid (C18:3) in which at least two double bonds are conjugated (contiguous), rather than separated by methilene groups, as occurs in linolenic acid (Fig. 1).16 These isomers only differ from CLA isomers in that they have a third double bond.
The presence of three double bonds in the linolenic acid molecule leads to multiple positional and geometric isomers. It should be noted that studies by Bassaganya-Riera et al. suggest that the presence of double conjugated bonds in fatty acids increases their biological activity and enables them to act as agonists of nuclear receptors.17 More specifically, in vitro studies have suggested that certain plant-derived CLNA isomers (such as punicic acid or α-eleostearic acid) may act as natural agonists of peroxisome proliferator-activated receptors (PPAR).18,19
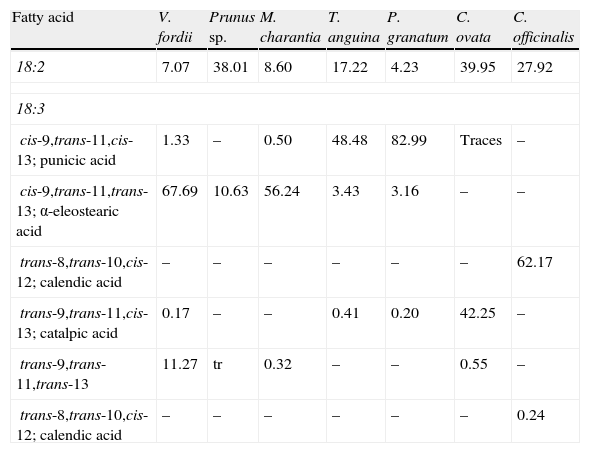
While CLA naturally occurs in food from ruminants (mainly cattle, sheep, and goats), and in milk and dairy products in very small amounts which account for up to 0.65% of all fatty acids,20 CLNA isomers occur in greater amounts in plant-derived food and products. As documented by Takagi and Itabashi (1981),21 Tung (Vernicia fordii) and bitter gourd (Momordica charantia) seed oils contain 67.7% and 56.2% of α-eleostearic acid (cis-9,trans-11,trans-13 CLNA) respectively, while those extracted from pomegranate (Punica granatum), catalpa (Catalpa ovata), and pot marigold (Calendula officinalis) contain 83% of punicic acid (cis-9,trans-11,cis-13 CLNA), 42.3% of catalpic acid (trans-9,trans-11,cis-13 CLNA), and 62.2% of calendic acid (trans-8,trans-10,cis-12 CLNA) respectively (Table 2).
Contents of conjugated linoleic acid and conjugated linolenic acid isomers in several seed oils.
| Fatty acid | V. fordii | Prunus sp. | M. charantia | T. anguina | P. granatum | C. ovata | C. officinalis |
| 18:2 | 7.07 | 38.01 | 8.60 | 17.22 | 4.23 | 39.95 | 27.92 |
| 18:3 | |||||||
| cis-9,trans-11,cis-13; punicic acid | 1.33 | – | 0.50 | 48.48 | 82.99 | Traces | – |
| cis-9,trans-11,trans-13; α-eleostearic acid | 67.69 | 10.63 | 56.24 | 3.43 | 3.16 | – | – |
| trans-8,trans-10,cis-12; calendic acid | – | – | – | – | – | – | 62.17 |
| trans-9,trans-11,cis-13; catalpic acid | 0.17 | – | – | 0.41 | 0.20 | 42.25 | – |
| trans-9,trans-11,trans-13 | 11.27 | tr | 0.32 | – | – | 0.55 | – |
| trans-8,trans-10,cis-12; calendic acid | – | – | – | – | – | – | 0.24 |
Values are percentages. Modified from Takagi and Itabashi.21
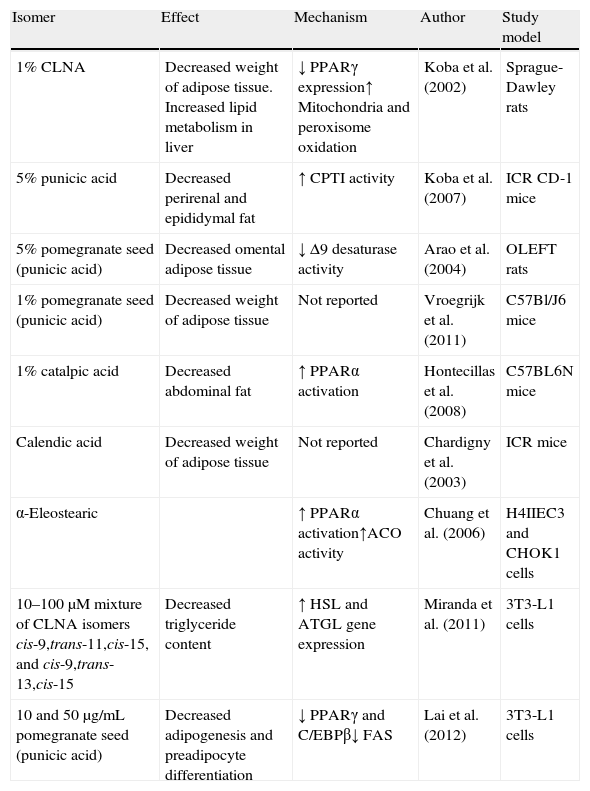
Since, as previously noted, the efficacy of conjugated linoleic acid as an anti-obesity molecule in human beings continues to be controversial, some scientific studies have started to focus on the search for CLNA isomers which can prevent this condition (Table 3).
Summary of the positive effects of CLNA isomers on fat reduction.
| Isomer | Effect | Mechanism | Author | Study model |
| 1% CLNA | Decreased weight of adipose tissue. Increased lipid metabolism in liver | ↓ PPARγ expression↑ Mitochondria and peroxisome oxidation | Koba et al. (2002) | Sprague-Dawley rats |
| 5% punicic acid | Decreased perirenal and epididymal fat | ↑ CPTI activity | Koba et al. (2007) | ICR CD-1 mice |
| 5% pomegranate seed (punicic acid) | Decreased omental adipose tissue | ↓ Δ9 desaturase activity | Arao et al. (2004) | OLEFT rats |
| 1% pomegranate seed (punicic acid) | Decreased weight of adipose tissue | Not reported | Vroegrijk et al. (2011) | C57Bl/J6 mice |
| 1% catalpic acid | Decreased abdominal fat | ↑ PPARα activation | Hontecillas et al. (2008) | C57BL6N mice |
| Calendic acid | Decreased weight of adipose tissue | Not reported | Chardigny et al. (2003) | ICR mice |
| α-Eleostearic | ↑ PPARα activation↑ACO activity | Chuang et al. (2006) | H4IIEC3 and CHOK1 cells | |
| 10–100μM mixture of CLNA isomers cis-9,trans-11,cis-15, and cis-9,trans-13,cis-15 | Decreased triglyceride content | ↑ HSL and ATGL gene expression | Miranda et al. (2011) | 3T3-L1 cells |
| 10 and 50μg/mL pomegranate seed (punicic acid) | Decreased adipogenesis and preadipocyte differentiation | ↓ PPARγ and C/EBPβ↓ FAS | Lai et al. (2012) | 3T3-L1 cells |
ACO, acyl-CoA oxidase; ATGL, triglycerides lipase; C/EBP, CCAAT/enhancer binding protein beta; CPT:I, carnitine palmitoyltransferase-I; FAS, fatty acid synthase; HSL, hormone-sensitive lipase; PPAR, peroxisome proliferator-activated receptor.
In vitro studies conducted on 3T3-L1 adipocytes and HepG2 cells have demonstrated the activating effect of punicic acid and α-eleostearic acid on nuclear receptors PPARα and PPARγ.17–19 Research conducted by our group found similar effects with a mixture of CLNA isomers cis-9,trans-11,cis-15, and cis-9,trans-13,cis-15 in HEK293 cells. These isomers were able to activate the PPAR response element in cells overexpressing the PPARα protein.22 These results suggest that CLNA isomers may increase glucose tolerance and fatty acid oxidation, and decrease inflammation related to obesity.
Lai et al.23 recently showed a decreased expression of genes controlling the differentiation process, such as PPARγ and C/EBPβ, and fatty acid synthase, an enzyme that increases triglyceride accumulation in 3T3-L1 preadipocytes treated with punicic acid. The abovementioned study of our research group allowed us to propose another mechanism of action for CLNA isomers. Specifically, different doses (10–100μM) of the CLNA isomers cis-9,trans-11,cis-15 and cis-9,trans-13,cis-15 have been shown to decrease triglyceride contents in 3T3-L1 adipocytes, increasing the gene expression of key enzymes in lipolysis.22
In a study conducted to compare the effect of CLA and CLNA in rats, Koba et al.24 noted that the addition of a mixture of unidentified CLNA isomers at a 1% concentration in diet induced a reduction in adipose tissue weight even greater than that caused by CLA. Similarly, CLNA intake had a much greater effect than CLA on lipid metabolism in the liver. Specifically, CLNA increased to a greater extent the oxidation of both mitochondrial and peroxisomal fatty acids.
The same research group subsequently conducted additional studies with CLNA, but using a specific isomer, punicic acid (cis-9,trans-11,cis-13 CLNA).25 A study conducted on ICR CD-1 mice for four weeks found that, in order to see a reducing effect on body fat, this fatty acid should be included at a 5% concentration in diet. This concentration is higher than the CLA dose usually causing significant body fat reductions (0.5–1%). Punicic acid was also seen to decrease leptin production and to increase the activity of the enzyme carnitine palmitoyltransferase (CPT-I).25
Arao et al.26 conducted an additional two-week study on Otsuka Long Evans Tokushima Fatty (OLETF) rats with a 5% supplementation in the diet, not with punicic acid, but with a pomegranate seed oil rich in this fatty acid. With this dose, a significant reduction was seen in omental adipose tissue; by contrast, a 2% supplement was not sufficient to induce changes in this tissue. Yamasaki et al.27 also found no effects in adipose tissue from C57BL/6N mice fed diets supplemented with pomegranate oil providing 0.12% and 1.2% of punicic acid for three weeks. Our research group found similar results in rats fed on a high-fat diet supplemented with 0.5% pomegranate seed oil for six weeks (data submitted for publication).
With this lower dose range, McFarlin et al.28 also found no changes in fat mass, but did find weight gains in CD-1 mice fed with a fat-rich diet containing 61.79mg of pomegranate seed oil for 14 weeks. By contrast, another study of similar duration (12weeks) conducted in mice which were fed a diet supplemented with 1% of pomegranate seed oil found both fat mass and body weight reductions.29 Improved insulin sensitivity was also documented.
Although punicic acid is one of the most widely studied isomers, there are other isomers which also have this effect. Thus, Hontecillas et al.17 noted that the addition to the diet of 1% catalpic acid, trans-9,trans-11,cis-13 CLNA, decreased abdominal fat accumulation in C57BL/6N mice after 78days of treatment.
Chardigny et al.30 also found that the CLNA isomer trans-8,trans-10,cis-12 (calendic acid) achieved a greater body fat decrease in male mice when compared to animals fed on a control diet. It should be noted, however, that CLNA isomer trans-8,trans-10,cis-12 caused a lower body fat reduction than isomer trans-10,cis-12 of CLA.
Jacaric acid deserves special mention. Like calendic acid, this fatty acid maintains the trans-10,cis-12 as well as the carboxyl group in the first carbon. It has been suggested that this structure is indispensable for the inhibition of lipoprotein lipase (LPL), which is one of the most important mechanism involved in the body fat reduction induced by CLA.31 Despite this, jacaric acid has no reducing effect on body fat.32 Because of this lack of effect, added to the fact that this fatty acid alters insulin function, jararic acid cannot be proposed as an anti-obesity molecule.
Other benefits of conjugated linolenic acid isomers related to obesityInsulin resistanceObesity is a chronic disorder associated with various conditions, most of them chronic, which may increase mortality as compared to the non-obese population and cause a significant decrease in patient quality of life. Epidemiological studies have shown a clear relationship between obesity and insulin resistance.33 This metabolic change is defined as a decreased ability of insulin to exert its biological actions in target tissues such as skeletal muscle, liver, or adipose tissue. It has been found that increased free fatty acid levels and decreased adiponectin levels (insulin-sensitizing hormone) contribute to this resistance state.
As previously noted, CLNA isomers, in addition to reducing body fat, may also have other benefits for health. In this regard, it may be noted that one of the most significant effects of bitter gourd is its hypoglycemic potential, shown in normal34 and diabetic35,36 rats and in patients with type 2 diabetes.24 Although its mechanism of action has not been fully elucidated yet, it appears that it inhibits intestinal glucose absorption, promotes insulin utilization in the liver, and even increases the number of beta cells in the pancreas. Similarly, α-eleostearic acid has recently been identified in bitter gourd as a PPARα activator and as potentially responsible for the hypoglycemic effects.18 It is important to note that studies have related PPARα agonists to the protection of pancreatic beta cells against the hyperactivation of insulin production derived from a diet rich in saturated fatty acids,37 thus preventing type 2 diabetes mellitus as a result of hyperinsulinemia. Finally, it should be noted that, as stated above, Vroegrijk et al.29 found improved insulin sensitivity in mice fed on a diet supplemented with 1% pomegranate seed oil for 12 weeks.
Similarly, catalpic acid17 also appears to improve serum fasting glucose and insulin levels, and to increase the ability of mice to normalize plasma glucose levels after a glucose tolerance test. The effects of this CLNA isomer on glucose are believed to be mediated by a PPARα-dependent mechanism. In fact, catalpic acid increases the expression of this transcription factor and of the genes controlled by it.17,18
The pro-inflammatory cytokine TNF-α is one of the main factors responsible for insulin resistance induced by inflammation. Specifically, TNF-α inhibits the insulin signal by the phosphorylation of serine at the insulin receptor, thus inhibiting the ability of the receptor to bind to insulin receptor substrate 1 (IRS1). According to the results reported by the Bassaganya Riera group, the effectiveness of punicic acid in the treatment of insulin resistance associated with obesity is due to its activating PPARγ and therefore decreasing TNF-α expression.19
Various research studies have shown that body fat reduction induced by CLA may be associated with a dramatic decrease in the serum levels of both leptin and adiponectin in mice38 (adipokines related to blood glucose control). Studies conducted with punicic acid appear to suggest that it does not modify the serum levels of these adipokines nor change plasma insulin or glucose levels in mice,25 which could mean that this molecule is safer than CLA. However, it should not be forgotten that the different CLNA isomers may be metabolized to CLA.39,40
InflammationIn recent years, obese patients have been reported as having a low-grade chronic inflammatory state. This condition appears to be the consequence of an increased adipose tissue mass that leads to the increased production of pro-inflammatory mediators which are jointly stimulated by signals of exogenous and/or endogenous origin.41 Adipose tissue contains fibroblasts, preadipocytes, adipocytes, and macrophages. The latter contribute significantly to the systemic inflammatory process by the production of pro-inflammatory mediators such as inflammatory cytokines (TNF-α, IL-6, IL-1β, IL-8, IL-18).42
It has been noted that excess calorie intake, some infections, and oxidative stress may cause the increased secretion of these adipokines leading to chronic inflammation in white adipose tissue which promotes the activation and infiltration of mature macrophages. The fact that these macrophages, as well as adipocytes and other cell types, start to secrete cytokines and chemokines makes possible the maintenance of an endless loop of macrophage recruitment and the production of inflammatory mediators, which initially leads to local primary inflammation in adipose tissue and may trigger the low-grade systemic inflammation seen in obesity.43
Conjugated fatty acids have been directly related to anti-inflammatory properties. While these properties were mostly attributed to CLA isomers in the past, according to recent research there are CLNA isomers which have the same effect. As for CLAs (in which discrepancies now exist), three mechanisms have been proposed as accounting for the effect of CLNA on inflammatory response:
- (a)
Decreased genesis of inducible eicosanoids involved in inflammatory response, including prostaglandins and leukotrienes.
Prostaglandins are a group of oxygenated fatty acids occurring in most mammalian tissues positively correlated to inflammation.44 As documented by Nugteren and Christ-Hazelhof in 1987,45 various CLNA isomers (jacaric acid, calendic acid, punicic acid, catalpic acid, and eleostearic acid) have anti-inflammatory activity mediated by inhibition of the activity of cyclooxygenases, enzymes that catalyze prostaglandin synthesis. Some of these data were confirmed by a subsequent study which concluded that pomegranate extract rich in punicic acid markedly inhibits both lipogenase and cyclooxygenase activity.46
- (b)
Interaction with PPARsγ.
PPARγ is expressed in adipose tissue and macrophages, and plays a significant role in the regulation of inflammation.47 In fact, PPAR has been found to be a negative regulator of macrophage activation and to inhibit the expression of genes related to immune response.48 PPARγ activation similarly inhibits the production of pro-inflammatory cytokines such as TNF-α and IL-6.49 The activating and agonist effect of punicic acid on PPARγ receptors has been demonstrated in this review. Punicic acid, as shown by a recent report, may therefore be effective in reducing the chronic inflammation underlying obesity.19
- (c)
Inactivation of the transduction signal of NF-κβ (nuclear factor kappa light chain enhancer of activated β cells).
NF-κB is a nuclear transcription factor. Its activation upon cell exposure to external stimuli, such as oxidative stress or ultraviolet radiation, induces the expression of cell genes associated with inflammation,50 including different cytokines (TNF-α, IL-1, IL-6) and chemokines (IL-8 and macrophage inflammatory protein).
The finding that pomegranate extract is able to decrease and inhibit the activation of NF-κB, and to inhibit the phosphorylation of cytokines related to inflammation and mitogen-activated protein kinases (MAPK) is, therefore, of great interest.51,52 The fact that patients with periodontitis (inflammation in the mouth) respond to treatment with pomegranate extract with a reduction in cytokines with a marked inflammatory nature (IL-1β and IL-6) should also not be overlooked.53 The molecule in pomegranate extract responsible for the inflammatory effect has not yet been identified, but it should be borne in mind that punicic acid is one of the most important functional molecules contained in this extract. In fact, as confirmed by Hontecillas et al.,19 punicic acid decreases the inflammation related to obesity by activating PPARγ and therefore inhibiting TNF-α expression and NF-κB activity. Since PPAR agonists have been shown to decrease the expression of pro-inflammatory cytokines by antagonizing NF-κB activity, it is natural to think that other CLNA isomers which are agonists of these PPAR receptors, such as α-eleostearic acid, may be effective in inhibiting this nuclear transcription factor and, thus, in reducing the inflammation associated with obesity.48,54,55
A clear example of this is found in the experiments conducted by Saha et al.56,57 in diabetic rats (streptozotocin-induced diabetes) treated with 0.5% α-eleostearic acid and 0.5% punicic acid. In these rats, the increased expression of inflammatory cytokines such as tumor necrosis factor (TNF-α) and interleukin 6 (IL-6) in blood, and the hepatic expression of transcription factor NF-kB after treatment with streptozotocin, due to increased inflammation, was restored by the administration of CLNA isomers.
LipidemiaIn obese people, increased body fat, especially visceral fat, may lead to hyperglycemia and insulin resistance, but may also affect lipid metabolism. Thus, the metabolic changes usually associated with central obesity may include massive access to the liver of free fatty acids, the stimulation of hepatic synthesis of triglycerides, and the secretion of very low density lipoproteins (VLDL) as well as the decreased clearance of triglyceride-rich lipoproteins, the presence of small, dense low density lipoproteins (LDL), and decreased levels of high density lipoproteins (HDL). This decrease in HDLs, together with the increase in LDLs, which are able to enter the arterial wall, where they are oxidized, creates the adequate metabolic conditions for the development of the atherogenic process.58
In relation to this subject, research conducted in recent years suggests that some CLNA isomers could be effective in lipid profile control. Thus, the cis-9,trans-11,cis-13 isomer of CLNA appears to reduce the secretion of apoB100 by human HepG2 liver cells, which could be due to decreased triglycerides in this type of cell.59 Studies such as the one reported by Koba et al.25 suggest that this decrease is due to a marked increase in the β-oxidation of fatty acids. This is not an unexpected effect, considering the potential activating effect shown by CLNA isomers at PPARα, the main regulator of β-oxidation in peroxisomes and mitochondria.18 Thus, a reduction in apoB100 production could be related to in vivo VLDL reduction.
However, it appears that CLNA does not only affect apoB100 secretion, but also plays a protective role against the peroxidation of plasma lipids. Plasma lipids, LDL, and erythrocyte membrane lipids may experience peroxidation, which may in turn lead to the development of atherosclerosis and diabetic vascular complications.60 According to a study conducted by Dhar et al.,61 in which lipid peroxidation and the antioxidant effect of two concentrations (0.1% and 0.05%) of α-eleostearic acid in diabetic and non-diabetic subjects were assessed, this substance was shown to be effective for reducing oxidation at both doses, the 0.1% concentration being the more effective.
In addition, in the study conducted by Arao et al.,59 the cis-9,trans-11,cis-13 isomer of CLNA was associated with an improved ratio between saturated and monounsaturated fatty acids, a significant marker not only of cardiovascular markers, but also of metabolic syndrome. The authors of this research postulated that, as occurred with the trans-10,cis-12 isomer of CLA, this improvement was the result of the inhibition of stearoyl CoA desaturase by the cis-9,trans-11,cis-13 isomer of CLNA.
In the Saha and Ghosh study,56 plasma cholesterol levels and cholesterol content in tissues returned to normal in rats with induced hypercholesterolemia on feeding a diet enriched with 0.5% and 1% concentrations of mixed CLNA isomers. This effect was due to decreased cholesterol synthesis in the liver, associated with the inhibition of hydroxymethylglutaryl coenzyme A reductase, the rate-limiting enzyme in cholesterol synthesis.
In the Mirmiran et al. study,62 although patients with dyslipidemia treated for four weeks with 400mg of pomegranate seed oil experienced no changes in total and LDL cholesterol levels in plasma, reductions did occur in triglyceride levels and triglyceride/HDL cholesterol and total cholesterol/LDL cholesterol ratios, with a resultant decrease in cardiovascular risk.
It should, however, be noted that studies have been conducted in rats and hamsters where the CLNA isomers tested had no effect on serum lipid profile63 or even worsened it, increasing total cholesterol levels, the LDL/HDL ratio, and triglycerides.64 These discrepancies may have been due to the use of different experimental models and different doses of CLNA isomers.
ConclusionsResearch conducted in recent years has shown that conjugated linolenic acid isomers are potential functional substances for the prevention of obesity and its associated conditions. The results achieved in the in vitro studies conducted show that punicic acid and α-eleostearic acid act as natural agonists of PPARα, which suggests that they may be effective in body fat reduction, in addition to having other positive effects related to obesity. These encouraging in vitro results are supported by the in vivo study conducted by Koba et al., where a mixture of unidentified CLNA isomers was added to the diet at a 1% concentration. It should be noted, however, that this mixture contained CLA isomers, which may have masked the actual effect of CLNA isomers on body fat reduction.
Subsequent studies with isolated CLNA isomers have reported that the fat-reducing effect usually occurs at doses higher than the effective CLA doses (0.5–1%). Therefore, CLNA does not appear to be a more promising molecule than CLA with regard to obesity. However, because of the multiple isomers encompassed by the term conjugated linolenic acid, additional experimental studies are needed to definitively rule out CLNA isomers as potential functional elements in the prevention of obesity through body fat reduction.
Obesity is a chronic disorder associated with various conditions, most of them chronic, which may increase mortality as compared to the non-obese population and may cause a significant decrease in patient quality of life. As regards the use of these conjugated fatty acids for the prevention of underlying changes in obesity, it should be borne in mind that only a few studies have been conducted to date (Tables 4 and 5). It is, therefore, difficult to draw clear-cut conclusions concerning the potential use of CLNA in insulin resistance, dyslipidemia, or inflammation.
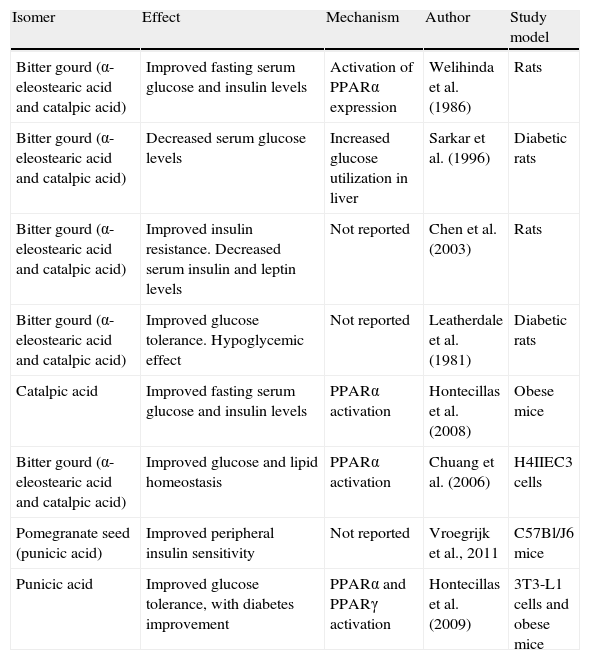
Summary of positive effects of CLNA isomers on diabetes and insulin resistance.
| Isomer | Effect | Mechanism | Author | Study model |
| Bitter gourd (α-eleostearic acid and catalpic acid) | Improved fasting serum glucose and insulin levels | Activation of PPARα expression | Welihinda et al. (1986) | Rats |
| Bitter gourd (α-eleostearic acid and catalpic acid) | Decreased serum glucose levels | Increased glucose utilization in liver | Sarkar et al. (1996) | Diabetic rats |
| Bitter gourd (α-eleostearic acid and catalpic acid) | Improved insulin resistance. Decreased serum insulin and leptin levels | Not reported | Chen et al. (2003) | Rats |
| Bitter gourd (α-eleostearic acid and catalpic acid) | Improved glucose tolerance. Hypoglycemic effect | Not reported | Leatherdale et al. (1981) | Diabetic rats |
| Catalpic acid | Improved fasting serum glucose and insulin levels | PPARα activation | Hontecillas et al. (2008) | Obese mice |
| Bitter gourd (α-eleostearic acid and catalpic acid) | Improved glucose and lipid homeostasis | PPARα activation | Chuang et al. (2006) | H4IIEC3 cells |
| Pomegranate seed (punicic acid) | Improved peripheral insulin sensitivity | Not reported | Vroegrijk et al., 2011 | C57Bl/J6 mice |
| Punicic acid | Improved glucose tolerance, with diabetes improvement | PPARα and PPARγ activation | Hontecillas et al. (2009) | 3T3-L1 cells and obese mice |
PPAR, peroxisome proliferator-activated receptor.
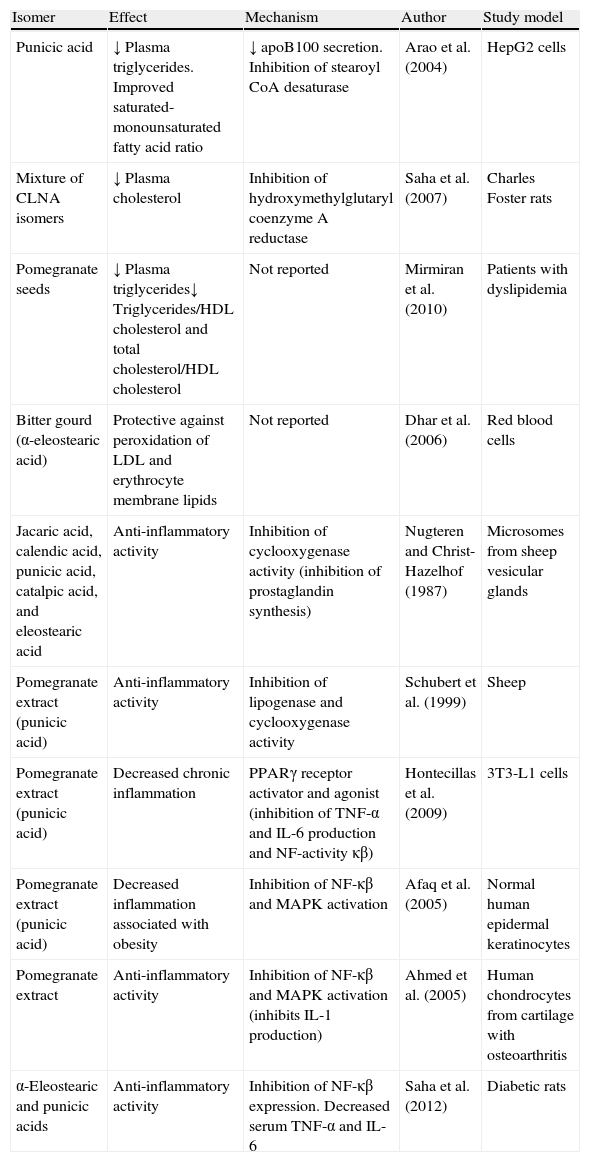
Summary of positive effects of CLNA isomers on lipidemia and inflammation underlying obesity.
| Isomer | Effect | Mechanism | Author | Study model |
| Punicic acid | ↓ Plasma triglycerides. Improved saturated-monounsaturated fatty acid ratio | ↓ apoB100 secretion. Inhibition of stearoyl CoA desaturase | Arao et al. (2004) | HepG2 cells |
| Mixture of CLNA isomers | ↓ Plasma cholesterol | Inhibition of hydroxymethylglutaryl coenzyme A reductase | Saha et al. (2007) | Charles Foster rats |
| Pomegranate seeds | ↓ Plasma triglycerides↓ Triglycerides/HDL cholesterol and total cholesterol/HDL cholesterol | Not reported | Mirmiran et al. (2010) | Patients with dyslipidemia |
| Bitter gourd (α-eleostearic acid) | Protective against peroxidation of LDL and erythrocyte membrane lipids | Not reported | Dhar et al. (2006) | Red blood cells |
| Jacaric acid, calendic acid, punicic acid, catalpic acid, and eleostearic acid | Anti-inflammatory activity | Inhibition of cyclooxygenase activity (inhibition of prostaglandin synthesis) | Nugteren and Christ-Hazelhof (1987) | Microsomes from sheep vesicular glands |
| Pomegranate extract (punicic acid) | Anti-inflammatory activity | Inhibition of lipogenase and cyclooxygenase activity | Schubert et al. (1999) | Sheep |
| Pomegranate extract (punicic acid) | Decreased chronic inflammation | PPARγ receptor activator and agonist (inhibition of TNF-α and IL-6 production and NF-activity κβ) | Hontecillas et al. (2009) | 3T3-L1 cells |
| Pomegranate extract (punicic acid) | Decreased inflammation associated with obesity | Inhibition of NF-κβ and MAPK activation | Afaq et al. (2005) | Normal human epidermal keratinocytes |
| Pomegranate extract | Anti-inflammatory activity | Inhibition of NF-κβ and MAPK activation (inhibits IL-1 production) | Ahmed et al. (2005) | Human chondrocytes from cartilage with osteoarthritis |
| α-Eleostearic and punicic acids | Anti-inflammatory activity | Inhibition of NF-κβ expression. Decreased serum TNF-α and IL-6 | Saha et al. (2012) | Diabetic rats |
IL, interleukin; MAPK, mitogen-activated protein kinases; NF-κβ, nuclear factor kappa light chain enhancer of activated β cells; PPAR, peroxisome proliferator-activated receptor; TNF, tumor necrosis factor.
The authors state that they have no conflicts of interest.
Please cite this article as: Miranda J, Arias N, Fernández-Quintela A, del Puy Portillo M. ¿Son los isómeros del ácido linolénico conjugado una alternativa a isómeros del ácido linoleico conjugado en la prevención de la obesidad?. Endocrinol Nutr. 2014;61:209–219.